Introduction
Acquired reactive perforating collagenosis (ARPC) is a type of perforating disorder that is often associated with underlying systemic diseases, such as diabetes mellitus, renal failure, or malignancy. Giant variants of ARPC may reach up to 2 cm in size and have been reported in only a handful of cases. We present a patient with giant ARPC in the setting of metastatic breast cancer and uncontrolled diabetes mellitus.
Case report
A 46-year-old woman presented with a 3-month history of nonpruritic, nontender, hyperkeratotic papules and plaques that involved the upper and lower extremities. She recently had a diagnosis of de novo metastatic hormone receptor–positive breast cancer involving the brain, liver, bone, and lymph nodes and uncontrolled diabetes mellitus. She received dexamethasone, whole-brain radiation, tamoxifen, leuprolide, and insulin with excellent control of metastatic disease and diabetes. The patient was referred to the dermatology department from her oncologist to rule out disseminated zoster, as she had significant lymphopenia from long-term steroid use.
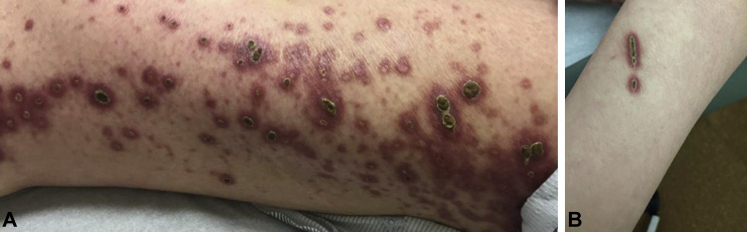
On examination, discrete, erythematous, umbilicated papules and plaques with central, adherent, 1- to 2-cm hyperkeratotic cores were seen on the upper and lower extremities (Fig 1, A). Koebnerization was apparent on the forearms (Fig 1, B). Laboratory analysis found a normal creatinine level of 0.48 mg/dL, an elevated glucose level of 217 mg/dL, and a hemoglobin A1c of 11.5%. A punch biopsy of a lesion on the lower extremity was performed.
Fig 1.
Giant ARPC. A, Erythematous, umbilicated papules and plaques with central adherent thick hyperkeratotic cores are seen on the upper and lower extremities. Representative lesions are seen on her left lateral thigh. The largest keratotic cores measure up to 2 cm. B, Evidence of koebnerization on the forearm.
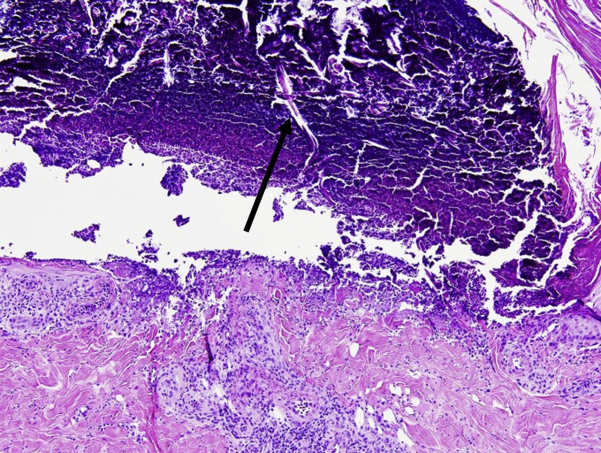
Histologic analysis found a large epidermal ulcer with overlying prominent scale crust. Vertically oriented, brightly eosinophilic collagen fibers were seen in the dermis, epidermis, and scale crust (Fig 2), confirming the diagnosis of ARPC.
Fig 2.
Histopathologic sections from punch biopsy specimen. A large epidermal ulcer is evident with vertically oriented collagen fibers (arrow) extruding from the dermis into the overlying thick scale-crust. (Hematoxylin-eosin stain.)
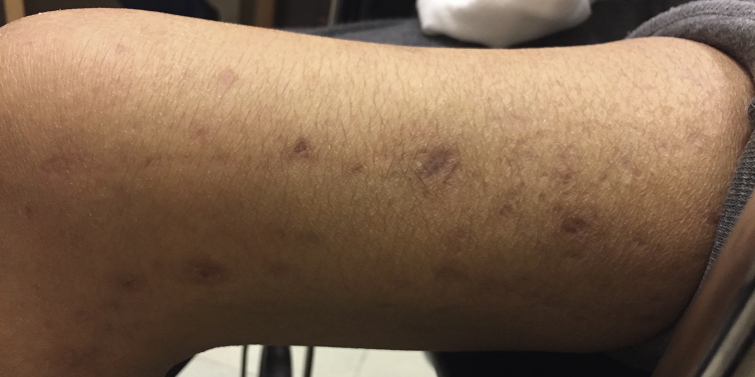
Because of her concurrent cancer therapies, the patient declined any dermatologic intervention for her lesions. However, after 10 months of glycemic control and chemotherapy, her lesions were significantly improved, with resolution of the hyperkeratotic cores and residual postinflammatory hyperpigmented macules (Fig 3).
Fig 3.
