Abstract
The Ehrlichia muris–like agent (EMLA) is a newly recognized human pathogen found in Wisconsin and Minnesota. Ecological investigations have implicated both the blacklegged tick, Ixodes scapularis, and the white-footed mouse, Peromyscus leucopus, as playing roles in the maintenance of EMLA in nature. The work presented here shows that I. scapularis is an efficient vector of EMLA in a laboratory mouse model, but that Dermacentor variabilis, another frequent human biting tick found in EMLA endemic areas, is not. Additionally, I. scapularis larvae are able to acquire EMLA through co-feeding with infected nymphs. As EMLA only persists in mouse blood for a relatively short period of time, co-feeding transmission may play an important role in the maintenance of EMLA in ticks, and subsequently may play a role in limiting the geographic distribution of this pathogen in areas where co-feeding of larvae and nymphs is less common.
Keywords: Ehrlichia muris–like agent (EMLA), Ixodes scapularis, Peromyscus leucopus, Co-feeding, Transmission
Introduction
Ehrlichia are Gram-negative obligate intracellular bacteria that exist in nature in a complex lifestyle involving both arthropod vectors and mammalian hosts (All-sopp and McBride 2009). Until recently, the two predominant agents of human ehrlichiosis in the United States were Ehrlichia chaffeensis and Ehrlichia ewingii, which are transmitted by the lone star tick, Amblyomma americanum (Paddock and Yabsley 2007). In 2011, another Ehrlichia species was reported as a cause of disease in humans in Wisconsin and Minnesota (Pritt et al. 2011). This newly recognized pathogen, referred to as the Ehrlichia muris–like agent (EMLA), most closely resembles E. muris, an ehrlichial species not previously known to exist in the Western Hemisphere. E. muris was first isolated from the spleen of a wild mouse, Eothenomys kageus, in Japan in 1983 (Kawahara et al. 1993, Wen et al. 1995). Both humans and wild animals seroreact to E. muris antigens, indicating exposure to and potential infection caused by this bacterium (Kawahara et al. 1999). Outside of Japan, E. muris has been reported in Russia, Slovakia, and Korea (Alekseev et al. 2001, Smetanov et al. 2007, Kang et al. 2013).
To identify potential vectors of EMLA, ticks were collected at or near case-patient households. The environmental investigation collected Ixodes scapularis and Dermacentor variabilis ticks, but no specimens of A. americanum. No EMLA DNA was detected in any of 88 D. variabilis ticks, but EMLA DNA was detected in 17 (2.8%) of 609 I. scapularis ticks, identifying I. scapularis as a potential vector for this pathogen (Pritt et al. 2011).
Shortly after the initial reports of EMLA as a cause of disease in humans in Wisconsin and Minnesota, Telford et al. analyzed 760 I. scapularis adult ticks collected in Wisconsin between 1992 and 1997. The authors report that seven pools of six ticks each are positive for EMLA DNA for a minimum prevalence of infection of 0.94% (Telford III et al. 2011). Stromdahl et al. tested 2783 I. scapularis adults and nymphs collected by the Department of Defense from 17 states in the eastern and midwestern United States (collected during 2007–2012). They reported that 4.6% of 196 ticks from Wisconsin and 0.5% of 365 ticks from Minnesota were positive for EMLA DNA, but all of the 2222 I. scapularis from 15 other states were negative (Stromdahl et al. 2014). These data strongly suggest that I. scapularis plays a primary role in the maintenance of EMLA in Wisconsin and Minnesota.
In the laboratory, I. scapularis is able to both acquire and transmit EMLA through feeding on mice (Saito and Walker 2015). However, EMLA is only detected in the blood of mice for a relatively short time, raising the possibility that other forms of EMLA transfer, such as co-feeding transmission, may be important. In co-feeding transmission, the salivary transfer of agent from an infected vector to a different un-infected vector occurs without a requirement for bacteremia in the host; this has been demonstrated to occur with both Borrelia and Rickettsia (Voordouw 2015). Alternatively, another human biting tick, D. variabilis, is also commonly present in Minnesota and Wisconsin and is a confirmed vector of E. chaffeensis (Stoffel et al. 2014), raising the possibility that this species may play a role in the transmission of EMLA.
