Abstract
We have developed a stereospecific, nickel-catalyzed cross-coupling of secondary benzylic ammonium salts and diboronate esters to deliver highly enantioenriched benzylic boronates. This reaction utilizes amine-derived electrophiles, which are readily available in high enantiopurity, and simple, inexpensive nickel catalysts. This reaction has broad scope, enabling synthesis of a variety of secondary benzylic boronates in good yields and excellent ee’s.
Graphical Abstract
Enantioenriched secondary benzylic boronic esters are valuable synthetic intermediates for the construction of complex molecules. Their C–B bonds can be converted with enantiomeric fidelity to C–O bonds by oxidation1 or C–C bonds by cross couplings2 or Matteson-type homologations.1b, 1d, 3 Due to their utility, various methods have been developed to deliver these compounds in high enantiomeric enrichment (ee, Scheme 1). From alkenes, catalytic, enantioselective hydroboration,4 β-boration of α, β-unsaturated carbonyls,5 diboration,6 and 1,1-arylboration7 methods have been developed. Borylation of allylic electrophiles also delivers enantioenriched benzylic boronates.8 Boronate starting materials can also be utilized to prepare enantioenriched benzylic boronates. Methods utilizing boronate substrates include cross couplings of diboranes;9 hydrogenation, hydroboration, and conjugate additions of vinyl boronates;10 and homologation approaches, such as Aggarwal’s asymmetric deprotonation/borylation route.11
Scheme 1. Asymmetric synthesis of secondary benzylic boronates.
Conspicuously absent from the previously reported asymmetric syntheses of secondary benzylic boronates is a Miyaura borylation of a benzylic electrophile.12,13 This approach would offer a powerful alternative route from readily available starting materials. Notable methods for C–B cross couplings of alkyl halides, tosylates and ethers have been developed using copper and nickel catalysts; however, to date these methods only deliver achiral or racemic products.14,15,16 Miyaura borylations of alkyl electrophiles to deliver enantioenriched products are currently limited to allylic electrophiles.8, 17
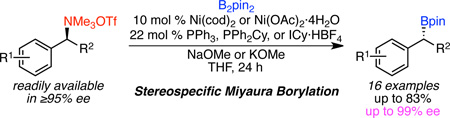
We have reported a stereospecific, nickel-catalyzed Suzuki–Miyaura cross coupling of benzylic ammonium triflates with arylboronic acids.18,19 Amine-derived substrates are ideal electrophiles for stereospecific cross couplings, because amines can be readily prepared in near perfect enantiomeric excess via a variety of methods.20 These amines can then be readily transformed to trimethylammonium salts via a two-step alkylation sequence, which requires only a single column chromatography purification.21,22 In addition, amine-derived substrates offer a functional group handle orthogonal to ethers, and both amines and ammonium salts are stable to long-term storage. We envisioned that a stereospecific Miyaura borylation of these attractive amine-derived electrophiles would be possible by changing the arylboronic acid to a diborane coupling partner.23 Herein we report an enantiospecific, nickel-catalyzed Miyaura borylation of secondary benzylic ammonium salts to deliver benzylic boronate esters in high yields and ee’s. This reaction represents the first example of a cross coupling of a benzylic electrophile to deliver a highly enantioenriched borane, and is one of the first examples of a stereospecific cross coupling of a benzylic electrophile and a heteroatomic nucleophile.14,13 Furthermore, it offers complementary scope to existing methods for asymmetric synthesis of benzylic boronate esters, such as asymmetric hydroboration of styrenes.
