Introduction
Human papillomavirus (HPV) is the causative agent of condyloma acuminata (CA). Although it is often assumed that CA are caused by HPV subtypes distinct from those that lead to squamous cell carcinoma, this is not always the case, particularly in immunocompromised patients. A greater percentage of HIV-positive men are infected with multiple HPV subtypes within their CA than their HIV-negative counterparts, with lower CD4 counts associated with higher levels of oncogenic HPV DNA. In addition, HIV-positive individuals have a higher prevalence and incidence of HPV-associated anal intraepithelial neoplasia (AIN) and anogenital cancers than their HIV-negative counterparts. Furthermore, many instances of malignant transformation of CA have been described. Here we show the spectrum and progression of disease from CA to aggressive anogenital squamous cell carcinoma in 3 HIV-positive patients and stress the diversity of clinical presentations that can exist in this patient population. Immunosuppression from HIV infection, co-infection with multiple HPV subtypes including oncogenic strains, a history of a low CD4 nadir, and the inability to adequately clear the HPV puts patients at a much greater risk of malignant progression of HPV-associated skin lesions, which can be rapid in some cases. Hence, it is critical that dermatologists screen patients and those affected receive close clinical monitoring, disease surveillance, and appropriate prompt treatment.
Case reports
Patient 1
An HIV-positive man in his 20s with a history of smoking, asthma, and pneumocystis pneumonia presented with numerous raised 2-mm condylomata on his penile shaft and anus. The patient was recently incarcerated and had sexual activity with more than 20 male and female partners. The patient had been inconsistent with his antiretroviral (ARV) regimen (efavirenz, emtricitabine, tenofovir, and ritonavir-boosted atazanavir) since his HIV diagnosis (baseline CD4 count of 22 cells per microliter). After restarting ARV therapy, his CD4 count increased and his viral load decreased; however, 8 months later the condylomata remained stable and continued to be unresponsive to imiquimod 5% 3 times weekly.
The patient did not return for 18 months. He presented with more than 20 skin-colored and hyperpigmented verrucous papules on his penile shaft, grouped hyperpigmented papules extending to the anal verge on his perianal skin, and a pedunculated 1-cm verrucous nodule on his right perianal skin. He underwent cryotherapy to the lesions on his penile shaft and perianal area and shave excision of the pedunculated lesion on his perianal skin. The biopsy found a pedunculated condyloma with a transition zone to squamous cell carcinoma in situ (Fig 1) with HPV-16 positivity (Fig 2). Imiquimod 5% was prescribed for treatment 5 times weekly for the remaining condyloma. Although we emphasized to the patient the importance of complete treatment, he was once again lost to follow-up.
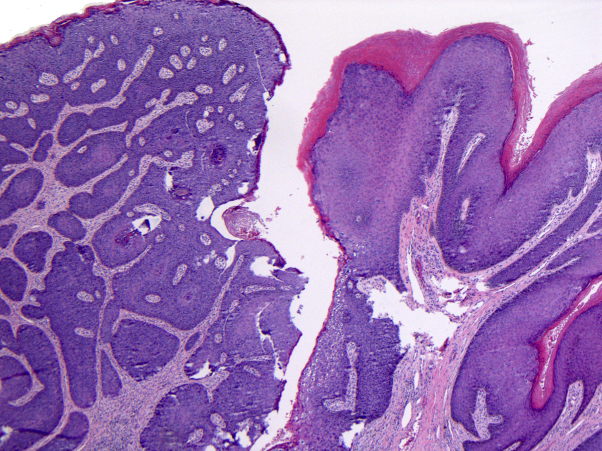
Fig 1.
Biopsy of pedunculated perianal lesion from patient 1. The biopsy shows a pedunculated condyloma with a transition zone to SCCIS (Hematoxylin-eosin stain; original magnification: ×40.)
