Introduction
Ipilimumab, an immune checkpoint inhibitor, is approved for the treatment of advanced melanoma. Its potential activity in keratinocytic cancers, however, is not well known. We present a case of a man in his 60s with advanced basal cell carcinoma (BCC) that regressed after incidental exposure to ipilimumab to treat the patient's concurrent metastatic melanoma.
Case report
A man in his 60s presented to the dermatology clinic with a 40-year history of a slowly growing plaque behind his left ear that had recently auto amputated the pinna. He had not previously sought medical care, and on physical examination, there was a 13-cm ulcerated plaque around the external auditory meatus. A biopsy found nodular BCC, and magnetic resonance imaging (MRI) found infiltration of the 7th cranial nerve, parotid gland, masseter muscle, and left pterygoid bone with enhancement surrounding the left mandibular condyle and within the infratemporal fossa. The patient declined surgical treatment and started vismodegib at 150 mg daily with excellent clinical response with regression to 2-cm longest diameter after 1 year of treatment. However, a biopsy of the remaining tumor found residual BCC.
At this time, a new 4-mm erythematous papule at least 2 cm removed from the residual BCC was noted and confirmed not to be previously present by photographic documentation. A biopsy of this lesion found an amelanotic nodular melanoma with Breslow depth to 2.75 mm, no ulceration, and a mitotic index of 5 per square millimeter. The patient was not considered a good candidate for sentinel lymph node biopsy because of extensive scarring around the melanoma owing to BCC regression while on vismodegib. He underwent wide local excision with 1-cm margins that that showed negative margins on pathologic analysis.
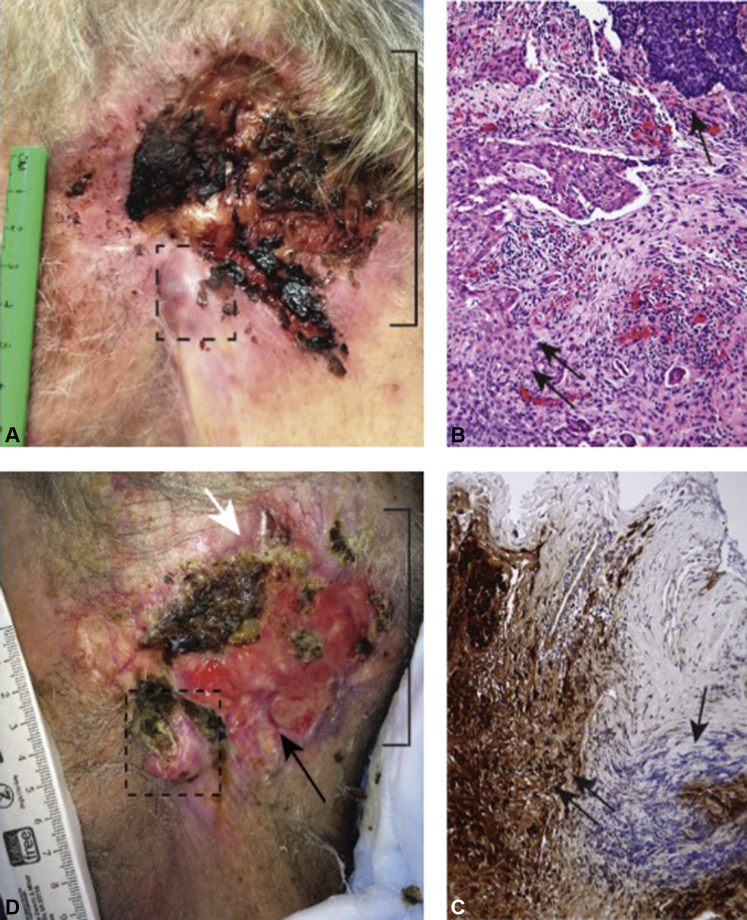
The patient did not return for recommended routine skin cancer surveillance until 14 months later, at which time there was clinically visible recurrence of his melanoma at the same site and increased growth in his adjacent BCC to 9 × 8 cm, as he had not been taking his vismodegib. A 1-cm nodule was visible in the location of his prior melanoma (Fig 1, A, box), confirmed to be the same nodular melanoma histology on biopsy (Fig 1, B, single arrow) and confirmed by S100 staining (Fig 1, C). The BCC had also regrown to abut the melanoma (Fig 1, B, double arrow). Positron emission tomography–computed tomography and MRI scans found lesions in the brain, bone, lung, and liver, which were subsequently confirmed by biopsy to be melanoma metastases.
Fig 1.