We selected the borylation of ammonium triflate 1a for our optimization. The amine precursor of 1a is commercially available in >99% ee. We assume no loss in ee during its conversion to ammonium triflate 1a. Using bis(pinacolato)diboron (B2pin2) under conditions similar to those optimized for the arylation of secondary benzylic ammonium triflates,18a low yield of boronate 2 was observed (Table 1, entry 1). However, by changing the base from K3PO4 to NaOMe, 78% yield and excellent stereochemical fidelity (95% ee) was achieved (entry 2).24 By lowering the reaction temperature, protodeboration is minimized, leading to even higher yield (entry 3). Investigation of the ligand showed that P(o-Tol)3 can be replaced with PPh3 (entry 4). Phosphine-less conditions are also successful, but provide 2 in slightly lower yield and ee (entry 5). In addition, air-stable Ni(OAc)2·4H2O can be used, with only a slight reduction in yield for the model reaction (entry 6). However, with some other substrates, Ni(OAc)2·4H2O proved inferior to Ni(cod)2 (not shown). Control experiments confirmed that both nickel and base are required for this reaction (entries 7 and 8). To obtain the highest levels of reactivity and enantiospecificity across a range of substrates, Ni(cod)2/PPh3 was selected as the optimal catalyst system (entry 4). Under these conditions, lower yields were obtained when iodide or methyl sulfate replaced triflate in the ammonium salt (30% and 72%, respectively, not shown). Consistent with the Suzuki arylation of benzylic ammonium triflates, this borylation proceeds with inversion of configuration.22 These results compare favorably with asymmetric hydroboration of 2-vinylnaphthalene to deliver 2a; via Rh- or Cu-catalyzed hydroboration, 2a is formed in only 85–86% ee.4b, 4d
Table 1.
Optimization of borylationa
 | |||||
|---|---|---|---|---|---|
| entry | [Ni] | L | temp (°C) |
yield (%)b |
ee (%)c |
| 1d | Ni(cod)2 | P(o-Tol)3 | 70 | 12 | n.d.e |
| 2 | Ni(cod)2 | P(o-Tol)3 | 70 | 78 | 95 |
| 3 | Ni(cod)2 | P(o-Tol)3 | rt | 84 | 98 |
| 4 | Ni(cod)2 | PPh3 | rt | 86 | 99 |
| 5 | Ni(cod)2 | None | rt | 81 | 97 |
| 6 | Ni(OAc)2·4H2O | PPh3 | rt | 80 | 99 |
| 7f | none | PPh3 | rt | trace | n.d.e |
| 8g | Ni(cod)2 | PPh3 | rt | 0 | n.d.e |
Conditions: ammonium triflate 1a (>99% ee, 0.1 mmol, 1.0 equiv), B2pin2 (1.5 equiv), [Ni] (10 mol %), ligand (L, 22 mol %), NaOMe (1.5 equiv), THF (0.2 M), 24 h, unless otherwise noted.
Determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard.
ee’s of the subsequent alcohol, formed via oxidation with H2O2and NaOMe. Determined by HPLC analysis using a chiral stationary phase.
K3PO4 replaced NaOMe.
n.d. = not determined.
11 mol % PPh3.
No base. 5 mol % Ni(cod)2, 11 mol % PPh3.