Resolving lesions of reactive perforating collagenosis after 10 months of glycemic control. Residual postinflammatory hyperpigmented macules at the site of prior lesions on the left lateral thigh.
Discussion
Reactive perforating collagenosis (RPC) is a type of perforating disorder in which degenerated collagen is transepidermally eliminated. Although initially described as a childhood disorder,1 acquired adult RPC has been reported2 particularly in association with underlying systemic disease.
Clinically, the lesions appear as erythematous-to-hyperpigmented papules, plaques, and nodules with a central umbilicated or crateriform hyperkeratotic core. The lesions are commonly pruritic and exhibit koebnerization. ARPC typically is distributed on the extensor aspects of the extremities, trunk, buttocks, and occasionally the face.3 Rare variants include giant lesions that measure 1 to 2 cm.4
Histopathologic features include a central crater, with epidermal ulceration and extrusion of vertically oriented connective tissue elements, such as collagen, keratin, and debris. A hyperkeratotic crust may overlie the ulcer.3
The differential diagnosis for ARPC largely encompasses other perforating disorders, such as perforating folliculitis, elastosis perforans serpiginosa, and Kyrle's disease. A more generalized term, acquired perforating dermatosis, is sometimes used to refer to such cases that occur in adults with systemic disease. The clinical spectrum of acquired perforating dermatosis includes erythematous follicular papules and pustules (perforating folliculitis–like), hyperkeratotic papules and nodules (Kyrle's disease–like), and umbilicated papules with keratotic plugs (RPC-like).5 Therefore, histologic analysis is necessary to identify the perforating element.
Although the pathogenesis is unknown, superficial trauma and diabetic microvasculopathy6 are hypothesized to be contributing factors to ARPC. In a review of approximately 100 cases, 62% were associated with diabetes mellitus and its complications.3 In 4 reported patients with giant ARPC, 3 had coexisting diabetes mellitus.4 ARPC is also associated with malignant conditions including leukemia, lymphoma, prostate carcinoma, hepatobiliary carcinomas, and papillary thyroid cancer.3 In at least 2 cases, ARPC developed in patients with diabetes mellitus and malignancy.7 Breast cancer–associated ARPC has never been reported in literature. Although our patient's ARPC is most likely associated with diabetes mellitus, we cannot exclude the possibility of an association between ARPC and breast cancer.
Treatments include topical glucocorticoids, topical and oral retinoids, oral antibiotics, and ultraviolet-B phototherapy, although the efficacy is often varied or unsatisfactory.3 Lesions may improve after treatment of the coexisting systemic condition. In our patient, her lesions dramatically improved with insulin and chemotherapy. As her cancer burden has continued to progress, we attribute the improvement of her ARPC with better glycemic control. Recently, allopurinol has been used successfully, particularly in the setting of diabetes mellitus8 and in giant ARPC.4 It is proposed that allopurinol may inhibit collagen cross-linking that is elevated in hyperglycemic states and may inhibit xanthine oxidase to decrease reactive oxygen species formation that leads to damaged collagen.8 In reported cases of giant ARPC, allopurinol 100 mg daily for 2 to 4 months resulted in appreciable improvement in pruritus and the number of lesions4 and is a reasonable treatment option to consider in such cases.
Acknowledgments
The authors thank Marianna Shvartsbeyn, MD, from The Ronald O. Perelman Department of Dermatology at New York University School of Medicine, for her assistance on the histopathologic photographs in this case.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Mehregan A.H., Schwartz O.D., Livingood C.S. Reactive perforating collagenosis. Arch Dermatol. 1967;96(3):277–282. [PubMed] [Google Scholar]
- 2.Faver I.R., Daoud M.S., Su W.P. Acquired reactive perforating collagenosis. Report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(4):575–580. doi: 10.1016/s0190-9622(94)70065-6. [DOI] [PubMed] [Google Scholar]
- 3.Karpouzis A., Giatromanolaki A., Sivridis E., Kouskoukis C. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37(7):585–592. doi: 10.1111/j.1346-8138.2010.00918.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoque S.R., Ameen M., Holden C.A. Acquired reactive perforating collagenosis: four patients with a giant variant treated with allopurinol. Br J Dermatol. 2006;154(4):759–762. doi: 10.1111/j.1365-2133.2005.07111.x. [DOI] [PubMed] [Google Scholar]
- 5.Saray Y., Seckin D., Bilezikci B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20(6):679–688. doi: 10.1111/j.1468-3083.2006.01571.x. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami T., Saito R. Acquired reactive perforating collagenosis associated with diabetes mellitus: eight cases that meet Faver's criteria. Br J Dermatol. 1999;140(3):521–524. doi: 10.1046/j.1365-2133.1999.02722.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsuboi H., Katsuoka K. Characteristics of acquired reactive perforating collagenosis. J Dermatol. 2007;34(9):640–644. doi: 10.1111/j.1346-8138.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 8.Munch M., Balslev E., Jemec G.B. Treatment of perforating collagenosis of diabetes and renal failure with allopurinol. Clin Exp Dermatol. 2000;25(8):615–616. doi: 10.1046/j.1365-2230.2000.00720.x. [DOI] [PubMed] [Google Scholar]