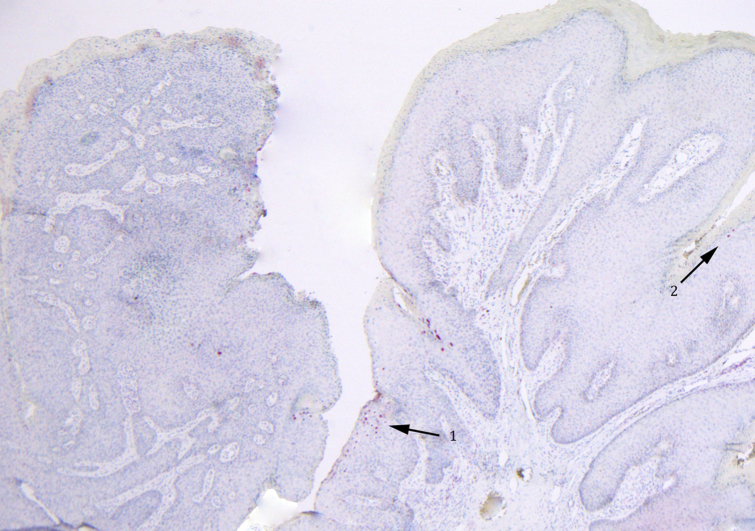
Fig 2.
HPV-16 immunostaining of the lesion in Fig 1. Significant HPV-16 nuclear staining is observed within the pedunculated lesion excised from patient 1. HPV-16 staining can be seen within the SCCIS region of the specimen (arrow 1) and within the adjacent condyloma (arrow 2) (original magnification: ×40.)
Patient 2
A man in his 40s with a long history of HIV positivity, moderate-to-heavy smoking, disseminated Kaposi's sarcoma, HPV-related oral leukoplakia (and subsequent invasive squamous cell carcinoma; Fig 3), HPV-related rectal cancer in remission, and a CD4 count less than 200 cells per microliter presented for evaluation and treatment of condylomata on the penis and perianal region. A diagnosis of CA was rendered; however, 1 lesion on the penis was larger and more atypical than the others, and a biopsy was performed. Histologically, the penile lesion showed squamous cell carcinoma in situ (SCCIS). The patient was treated with a combination of cryotherapy and imiquimod 5%.
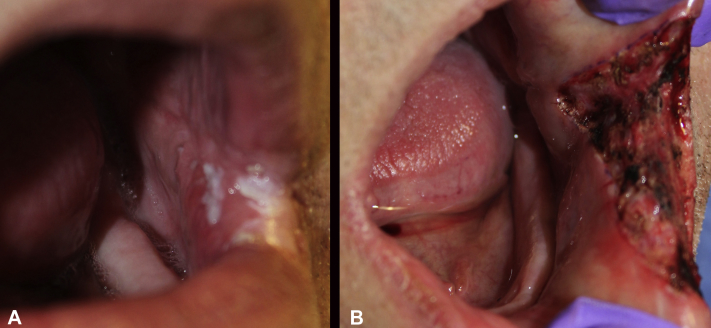
Fig 3.
HPV-related oral leukoplakia with biopsy-proven invasive squamous cell carcinoma. This is the clinical appearance before Mohs surgery (A) and after clearance of the tumor (B).
Over the next few months, the patient continued to have an excellent response to imiquimod 5%, with complete resolution of his penile CA. However, the patient then began experiencing perianal bleeding, and on physical examination was found to have a new perianal erosion suspicious for malignancy. Histologically, biopsies found SCCIS in the perianal area, and the patient was treated with imiquimod. Over the next year, the patient's CD4 count increased to 217 cells per microliter, and his viral load became undetectable, and all evidence of his perianal CA resolved.
The patient remained clear of his perianal CA for 3 years, at which point a concerning new perianal thin red plaque was noted. A biopsy of the lesion found recurrent SCCIS, and an excision was performed. Results of a follow-up anoscopy were normal, and the patient was counseled to undergo an annual anoscopy because of his high risk of anal dysplasia and recurrent anal cancer.