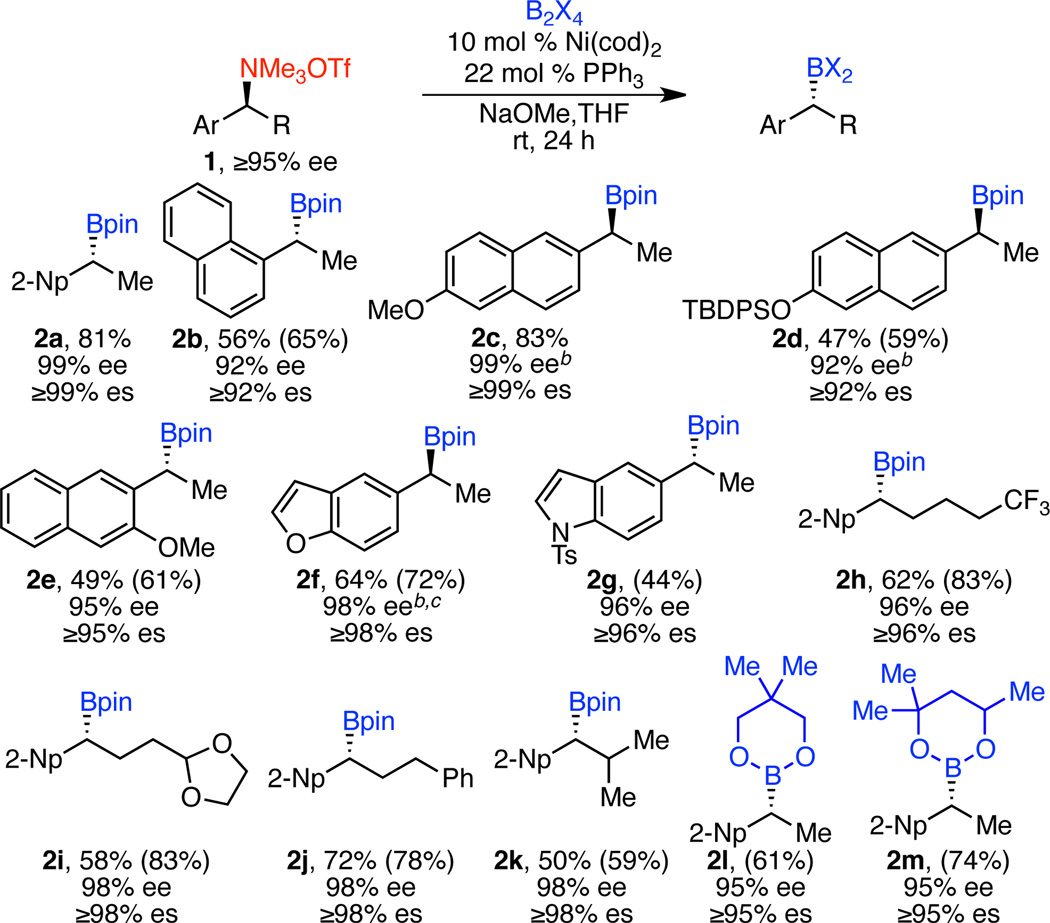
Under the optimized conditions, a variety of benzylic ammonium triflates successfully underwent borylation, delivering boronate products in good yields and excellent ee’s (Scheme 2).24 In every case, the benzylic amine precursors were prepared or purchased in high enantiopurity, highlighting an advantage of amine-derived electrophiles. Amines that were not commercially available were prepared via additions to Ellman’s sulfinimines.20b–d The sulfinamide intermediates were isolated as single diastereomers (≥95% de), as determined by 1H NMR, and we assume the subsequent ammonium triflates are prepared in ≥95% ee.22 As shown by the comparison of yields determined by 1H NMR analysis and isolated yields, the purification of some of these benzylic boronates often resulted in 10–15% loss of product due to unavoidable decomposition of these sensitive compounds. Nonetheless, synthetically useful yields are obtained.
Scheme 2. Scope of ammonium triflatea.
aConditions: ammonium triflate 1 (≥95% ee, 0.30 mmol, 1.0 equiv), B2X4 (1.5 equiv), Ni(cod)2 (10 mol %), PPh3 (22 mol %), Na-OMe (1.5 equiv), THF (0.2 M), rt, 24 h, unless otherwise noted. Isolated yields. Yields in parentheses determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard. Ee’s of the subsequent alcohol, formed via oxidation with H2O2 and NaOMe, determined by HPLC analysis using a chiral stationary phase. Enantiospecificity (es) = eeprod/eesm × 100. bOpposite enantiomer of 1 used. c50 °C.
In addition to the 2-naphthyl substituent, a variety of other arenes with extended π-systems are well tolerated, including the more sterically hindered 1-naphthyl (2b) and both methyl and silyl ether substituted naphthyls (2c–e). Substrates with heteroaryl groups also undergo borylation. Benzofuran 2f was formed in 64% yield and 98% ee. Indoles can also be utilized, providing excellent levels of stereochemical fidelity, albeit in lower yield (2g).