This study was undertaken to evaluate the ability of I. scapularis and D. variabilis to acquire and transmit EMLA to a susceptible host, Mus musculus, and to evaluate the ability of these ticks to acquire this pathogen through co-feeding by different tick life stages.
Materials and Methods
Propagation of EMLA
EMLA strain Wisconsin (generously provided by Dr. Ulrike Munderloh, University of Minnesota) was propagated in RF/6A rhesus macaque endothelial cells (CRL-1780; American Type Culture Collection [ATCC], Manassas, VA) grown at 37°C in 5% CO2. The cells were fed with Minimal Essential Media (MEM) (Gibco, Carlsbad, CA) supplemented with 0.1 mM MEM non-essential amino acids (NEAA; Gibco), 10 mM HEPES buffer (Gibco), 2 mM L-glutamine (Gibco), 10 mM sodium pyruvate (Gibco), and 10% heat-inactivated fetal bovine serum (FBS; Atlas Biologicals, Fort Collins, CO).
Mouse infection
All studies were performed in accordance with a Centers for Disease Control and Prevention (CDC) Institutional Animal Care and Use Committee–(IACUC) approved animal use protocol 2391LEVMOUC-A2. Female C57BL/6J mice (6–10 weeks old) (Jackson Laboratories, Bar Harbor, ME) were injected intraperitoneally (i.p.) with RF/6A cells infected with EMLA and were bled via submandibular vein puncture with a lancet. Mice were euthanized via inhalation of CO2 followed by cervical dislocation, and tissues were aseptically removed during necropsy. To investigate ehrlichial levels in the blood a total of 20 mice were inoculated i.p. with homogenized lung from an EMLA-infected mouse (10 each with 103 and 102 genomic copies) suspended in Snyder I (sucrose phosphate glutamate buffer) medium. Each group of 10 mice was further subdivided into three groups of three mice each, with one group being bled each day so that each mouse was only bled every third day; one mouse of each group of 10 was held as a replacement in case a mouse succumbed to infection. Daily blood collection continued for 2 weeks, at which time the daily collections ceased and blood was collected from every mouse twice weekly. Mice were euthanized on day 28 postinfection (PI), and blood, lung, and liver tissues were collected for analysis.
Propagation of ticks
Uninfected colonies of both I. scapularis and D. variabilis are maintained in the Medical Entomology Laboratory at the CDC by feeding all developmental stages upon specific pathogen-free New Zealand white rabbits (Oryctolagus cuniculus), as previously described (Troughton and Levin 2007). The I. scapularis colony originated from adult ticks collected from vegetation in Connecticut, whereas the D. variabilis colony originated from adult ticks collected from vegetation in Georgia. Between feedings, ticks were held in incubators at 22°C ± 1° with a photoperiod of 16 h light/8 h dark and 90% relative humidity. The ticks in these colonies are monitored routinely, and every generation is confirmed to be free of known ehrlichial pathogens through PCR of representative samples of larvae in progeny of every female and by immunofluorescence assay (IFA) of serum samples from every animal used for colony maintenance.
Tick transmission studies
For all tick transmission studies, two duplicate cohorts of ticks were used, one comprised of I. scapularis ticks and one of D. variabilis ticks. C57BL/6J mice were inoculated i.p. with 0.5 mL of homogenized infected lung tissue (103 genomic copies EMLA) suspended in Snyder I buffer. Approximately 300 colony-reared larval ticks were placed on the neck, head, and back of each mouse. Larva were placed on the mice 7 days postinoculation so that tick feeding would coincide with the established peak of EMLA bacteremia (≈10–11 days postinoculation), as determined from mouse bacteremia studies. Ticks were allowed to feed to repletion and detach from the mice (3–6 days).
Engorged larvae were collected daily and placed in an environmental incubator to molt to nymphs. For nymphal feeding, 10–12 infected ticks were placed in a feeding capsule (modified Eppendorf tube glued with surgical glue to the shaved back of the mouse) on each naïve mouse. The nymphs were allowed to feed to repletion (3–6 days) until they detached from the mouse. The detached nymphs were collected daily and placed in the environmental chamber and allowed to molt to adults. To infect additional larvae via acquisition feeding from infected animals, ≈300 larvae were placed on each mouse 7 days after placement of infected nymphs. The larval infestation was timed to ensure the ticks were feeding during the peak of bacteremia transmitted by nymphal tick feeding. The engorged larvae were collected and allowed to molt to nymphs as previously described.