Patient 3
An HIV-positive (baseline CD4 count of 138 cells per microliter [20%], viral load of 33,453 copies per milliliter) man in his 20s with a history of central nervous system toxoplasmosis and perianal CA presented in acute renal failure and with a recent history of rectal bleeding. The patient identified as a man who has sex with men (MSM). Upon admission, random biopsies of his rectum during colonoscopy found histologic changes consistent with HPV and low-grade AIN-1. The patient's CD4 count at this time was 77 cells per microliter, and he was started on ARV therapy (Truvada and ritonavir-boosted darunavir).
Over the next 6 months, the patient tolerated his ARV regimen well, with his CD4 count increasing to 120 cells per microliter and his viral load decreasing. However, the patient's lesions did not improve, and he presented for evaluation of a new painful partially prolapsed ulcerated lesion emanating from his anal canal. The patient underwent successful transanal excision of this anal canal mass and distal anal canal condyloma fulguration and excision. He also had new anal and perianal condyloma lesions. Excisional biopsies of these lesions were found to be a mixture of focal high-grade and low-grade squamous intraepithelial lesions. Imiquimod 5% was also prescribed, as the patient's lesions started to involve the perianal skin as well. However, the aggressive course that the patient's disease ultimately followed led him to require a systemic therapy for his disease.
The patient again presented to the emergency room with rectal bleeding and on flexible sigmoidoscopy was found to have a rectal mass that was partially obstructing and bleeding. On digital rectal examination, a large friable bleeding sessile fixated mass approximately 4 cm from the anal verge was found tethered to the rectal wall, encompassing 75% of the circumference of the lumen. Histologically, biopsies results of the lesion were conclusive for invasive squamous cell carcinoma.
The patient underwent computed tomography scanning for staging, which showed multiple pulmonary nodules and liver lesions in keeping with stage IV squamous cell carcinoma. The patient was referred for palliative systemic chemotherapy with cisplatin and fluorouracil (dose adjusted) plus definitive local radiation to the anorectal mass.
Discussion
HPV is the causative agent of CA, and certain HPV subtypes are also responsible for causing some forms of genital cancers.1 Although CA are often considered to be caused by HPV subtypes distinct from those that lead to squamous cell carcinoma, we now know this to be a dangerous assumption to make clinically, particularly in certain populations such as immunocompromised patients. Infections with multiple HPV types in CA are common,2 and many instances of co-infection with oncogenic HPV and malignant transformation of condyloma have been described. We now have a better understanding that sexual transmission of oncogenic HPV subtypes is likely responsible for many squamous cell malignancies that occur in the anogenital tract of both men and women.3
Previous studies have reported a higher prevalence and incidence of anogenital HPV infection in HIV-positive MSM than in HIV-negative MSM.4, 5 Consistent with this, HIV-positive men and women have a higher prevalence and incidence of HPV-associated AIN and anogenital cancers than their healthy HIV-negative counterparts.6 The incidence of anal cancer in HIV-positive MSM was found to be twice as high than in their HIV-negative counterparts in one study.7 The relative risk of anal cancer among HIV-positive men and women was reported to be 37.9-fold and 6.8-fold higher, respectively, than that in the general population; the relative risk of penile cancer among HIV-positive men was 3.7-fold higher than that in the general population.8
Furthermore, although the HPV subtypes detectable in the anal canal of HIV-positive and HIV-negative men are similar, one study found that 73% of HIV-positive men were infected with multiple HPV types compared with only 23% of HIV-negative men, with HPV-16 the most common subtype.9 Among these HIV-positive men, lower CD4 counts were associated with higher levels of oncogenic HPV subtypes DNA (HPV-16/18/31/33/35/39/45/51/52/56/58), suggesting increased replication of these subtypes with more advanced immunosuppression.9 Evidence suggests that this increased risk may be a result of HIV-associated disruption of epithelial tight junctions, which, in the context of attenuated immunity, may potentiate HPV infection, reduce viral clearance, and ultimately contribute to the higher prevalence and incidence of HPV-associated neoplasia in this population.10 Other risk factors for anogenital cancers include a history of CA (3- to 5-fold increased risk), a history of sexually transmitted infections, and smoking.11
In our series, we found that it is often unclear whether the development of squamous cell malignancies from CA are caused by true transformation or the concomitant presence of multiple HPV subtypes (including oncogenic subtypes) in adjacent skin, but true transformation does occur in some instances. Although the ARV regimens now available are undoubtedly more efficacious than ever before, with HIV patients recovering CD4 counts and suppressing viral loads, a clinician must take care not to assume the CA of an HIV patient will behave in a benign manner, as they likely would in their otherwise healthy counterparts. Existing data suggest that even if a patient has been on an ARV regimen for an extended period, a history of HPV infection at the time of a low CD4 nadir puts them at a much greater risk of malignant progression of HPV-associated skin lesions.