With respect to the alkyl substituent (R, Scheme 2), a wide variety of groups can be used. Linear alkyl groups are well tolerated, including those with trifluoromethyl and acetal functional groups (2h–j). Impressively, the borylation is also effective with branched alkyl substituents, such as a bulky i-Pr group (2k). Notably, asymmetric hydroboration cannot deliver benzylic boronates with branched substituents in this position. Dibenzylic ammonium salts (R = aryl) were unsuccessful in this borylation; no desired product was formed.
These reaction conditions are also amenable to the use of alternative diboranes. Borylation with bis(neopentylglycolato)diboron (B2neop2) and bis(hexyleneglycolato)diboron (B2hex2) delivered benzylic boronates 2l and 2m in good yields and excellent levels of stereochemical fidelity (Scheme 2). However, these benzylic boronates proved unstable to isolation, limiting their utility to downstream reactions that do not require purification after the Miyaura borylation.
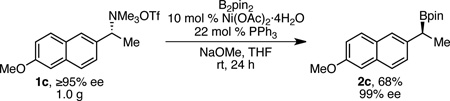
To demonstrate the synthetic utility and robustness, we conducted the borylation of ammonium triflate 1c, prepared in ≥95% ee, on a 1-gram scale (eq 1). We employed air-stable Ni(OAc)2·4H2O/PPh3 as the catalyst, allowing bench-top set-up. Boronate 2c was isolated in 68% yield and near perfect enantiopurity (99% ee). In contrast, asymmetric hydroboration resulted in only 88% ee of 2c.4b This boronate is a direct precursor to the anti-inflammatory drug (S)-naproxen, as shown by the Crudden group.4b
 |
(1) |
A current limitation of these conditions is that substrates must contain an aryl substituent with an extended π-system, such as naphthyl.25 The borylation to form p-fluorophenyl-substituted 2n under the standard Ni(cod)2/PPh3 conditions led to only 5% yield after 6 h. Hypothesizing that the lower reactivity of these substrates stems from difficulty in the oxidative addition, we investigated the use of more electron-donating ligands. By using a catalyst generated from Ni(cod)2 and either 1,3-bis(cyclohexyl)imidazolium tetrafluoroborate (ICy·HBF4) or PPh2Cy with KOMe as base at increased reaction temperature, synthetically useful yields and good ee’s of benzylic boronates 2n–p were achieved (Scheme 3).
Scheme 3. Borylation of non-naphthyl substratesa.
aConditions: ammonium triflate 1 (≥95% ee, 0.3 mmol, 1.0 equiv), B2pin2 (1.5 equiv), Ni(cod)2 (10 mol %), PPh2Cy (22 mol %), KOMe (1.7 equiv), THF (1.0 M), 70 °C, 24 h, unless otherwise noted. Average isolated yields (±3%). Yields in parentheses determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard. Average ee’s (±1%) of the subsequent alcohol, formed via oxidation with H2O2 and NaOMe, determined by HPLC analysis using a chiral stationary phase. b0.5 mmol scale. ICy·HBF4 (12 mol %) in place of PPh2Cy. Result of a single experiment.