For the co-feeding studies, 10 nymphs previously infected with EMLA by feeding on infected mice as larvae were placed in feeding capsules along with approximately 100 noninfected larvae. Both tick stages were allowed to feed to repletion and allowed to molt as previously stated.
DNA Extraction and PCR
DNA was extracted from a small portion of each necropsied tissue, and the remaining homogenized tissue was suspended in Snyder I and frozen at −80°C for later use. For DNA extraction, the tissue was minced with a sterile scalpel, and total DNA was extracted using a QiaAmp Mini DNA Kit (Qiagen, Valencia, CA). Extraction blanks were included in every set of samples extracted. DNA was extracted from 100 μL of blood using a FlexiGene DNA Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Ticks were placed in 1.8-mL Sarstedt vials (larvae in pools of five, while nymphs and adults were processed individually), and the surface disinfected as previously published (Bermúdez et al. 2009). Total DNA was extracted using the Wizard SV96 Genomic DNA Kit (Promega, Madison, WI) and eluted in 120 μL of sterile nuclease free water.
EMLA DNA was detected using a qPCR assay targeting the 16S rRNA gene, as previously described (Li et al. 2002, Eremeeva et al. 2007, Pritt et al. 2011), but using the SsoFast EvaGreen Supermix instead of SYBR Green (BioRad, Hercules, CA). No template control samples consisting of the reaction mix and sterile water in place of template DNA were included on every reaction plate. A control plasmid was constructed by ligating the PCR product into pCR2.1 using the TA Cloning Kit (Invitrogen, Carlsbad, CA) following the manufacturer’s recommendations. Absolute quantification was based on plasmid copy number determined using a NanoDrop 1000 (NanoDrop Technologies, Wilmington, DE). Viable counts were estimated from the genomic DNA content, with one genomic copy of DNA representing one organism.
Co-feeding tick samples were tested using an EMLA-specific real-time PCR assay targeting the outer membrane protein p13 (Allerdice et al. 2015). The PCR was performed in a BioRad CFX 96 thermal cycler using the Quantitect Multiplex PCR Kit (Qiagen, Valencia, CA). As above, no template controls were included on every reaction plate.
Results
Mouse infection
C57BL/6J mice injected i.p. with either 105 or 104 genomic copies of EMLA in infected RF/6A cells exhibited huddling and lethargy 4 days PI. Three of five mice injected with 105 genomic copies died 7–8 days PI and one of five mice inoculated with 104 genomic copies of EMLA died on day 8 PI. All surviving mice exhibited signs of illness and were euthanized on day 8 PI to minimize suffering; the spleen, liver, kidney, and lungs were removed aseptically. Sections of each tissue were homogenized, and the total DNA was extracted and tested for the presence of EMLA DNA, as previously described (Li et al. 2002, Eremeeva et al. 2007, Pritt et al. 2011). All six liver and lung, five of six kidney, and two of six spleen samples were positive for EMLA DNA (data not shown). However, due to the rapid mortality seen in this initial experiment, the bacterial doses were determined to be too high and lower concentrations were used in subsequent experiments.
To determine a more precise dose for mouse infection studies, 15 mice were inoculated i.p. with RF/6A cells infected with EMLA; five mice each with 7.25 × 103, 7.25 × 102, and 7.25 × 101 genomic copies of EMLA. All mice were euthanized on day 6 PI and tissues were collected. DNA of EMLA was detected in the lungs and blood of 14 mice, in the kidney of 13 mice, and in the spleen and liver of eight mice. When the number of genomic copies detected were compared, the lungs had the highest average bacterial load per mg tissue weight tested followed by the liver, spleen, and kidney (Table 1).
Table 1.
