Introduction
Hydroquinone is the best studied and most widely used topical depigmenting agent, and represents the gold standard for treatment of disorders of pigmentation. Although ochronosis is a known sequela of long-term hydroquinone use, pigmentation of the nails is rarely a reported side effect. We present a case of identical twins who developed hyperpigmentation of identical nails after the use of a compounded cream containing 10% hydroquinone and 0.05% tretinoin to improve facial dyschromia.
Case report
Sixty-two-year-old identical twin males presented with hyperpigmentation after the use of hydroquinone. Both twins noticed nail dyspigmentation on their distal fourth and fifth fingernails 3 months after starting treatment with hydroquinone. In both patients the hyperpigmentation was asymptomatic, and neither had any underlying onychodystrophy. Over the next 2 months, the brown dyspigmentation continued to extend proximally eventually measuring as much as 7 mm on the first patient's hand and 9 mm on the second patient's hand (Figs 1 and 2). At a follow-up visit 2 months later, the nails for both patients were a dusky brown color. Attempts to scrape off the pigment were unsuccessful. Periodic acid–Schiff stain was negative, and cultures showed no growth. Complete blood count and comprehensive metabolic panel were both within normal limits.
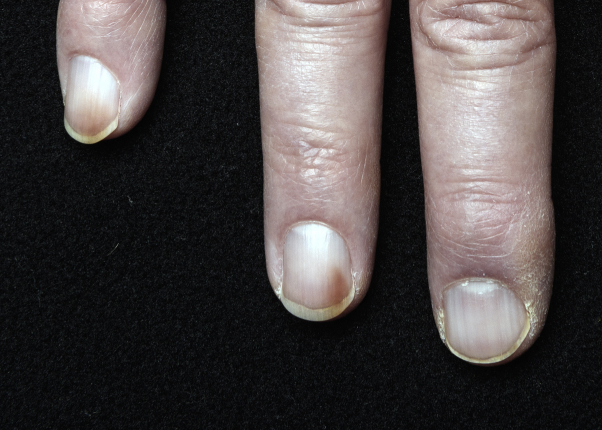
Fig 1.
Brown discoloration on the distal medial aspects of the nails on the fourth and fifth digits of twin A's hand.
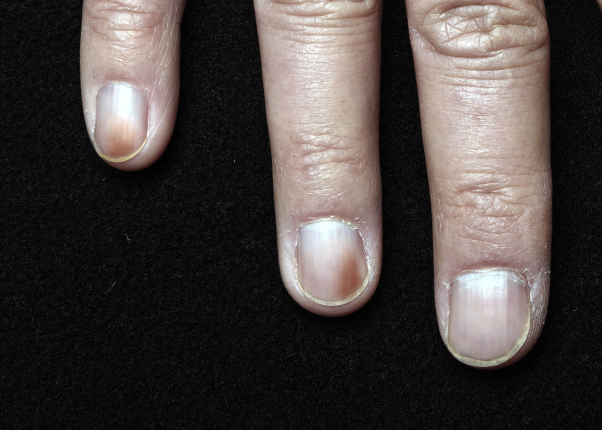
Fig 2.
Prominent brown discoloration on the distal medial aspects of the nails on the fourth and fifth digits of twin B's hand.
Both patients were using a mixture of 10% hydroquinone and tretinoin 0.05% cream for cosmetic improvement of facial lentigines. The dyschromia for both patients improved significantly after twice-daily use, applied using their fifth digit, for 2 months. Because of the unique temporal association of both individuals using the same compounded cream, it was thought that hydroquinone was the most likely causal agent and was therefore discontinued. The patients were seen again 3 months after stopping the hydroquinone cream, and the nail dyspigmentation on both individuals had resolved. One subject restarted the hydroquinone cream because of the asymptomatic and cosmetic nature of the nail dyspigmentation, and the same nail developed hyperpigmentation within 2 months of daily use.
Discussion
Fitzpatrick first recognized hydroquinone as a depigmenting agent in the 1960s.1 Hydroquinone is known to be a strong inhibitor of melanin production, but its mechanism of action is poorly understood. It is proposed that hydroquinone acts as a structural analog of melanin precursors that inhibits the conversion of L-DOPA to melanin by tyrosinase.