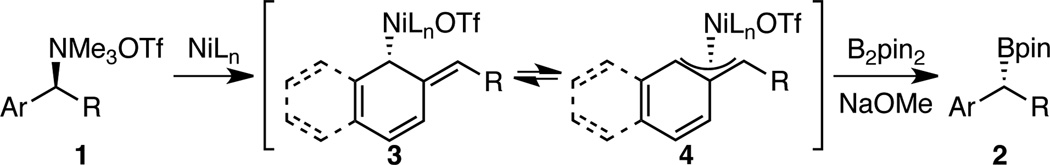
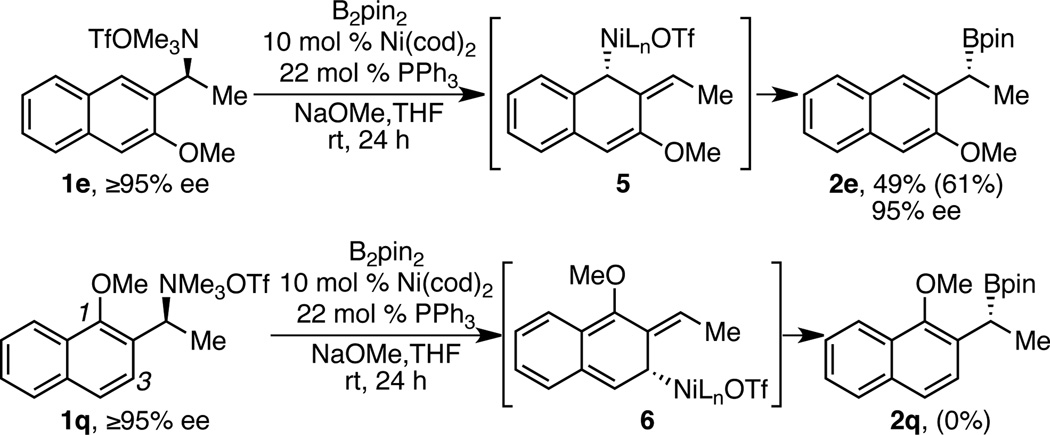
This reaction likely proceeds via an oxidative addition to generate a benzylic nickel species, which then undergoes transmetallation and reductive elimination to deliver enantioenriched benzylic boronate product (Scheme 4). As is commonly observed in stereospecific cross couplings of benzylic electrophiles, greater reactivity is observed for substrates containing aryl substituents with extended π systems.25 This observation, along with the overall inversion of configuration, is consistent with oxidative addition occurring in an SN2’ fashion via attack of the nickel catalyst at the ortho carbon to generate nickel intermediates 3 and/or 4, at least when the loss of aromaticity is not too costly. Further support for this hypothesis is seen by comparing the borylations of 1e and 1q (Scheme 5). The borylation of 1e is effective, but borylation of 1q resulted in no desired product. In this case, the methoxy group may hinder attack at C1. Attack at C3 would result in a less stable syn-allyl nickel intermediate 6 and a complete loss of aromaticity.26
Scheme 4. Proposed mechanism.
Scheme 5. Substituent effects on reactivitya.
aIsolated yields. Yields in parentheses determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard. Ee’s of the subsequent alcohol, formed via oxidation with H2O2 and NaOMe, determined by HPLC analysis using a chiral stationary phase.
In conclusion, we have developed a stereospecific, nickel-catalyzed Miyaura borylation of secondary, benzylic ammonium triflates to deliver benzylic boronates in good yields and excellent ee’s. For naphthyl-substituted substrates, this reaction utilizes a simple and inexpensive Ni(cod)2/PPh3 or Ni(OAc)2·H2O/PPh3 catalyst. By using a more electron-rich ligand, borylation of nonnaphthyl substrates has also been accomplished. This reaction represents the first example of a Miyaura borylation of a benzylic electrophile to deliver highly enantioenriched benzylic boronates, which represent a valuable class of intermediates for organic synthesis.
Supplementary Material
Acknowledgments
NIH (R01 GM111820) and the University of Delaware Research Foundation Strategic Initiatives program are gratefully acknowledged. For instrumentation support we are thankful for NSF CHE0421224, NSF CHE1229234, NSF CHE0840401, NIH P20 GM104316, NIH P20 GM103541, and NIH S10 RR02692.
Footnotes
ASSOCIATED CONTENT
Full experimental data, details on methods and starting materials, and copies of spectral data. The Supporting Information is available free of charge on the ACS Publications website.