EMLA in Mouse Tissues Following Intraperitoneal Inoculation
| Inoculation titer (GC) |
Spleen
|
Liver
|
Lung
|
Kidney
|
Blood
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| EMLA copies/mg tissue |
Pos/total samples |
EMLA copies/mg tissue |
Pos/total samples |
EMLA copies/mg tissue |
Pos/total samples |
EMLA copies/mg tissue |
Pos/total samples |
EMLA copies/mg tissue |
Pos/total samples |
|
| 7.25 × 103 | 61.0 | 2/5 | 81.3 | 2/5 | 956.1 | 5/5 | 16.8 | 4/5 | 1342.5 | 4/5 |
| 7.25 × 102 | 88.7 | 3/5 | 20.7 | 3/5 | 600.1 | 5/5 | 27.8 | 4/5 | 47.5 | 5/5 |
| 7.25 × 101 | 87.5 | 3/5 | 31.9 | 3/5 | 750.5 | 4/5 | 27.7 | 5/5 | 35.0 | 5/5 |
Number of genomic copies per milligram tissue weight for selected mouse tissues following i.p. inoculation with EMLA-infected RF/6A cells.
GC, genomic copies; EMLA, Ehrlichia muris–like agent; i.p., intraperitoneal.
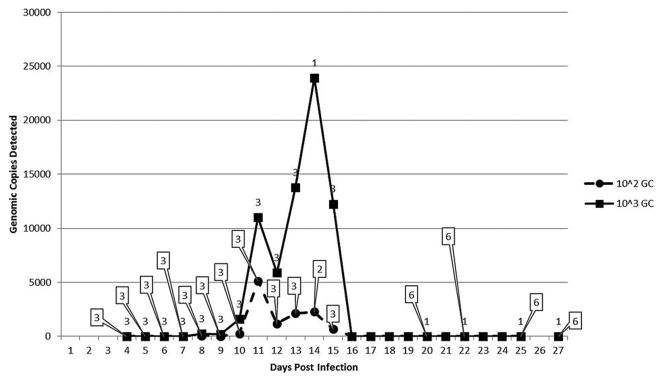
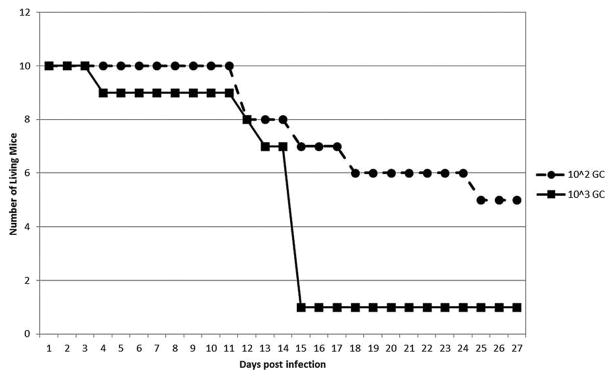
To determine the time course of EMLA bacteremia for use in subsequent tick feeding experiments, a total of 20 mice were inoculated i.p. with homogenized lung from an EMLA-infected mouse (10 each with 103 and 102 genomic copies) (Fig. 1). One mouse from the 103 dosage group died on day 10 PI, and all but one mouse from this group were dead by day 15 PI. Two of the 102 mice died on day 12 PI, both of which were blood PCR positive on day 11, and a third mouse from this group died on day 18 PI. This mouse’s blood titer peaked on day 12, and the EMLA levels had decreased on day 15. The remaining mice in this group survived until euthanized on day 28 PI. The 103 dosage group exhibited a peak in blood EMLA DNA levels between days 10–14 PI (Fig. 1), which corresponded to the period of high mouse mortality (Fig. 2). The levels of EMLA DNA in the blood of the 102 group also peaked at this time, although the amount of DNA detected was much lower than for the 103 group.
FIG. 1.
Detection of Ehrlichia muris–like agent (EMLA) in mouse blood following intraperitoneal (i.p.) infection with infected mouse lung. Shown is the average number of genomic copies (GC) of EMLA DNA detected in mouse blood following i.p. inoculation with 103 and 102 genomic copies. The numbers in boxes pointing to the 102 data points indicate the number of animals tested at that time point. The numbers above the 103 data points indicate the number of animals tested at that time point.
FIG. 2.