Because the rate of progression from CA to squamous cell malignancy is unpredictable, a patient may initially present with benign-appearing CA but in reality be infected with an oncogenic HPV subtype masquerading as benign CA. A patient's CA may remain stable for years without transformation, as in the case of our first patient, but could also progress as far as incurable metastatic squamous cell carcinoma in a manner of months, as in the case of our third patient. Others may have a continuous long-term battle with HPV-related dysplasia, as in the case of our second patient. Hence, it is critical that all HIV patients, especially those with a history of HPV, be examined in the genital and anal areas on a regular basis to evaluate for new or recurrent disease and the need for treatment. There should be a low threshold for biopsies to rule out malignant progression and regular clinical surveillance through physical examination and malignancy screening.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Insinga R.P., Dasbach E.J., Elbasha E.H. Epidemiologic natural history and clinical management of human papillomavirus (HPV) disease: A critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC Infect Dis. 2009;9:119. doi: 10.1186/1471-2334-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anic G.M., Giuliano A.R. Genital HPV infection and related lesions in men. Prev Med. 2011;53(Suppl 1):S36–S41. doi: 10.1016/j.ypmed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson R.A. Human papillomavirus: Confronting the epidemic-a urologist's perspective. Rev Urol. 2005;7(3):135–144. [PMC free article] [PubMed] [Google Scholar]
- 4.Breese P.L., Judson F.N., Penley K.A., Douglas J.M., Jr. Anal human papillomavirus infection among homosexual and bisexual men: prevalence of type-specific infection and association with human immunodeficiency virus. Sex Transm Dis. 1995;22(1):7–14. doi: 10.1097/00007435-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Palefsky J.M., Shiboski S., Moss A. Risk factors for anal human papillomavirus infection and anal cytologic abnormalities in HIV-positive and HIV-negative homosexual men. J Acquir Immune Defic Syndr. 1994;7(6):599–606. [PubMed] [Google Scholar]
- 6.Palefsky J.M. Anal cancer prevention in HIV-positive men and women. Curr Opin Oncol. 2009;21(5):433–438. doi: 10.1097/CCO.0b013e32832f511a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreuter A., Potthoff A., Brockmeyer N.H. Anal carcinoma in human immunodeficiency virus-positive men: Results of a prospective study from Germany. Br J Dermatol. 2010;162(6):1269–1277. doi: 10.1111/j.1365-2133.2010.09712.x. [DOI] [PubMed] [Google Scholar]
- 8.Frisch M., Biggar R.J., Goedert J.J. Human papillomavirus-associated cancers in human with human immunodeficiency virus and acquired immunodeficiency syndromes. J Natl Cancer Inst. 2000;92(18):1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 9.Palefsky J.M., Holly E.A., Ralston M.L., Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis. 1998;177(2):361–367. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 10.Tugizov S.M., Herrera R., Chin-Hong P. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013;446(1-2):378–388. doi: 10.1016/j.virol.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross G., Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med Microbiol Immunol. 2004;193(1):35–44. doi: 10.1007/s00430-003-0181-2. [DOI] [PubMed] [Google Scholar]