REFERENCES
- 1.(a) Farthing CN, Marsden SP. Tetrahedron Lett. 2000;41:4235. [Google Scholar]; (b) Chen A, Ren L, Crudden CM. J. Org. Chem. 1999;64:9704. [Google Scholar]; (c) Bagutski V, Ros A, Aggarwal VK. Tetrahedron. 2009;65:9956. [Google Scholar]; (d) Larouche-Gauthier R, Elford TG, Aggarwal VK. J. Am. Chem. Soc. 2011;133:16794. doi: 10.1021/ja2077813. [DOI] [PubMed] [Google Scholar]; (e) Werle S, Fey T, Neudorfl M, Jr, Schmalz H-G. Org. Lett. 2007;9:3555. doi: 10.1021/ol071228v. [DOI] [PubMed] [Google Scholar]
- 2. Wang C-Y, Derosa J, Biscoe MR. Chem. Sci. 2015;6:5105. doi: 10.1039/c5sc01710f. Imao D, Glasspoole BW, Laberge VS, Crudden CM. J. Am. Chem. Soc. 2009;131:5024. doi: 10.1021/ja8094075. Doucet H. Eur. J. Org. Chem. 2008;2008:2013. Ohmura T, Awano T, Suginome M. J. Am. Chem. Soc. 2010;132:13191. doi: 10.1021/ja106632j. See also: Tellis JC, Primer DN, Molander GA. Science. 2014;345:433. doi: 10.1126/science.1253647.
- 3.Thomas SP, French RM, Jheengut V, Aggarwal VK. Chem. Rec. 2009;9:24. doi: 10.1002/tcr.20168. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hayashi T, Matsumoto Y, Ito Y. J. Am. Chem. Soc. 1989;111:3426. [Google Scholar]; (b) Crudden CM, Hleba YB, Chen AC. J. Am. Chem. Soc. 2004;126:9200. doi: 10.1021/ja049761i. [DOI] [PubMed] [Google Scholar]; (c) Noh D, Chea H, Ju J, Yun J. Angew. Chem., Int. Ed. 2009;48:6062. doi: 10.1002/anie.200902015. [DOI] [PubMed] [Google Scholar]; (d) Noh D, Yoon SK, Won J, Lee JY, Yun J. Chem. Asian J. 2011;6:1967. doi: 10.1002/asia.201100146. [DOI] [PubMed] [Google Scholar]
- 5.(a) Lee J-E, Yun J. Angew. Chem., Int. Ed. 2008;47:145. doi: 10.1002/anie.200703699. [DOI] [PubMed] [Google Scholar]; (b) Sim H-S, Feng X, Yun J. Chem. Eur. J. 2009;15:1939. doi: 10.1002/chem.200802150. [DOI] [PubMed] [Google Scholar]; (c) Chea H, Sim H-H-S, Yun J. Adv. Synth. Catal. 2009;351:855. [Google Scholar]; (d) Lillo V, Prieto A, Bonet A, Díaz-Requejo MM, Ramírez J, Pérez PJ, Fernández E. Organometallics. 2009;28:659. [Google Scholar]; (e) Shiomi T, Adachi T, Toribatake K, Zhou L, Nishiyama H. Chem. Commun. 2009:5987. doi: 10.1039/b915759j. [DOI] [PubMed] [Google Scholar]; (f) Fleming WJ, Müller-Bunz H, Lillo V, Fernández E, Guiry PJ. Org. Biomol. Chem. 2009;7:2520. doi: 10.1039/b900741e. [DOI] [PubMed] [Google Scholar]; (g) Park JK, Lackey HH, Rexford MD, Kovnir K, Shatruk M, McQuade DT. Org. Lett. 2010;12:5008. doi: 10.1021/ol1021756. [DOI] [PubMed] [Google Scholar]
- 6. Kliman LT, Mlynarski SN, Morken JP. J. Am. Chem. Soc. 2009;131:13210. doi: 10.1021/ja9047762. (b) for the related diboration of imines, see: Hong K, Morken JP. J. Am. Chem. Soc. 2013;135:9252. doi: 10.1021/ja402569j. Beenen MA, An C, Ellman JA. J. Am. Chem. Soc. 2008;130:6910. doi: 10.1021/ja800829y.