Mouse mortality after intraperitoneal (i.p.) inoculation with Ehrlichia muris–like agent (EMLA) in infected mouse lung. Mouse mortality following i.p. inoculation with two different doses (103 and 102 genomic copies [GC]) of EMLA.
Tick transmission
Two mice were injected i.p. with 103 genomic copies of EMLA in mouse lung. After infestation on day 7 with larval I. scapularis or D. variabilis ticks on different mice, the fed larvae were placed into incubators to molt. The mice were bled and euthanized 15 days after tick placement. Both of these blood samples were positive for EMLA DNA. Once the larvae molted, they were allowed to feed on naïve mice (two for each tick species) as nymphs. One week after the infected nymphs were placed, the mice were re-infested, but with naïve larvae. One of the two mice infested with I. scapularis nymphs began to show signs of illness (huddling and lethargy) 12 days after being infested. This mouse succumbed to its illness and died the next day. A blood sample for this mouse was positive for EMLA DNA. Both mice infested with D. variabilis as well as the other I. scapularis–infested mouse showed no signs of illness and were euthanized 14 days after nymphal placement, providing evidence that tick feeding alone had no detrimental effect on the health of the mice. Blood samples for these three mice were negative for EMLA DNA, as were samples from their livers, lungs, and spleens.
Once the larvae from the second round of transmission studies molted, nymphs from all four mice were used to infest a third group of naïve mice (five mice per tick species). Four of five mice infected with I. scapularis died between 10–13 days after tick placement, while the fifth mouse showed signs of illness and was euthanized on day 14. Blood from all five of these mice was positive for EMLA DNA. Tissues were collected from three of the five mice, and the livers, lungs, and spleen were all positive for EMLA. None of the mice infested with D. variabilis showed signs of illness and all five mice were euthanized 14 days after infestation. Blood and tissues (liver, lung, spleen) from these mice were all negative for EMLA DNA.
To test for the ability of I. scapularis to acquire EMLA via co-feeding, naïve larvae were fed on five naïve mice while infected nymphs were also allowed to feed. Both larvae and nymphs were allowed to feed to repletion and allowed to molt. A subset of the molted nymphs was screened, and 17/135 (12.6%) were positive for EMLA DNA.
Discussion
As occurs with classic E. muris, Ixodes ticks can acquire and transmit EMLA to mice in an experimental setting (Lynn et al. 2015, Saito and Walker 2015, Saito et al. 2015). However, the data we present here agrees with that of Saito et al. (2015). Unlike E. muris, EMLA can cause lethal infections in C57BL/6 mice in a dose-dependent manner, and this lethality is also observed via tick transmission (Olano et al. 2004, Saito and Walker 2015). Additionally, similar to what was previously reported (Saito et al. 2015), our data show that the bacteria can be found in multiple organs in the mouse, including the liver, spleen, and lungs, with the lungs having the highest bacterial load per milligram of tissue.
I. scapularis have been suggested to be a vector of EMLA (Pritt et al. 2011, Telford III et al. 2011, Stromdahl et al. 2014), and EMLA has been shown to disseminate throughout the tick (Lynn et al. 2015). However, D. variabilis, a common human-biting tick, is also present in areas endemic for EMLA, and has been previously shown to acquire other pathogenic Ehrlichia (Kramer et al. 1999, de los Santos et al. 2007, Pritt et al. 2011, Stoffel et al. 2014). We have shown that I. scapularis larvae, but probably not D. variabilis larvae, can acquire EMLA by feeding on an infected mouse, and that the bacterium is transmitted transstadially to subsequent life stages. However, Ehrlichia are typically not transmitted transovarially, and they require a mammalian reservoir to infect new ticks. The white-footed mouse, P. leucopus, has been implicated as a potential reservoir for EMLA in nature. Yet, only a small percentage of blood samples collected from wild mice are positive for EMLA DNA (two of 146; 1.4%) (Castillo et al. 2015). EMLA does not persist for very long in the blood of an infected mouse. Saito found that in a sublethal infection due to an intravenous inoculation of EMLA, the bacteria could only be detected in the blood for up to 30 days, despite being detected in the lung, lymph node, and bone marrow for at least 60 days (Saito et al. 2015). Our data suggest that the bacteria may be cleared from the blood even quicker following sublethal i.p. inoculation, with the bacteremia no longer detected after 15 days.