Hydroquinone also generates free radicals that further damage melanosomes.2 The paradoxical hyperpigmentation of fingernails is a rare phenomenon seen with hydroquinone use.
Very few cases reported of hydroquinone leading to hyperpigmentation of nails are reported.3, 4, 5 In these cases, the hyperpigmentation presented as an asymptomatic darkening of the distal fingernails on a variable number of nails on one or both hands. The pigment has been described as chestnut brown and typically worsened with continued use of hydroquinone. Sun exposure may lead to darkening and expansion of affected areas.5 Pigment changes typically resolve after cessation of hydroquinone.3, 4, 5 In our case, hyperpigmentation resolved in 3 months.
Hydroquinone is thought to be a safe and effective cream at low concentrations of 4% or less and is the gold standard for depigmenting agents in the United States. It can be found in concentrations up to 2% in over-the-counter products and up to 4% in prescription creams. Higher concentrations are available by compounding pharmacies and over the counter in other countries, but lower concentrations are typically used because of an increased potential for irritation and ochronosis. Although it is the most widely used depigmenting agent in the United States, that is not the case throughout the rest of the world where hydroquinone's safety has come into question.6 Although the most common side effects are typically self-limited, concerns remain about its potential mutagenicity and carcinogenicity. In vitro and animal models have shown DNA damage with use of hydroquinone. Animal models have also shown increased development of leukemia in some studies.7 This led some countries in Europe and Asia to ban hydroquinone over concerns about potential malignancy risk. Despite these claims, there are no documented cases of cutaneous or internal malignancy associated with hydroquinone use in humans.7 Reports of malignancy in animal models involved oral hydroquinone used at very high concentrations, much higher than those used topically. More than 25 cases of ochronosis were reported since hydroquinone's inception over 50 years ago, with none describing neoplastic induction. These studies show that there is no evidence that hydroquinone leads to malignancy when used topically and at low concentrations. Although nail hyperpigmentation is a rare side effect, we show here, similar to previous findings, that it is self-limited. It is important for providers to be aware of this potential complication and to discontinue hydroquinone use in a timely fashion if identified.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Arndt K.K., Fitzpatrick T.B. Topical use of hydroquinone as a depigmenting agent. JAMA. 1965;194:965. [PubMed] [Google Scholar]
- 2.Jimbow K., Obata H., Pathak M.A., Fitzpatrick T.B. Mechanism of depigmentation by hydroquinone. J Invest Dermatol. 1974;62:436–449. doi: 10.1111/1523-1747.ep12701679. [DOI] [PubMed] [Google Scholar]
- 3.Parlak A.H., Aydogan I., Kavak A. Discolouration of fingernails from using hydroquinone skin lightening cream. J Cosmet Dermatol. 2003;2(3-4):199–201. doi: 10.1111/j.1473-2130.2004.00085.x. [DOI] [PubMed] [Google Scholar]
- 4.Ozluer S.M., Muir J. Nail staining from hydroquinone cream. Australas J Dermatol. 2000;41(4):255–256. doi: 10.1046/j.1440-0960.2000.00448.x. [DOI] [PubMed] [Google Scholar]
- 5.Mann R.I., Harman R.R. Nail staining due to hydroquinone skin lightening creams. Br J Dermatol. 1983;108:363. doi: 10.1111/j.1365-2133.1983.tb03976.x. [DOI] [PubMed] [Google Scholar]
- 6.Chandra M., Levitt J., Pensabene C.A. Hydroquinone therapy for post-inflammatory hyperpigmentation secondary to acne: not just prescribable be dermatologists. Acta Derm Venereol. 2012;92(3):232–235. doi: 10.2340/00015555-1225. [DOI] [PubMed] [Google Scholar]
- 7.Nordlund J.J., Grimes P.E., Ortonne J.P. The safety of hydroquinone. J Eur Acad Dermatol Venereol. 2006;20:781–787. doi: 10.1111/j.1468-3083.2006.01670.x. [DOI] [PubMed] [Google Scholar]