- 7.Nelson HM, Williams BD, Miró J, Toste FD. J. Am. Chem. Soc. 2015;137:3213. doi: 10.1021/jacs.5b00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman-Martinez A, Hoveyda AH. J. Am. Chem. Soc. 2010;132:10634. doi: 10.1021/ja104254d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Sun C, Potter B, Morken JP. J. Am. Chem. Soc. 2014;136:6534. doi: 10.1021/ja500029w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee JCH, McDonald R, Hall DG. Nat. Chem. 2011;3:894. doi: 10.1038/nchem.1150. [DOI] [PubMed] [Google Scholar]
- 10.(a) Lee JCH, Hall DG. J. Am. Chem. Soc. 2010;132:5544. doi: 10.1021/ja9104057. [DOI] [PubMed] [Google Scholar]; (b) Ding J, Lee JCH, Hall DG. Org. Lett. 2012;14:4462. doi: 10.1021/ol301958x. [DOI] [PubMed] [Google Scholar]; (c) Morgan JB, Morken JP. J. Am. Chem. Soc. 2004;126:15338. doi: 10.1021/ja044396g. [DOI] [PubMed] [Google Scholar]; (d) Feng X, Jeon H, Yun J. Angew. Chem., Int. Ed. 2013;52:3989. doi: 10.1002/anie.201208610. [DOI] [PubMed] [Google Scholar]
- 11.(a) Stymiest JL, Dutheuil G, Mahmood A, Aggarwal VK. Angew. Chem., Int. Ed. 2007;46:7491. doi: 10.1002/anie.200702146. [DOI] [PubMed] [Google Scholar]; (b) Roesner S, Casatejada JM, Elford TG, Sonawane RP, Aggarwal VK. Org. Lett. 2011;13:5740. doi: 10.1021/ol202251p. [DOI] [PubMed] [Google Scholar]; (c) Matteson DS, Sadhu KM, Ray R, Peterson ML, Majumdar D, Hurst GD, Jesthi PK, Tsai DJS, Erdik E. Pure Appl. Chem. 1985;57:1741. [Google Scholar]; (d) Beckmann E, Desai V, Hoppe D. Synlett. 2004;2004:2275. [Google Scholar]; (e) Scott HK, Aggarwal VK. Chem. Eur. J. 2011;17:13124. doi: 10.1002/chem.201102581. [DOI] [PubMed] [Google Scholar]
- 12.Ishiyama T, Murata M, Miyaura N. J. Org. Chem. 1995;60:7508. [Google Scholar]
- 13.During the preparation of this manuscript, Shi reported a nickel-catalyzed borylation of aryl and benzylic ammonium salts, including one stereospecific example. See: Hu J, Sun H, Cai W, Pu X, Zhang Y, Shi Z. J. Org. Chem. 2015 doi: 10.1021/acs.joc.5b02557. just accepted.
- 14. Dudnik AS, Fu GC. J. Am. Chem. Soc. 2012;134:10693. doi: 10.1021/ja304068t. Yang C-T, Zhang Z-Q, Tajuddin H, Wu C-C, Liang J, Liu J-H, Fu Y, Czyzewska M, Steel PG, Marder TB, Liu L. Angew. Chem., Int. Ed. 2012;51:528. doi: 10.1002/anie.201106299. Zarate C, Manzano R, Martin R. J. Am. Chem. Soc. 2015;137:6754. doi: 10.1021/jacs.5b03955. See also: Zarate C, Martin R. J. Am. Chem. Soc. 2014;136:2236. doi: 10.1021/ja412107b.
- 15.One example of moderate enantioselectivity (61% ee) in the nickel-catalyzed borylation of a racemic alkyl halide has been reported. See ref 14a.
- 16.For an alternative approach to racemic secondary benzylic boronates, see: Cho SH, Hartwig JF. J. Am. Chem. Soc. 2013;135:8157. doi: 10.1021/ja403462b.