These studies were accomplished using an inbred laboratory mouse strain, C57BL/6J, that is not found in nature. It remains to be shown if the characteristics of EMLA infection and tick transmission data reported here and elsewhere are compatible with wild-type mice such as P. leucopus. Ecological studies are also needed to confirm the role of both P. leucopus and I. scapularis in the maintenance of EMLA in nature. It is striking that this mouse-and-tick pair is present in much of the northeastern United States, yet EMLA has only been reported in Wisconsin and Minnesota (Pritt et al. 2011, Stromdahl et al. 2014). Our experiments indicate that I. scapularis larvae can acquire EMLA while co-feeding in the presence of infected nymphs on a naïve mouse. Co-feeding transmission may play an important role in the maintenance of EMLA in nature given the relatively short amount of time the bacteria is found in the blood, as has been suggested for other rickettsial pathogens (Levin and Fish 2000, Hirunkanokpun et al. 2011, Levin et al. 2014).
Additionally, the phenology of I. scapularis may also play a role in limiting EMLA to Wisconsin and Minnesota. In the northeastern United States, there is a gap between peak nymphal activity in May to June and peak larval activity in August to September, limiting the role co-feeding could play in EMLA reservoir maintenance in nature (Levi et al. 2015, Voordouw 2015). This contrasts with what is observed in Minnesota and Wisconsin, where larvae and nymphs are both actively feeding in June (Stromdahl et al. 2014). With the peak of larvae and nymph activities overlapping, co-feeding transmission between these life stages could play a role in EMLA maintenance and could help explain the apparent geographic restriction of this pathogen. Finally, ecological investigations into additional tick vectors and mammalian reservoirs that may also account for this limited distribution are warranted.
Acknowledgments
The research reported here was supported in part by appointments of M. Allerdice and M. Sheth to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention (CDC). The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Alekseev AN, Dubinina HV, Van De Pol I, Schouls LM. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J Clin Microbiol. 2001;39:2237–2242. doi: 10.1128/JCM.39.6.2237-2242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allerdice MEJ, Pritt BS, Sloan LM, Paddock CD, et al. A real-time assay for detection of the Ehrlichia muris-like agent, a newly recognized pathogen of humans in the upper Mid-western United States. Ticks Tick Borne Dis. 2015;7:146–149. doi: 10.1016/j.ttbdis.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp BA, Mcbride JW. Ehrlichia. In: Nene V, Kole C, editors. Genome Mapping and Genomics in Animal-Associated Microbes. Berlin: Springer-Verlag; 2009. [Google Scholar]
- Bermúdez S, Eremeeva M, Karpathy S, Samudio F, et al. Detection and identification of rickettsial agents in ticks from domestic mammals in eastern Panama. J Med Entomol. 2009;46:856–861. doi: 10.1603/033.046.0417. [DOI] [PubMed] [Google Scholar]
- Castillo CG, Eremeeva ME, Paskewitz SM, Sloan LM, et al. Detection of human pathogenic Ehrlichia muris-like agent in Peromyscus leucopus. Ticks Tick Borne Dis. 2015;6:155–157. doi: 10.1016/j.ttbdis.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Delos Santos JR, Boughan K, Bremer WG, Rizzo B, et al. Experimental infection of dairy calves with Ehrlichia chaffeensis. J Med Microbiol. 2007;56:1660–1668. doi: 10.1099/jmm.0.47427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremeeva ME, Oliveira A, Moriarity J, Robinson JB, et al. Detection and identification of bacterial agents in Ixodes persulcatus Schulze ticks from the north western region of Russia. Vector Borne Zoonotic Dis. 2007;7:426–436. doi: 10.1089/vbz.2007.0112. [DOI] [PubMed] [Google Scholar]