A, Recurrent nodular melanoma (dashed box) adjacent to advanced BCC (bracket) before ipilimumab therapy. B, Histolopathologic analysis of the nodule shows amelanotic nodular melanoma adjacent to BCC. Melanoma cells (double arrows) consisting of atypical epithelioid and spindled cells abut the BCC cells (single arrow) indicated by palisading basaloid cells with artifactual clefting. C, S100 positivity on immunohistochemical analysis highlights spindled cells, supporting melanoma histology (double arrows). BCC does not display S100 positivity (single arrow) (100× magnification, hematoxylin and eosin stain). D, After 8 weeks of ipilimumab exposure, the melanoma showed increased ulceration and growth (dashed box); however, the BCC had regressed with granulation tissue (bracket) filling the prior BCC ulcer bed and re-epithelialization (white arrow). Black arrow indicates site of biopsy that showed granulation tissue and no residual BCC. (B and C, Hematoxylin-eosin stain; original magnifications: B, ×40; C, ×100.)
The patient's melanoma was negative for the BRAF V600E mutation; therefore, he was started on ipilimumab at 3 mg/kg every 3 weeks. Although we considered concurrent therapy with vismodegib to treat the adjacent BCC, the lack of data on its safety and tolerability with ipilimumab led us to not reintroduce this treatment.
After 6 weeks of ipilimumab, the patient reported mild memory loss and difficulty with balance. An MRI scan of the brain found melanoma disease progression and new intracranial lesions. In addition, the patient's lactate dehydrogenase level increased from 43 U/L before ipilimumab initiation to 2,864 U/L. After 8 weeks of ipilimumab therapy, on clinical examination, his melanoma had unfortunately grown visibly (Fig 1, D, dashed red box).
We were surprised to observe that the adjacent BCC had decreased in size from 9 × 8 cm before ipilimumab to 5 × 7 cm after ipilimumab, with significant granulation tissue replacing the previous BCC ulcer (Fig 1, D). Although it was not feasible to sample the entire granulation tissue area for residual BCC, we did perform a 4-mm biopsy by punch technique to confirm granulation tissue and lack of BCC (Fig 1, D, arrow). Unfortunately, the patient passed away 2 weeks later from progressive central nervous system deterioration caused by metastatic melanoma. Nevertheless, the differential response of these 2 tumors types in this case offered a rare glimpse into the potential for systemic immunotherapy for nonmelanoma skin cancers.
Discussion
To our knowledge, there are no reports of BCCs treated with immune checkpoint inhibitors. We describe a case of advanced BCC regressing in the setting of ipilimumab treatment for treatment of a concurrent metastatic melanoma. This case of incidental exposure and regression of an advanced BCC to ipilimumab suggests that ipilimumab may have activity against BCCs. Ipilimumab binds cytotoxic T-lymphocyte antigen–4 (CTLA4) and promotes T-cell recognition of tumors such as melanoma1 and non–small cell lung cancer.2 BCCs are well known to respond to immunotherapy in the form of the topical toll-like receptor–7 agonist,3 imiquimod, although the pathways stimulated by ipilimumab and imiquimod are likely distinct. However, emerging evidence shows that the injectable toll-like receptor–7/8 agonist, 3M-052, potentiates the checkpoint blockade therapy of anti-CTLA4 antibodies and can augment antitumor effects of anti-CTLA4 antibodies.4 Our observations suggest that ipilimumab may have activity against BCC, and clinical trials utilizing this drug either alone or in combination with other therapies are needed to confirm this observation.
Acknowledgments
The authors are indebted to Robert LeBlanc, MD, for assistance with pathologic interpretation.
Footnotes
Funding sources: None.
Conflicts of interest: Relevant: Dr Chang is a clinical investigator on studies funded by Genentech, Novartis and Eli Lilly. She is also a consultant for Genentech and Novartis. Dr Mohan has no disclosures. Not relevant: Dr Chang is a clinical investigator for studies funded by Galderma and NuSkin. Dr Mohan and Dr Kuo have no disclosures.
References
- 1.Lipson E.J., Drake C.G. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck M., Bondarenko I., Luft A. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann of Oncol. 2012;24(1):75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 3.Oldfield V., Keating G.M., Perry C.M. Imiquimod: in superficial basal cell carcinoma. Am J Clin Dermatol. 2005;6(3):195–200. doi: 10.2165/00128071-200506030-00006. discussion 201-2. [DOI] [PubMed] [Google Scholar]
- 4.Singh M., Khong H., Dai Z. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol. 2014;193(9):4722–4731. doi: 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]