- 17.Ito H, Kawakami C, Sawamura M. J. Am. Chem. Soc. 2005;127:16034. doi: 10.1021/ja056099x. [DOI] [PubMed] [Google Scholar]
- 18.(a) Maity P, Shacklady-McAtee DM, Yap GPA, Sirianni ER, Watson MP. J. Am. Chem. Soc. 2013;135:280. doi: 10.1021/ja3089422. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shacklady-McAtee DM, Roberts KM, Basch CH, Song Y-G, Watson MP. Tetrahedron. 2014;70:4257. doi: 10.1016/j.tet.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For related Suzuki cross-couplings of alcohol-derived electrophiles, see: Zhou Q, Srinivas HD, Dasgupta S, Watson MP. J. Am. Chem. Soc. 2013;135:3307. doi: 10.1021/ja312087x. Harris MR, Hanna LE, Greene MA, Moore C, Jarvo ER. J. Am. Chem. Soc. 2013;135:3303. doi: 10.1021/ja311783k.
- 20.(a) Nugent TC. Chiral Amine Synthesis. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2010. [Google Scholar]; (b) Tanuwidjaja J, Peltier HM, Ellman JA. J. Org. Chem. 2007;72:626. doi: 10.1021/jo0616512. [DOI] [PubMed] [Google Scholar]; (c) Procopiou G, Lewis W, Harbottle G, Stockman RA. Org. Lett. 2013;15:2030. doi: 10.1021/ol400720b. [DOI] [PubMed] [Google Scholar]; (d) Aggarwal VK, Barbero N, McGarrigle EM, Mickle G, Navas R, Suarez R, Unthank MG, Yar M. Tetrahedron Lett. 2009;50:3482. [Google Scholar]; (e) Mereyala HB, Pola P. Tetrahedron Asymmetry. 2003;14:2683. [Google Scholar]; (f) Savile CK, Janey JM, Mundorff EC, Moore JC, Tam S, Jarvis WR, Colbeck JC, Krebber A, Fleitz FJ, Brands J, Devine PN, Huisman GW, Hughes GJ. Science. 2010;329:305. doi: 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]; (g) Vries T, Wynberg H, van Echten E, Koek J, ten Hoeve W, Kellogg RM, Broxterman QB, Minnaard A, Kaptein B, van der Sluis S, Hulshof L, Kooistra J. Angew. Chem., Int. Ed. 1998;37:2349. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2349::AID-ANIE2349>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.(a) Icke RN, Wisegarver BB, Alles GA. Org. Synth. 1955;3:723. Coll. Vol. [Google Scholar]; (b) Borch RF, Hassid AI. J. Org. Chem. 1972;37:1673. doi: 10.1021/jo00979a032. [DOI] [PubMed] [Google Scholar]
- 22.Please see Supporting Information.
- 23.Nickel-catalyzed borylation via cleavage of aryl C–N bonds has been demonstrated. For example, see: Tobisu M, Nakamura K, Chatani N. J. Am. Chem. Soc. 2014;136:5587. doi: 10.1021/ja501649a.
- 24.Enantiospecificity (es) = eeprod/eesm × 100. See: Denmark SE, Vogler T. Chem. Eur. J. 2009;15:11737. doi: 10.1002/chem.200901377.
- 25.For discussion and solutions to this common limitation, see: Correa A, León T, Martin R. J. Am. Chem. Soc. 2014;136:1062. doi: 10.1021/ja410883p. Greene MAM, Yonova IMI, Williams FJF, Jarvo ERE. Org. Lett. 2012;14:4293. doi: 10.1021/ol300891k. Taylor BLH, Harris MR, Jarvo ER. Angew. Chem., Int. Ed. 2012;51:7790. doi: 10.1002/anie.201202527.
- 26.For a similar substituent effect study, see: Mendis SN, Tunge JA. Org. Lett. 2015;17:5164. doi: 10.1021/acs.orglett.5b02410.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.