- Hirunkanokpun S, Thepparit C, Foil LD, Macaluso KR. Horizontal transmission of Rickettsia felis between cat fleas, Ctenocephalides felis. Mol Ecol. 2011;20:4577–86. doi: 10.1111/j.1365-294X.2011.05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SW, Doan HT, Choe SE, Noh JH, et al. Molecular investigation of tick-borne pathogens in ticks from grazing cattle in Korea. Parasitol Int. 2013;62:276–282. doi: 10.1016/j.parint.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Suto C, Rikihisa Y, Yamamoto S, et al. Characterization of ehrlichial organisms isolated from a wild mouse. J Clin Microbiol. 1993;31:89–96. doi: 10.1128/jcm.31.1.89-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M, Ito T, Suto C, Shibata S, et al. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris as antigen. J Clin Microbiol. 1999;37:1123–1129. doi: 10.1128/jcm.37.4.1123-1129.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer VL, Randolph MP, Hui LT, Irwin WE, et al. Detection of the agents of human ehrlichioses in ixodid ticks from California. Am J Trop Med Hyg. 1999;60:62–65. doi: 10.4269/ajtmh.1999.60.62. [DOI] [PubMed] [Google Scholar]
- Levi T, Keesing F, Oggenfuss K, Ostfeld RS. Accelerated phenology of blacklegged ticks under climate warming. Philos Trans R Soci Lond B Biol Sci. 2015;370:20130556. doi: 10.1098/rstb.2013.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Fish D. Immunity reduces reservoir host competenance of Peromyscus leucopus for Ehrlichia phagocytophila. Infect Immun. 2000;68:1514–1518. doi: 10.1128/iai.68.3.1514-1518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Zemtsova GE, Montgomery M, Killmaster LF. Effects of homologous and heterologous immunization on the reservoir competence of domestic dogs for Rickettsia conorii (israelensis) Ticks Tick Borne Dis. 2014;5:33–40. doi: 10.1016/j.ttbdis.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JS-Y, Chu F, Reilly A, Winslow GM. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J Immunol. 2002;169:1419–1425. doi: 10.4049/jimmunol.169.3.1419. [DOI] [PubMed] [Google Scholar]
- Lynn GE, Oliver JD, Nelson CM, Felsheim RF, et al. Tissue distribution of the Ehrlichia muris-like agent in a tick vector. PLoS One. 2015;10:e0122007. doi: 10.1371/journal.pone.0122007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano JP, Wen G, Feng H-M, Mcbride JW, et al. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am J Pathol. 2004;165:997–1006. doi: 10.1016/S0002-9440(10)63361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Yabsley MJ. Ecological havoc, the rise of the white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. In: Childs JE, Mackenzie JS, Richt JA, editors. Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission. New York: Springer; 2007. pp. 289–324. [DOI] [PubMed] [Google Scholar]
- Pritt B, Sloan L, Johnson DKH, Munderloh U, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365:422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito TB, Walker DH. A tick vector transmission model of monocytotropic ehrlichiosis. J Infect Dis. 2015;15:968–977. doi: 10.1093/infdis/jiv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito TB, Thirumalapura NR, Shelite TR, Rockx-Brouwer D, et al. An animal model of a newly emerging human ehrlichiosis. J Infect Dis. 2015;211:452–461. doi: 10.1093/infdis/jiu372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetanov K, Boldis V, Kocianov E, Spitalsk E. Detection of Ehrlichia muris in a yellow-necked mouse (Apodemus flavicollis) in Central Slovakia. Acta Virol. 2007;51:69–71. [PubMed] [Google Scholar]
- Stoffel RT, Mcclure JC, Butcher MM, Johnson GC, et al. Experimental infection of Rhipicephalus sanguineus with Ehrlichia chaffeensis. Vet Microbiol. 2014;172:334–338. doi: 10.1016/j.vetmic.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromdahl E, Hamer S, Jenkins S, Sloan L, et al. Comparison of phenology and pathogen prevalence, including infection with the Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the midwestern and the northeastern United States over a 15 year period (1997–2012) Parasit Vectors. 2014;7:553. doi: 10.1186/s13071-014-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, III, Goethert HK, Cunningham JA. Prevalence of Ehrlichia muris in Wisconsin deer ticks collected during the mid 1990s. Open Microbiol J. 2011;5:18–20. doi: 10.2174/1874285801105010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troughton DR, Levin ML. Life cycle of seven ixodid tick species (Acari: Ixodidae) under standarized laboratory conditions. J Med Entomol. 2007;44:732–740. doi: 10.1603/0022-2585(2007)44[732:lcosit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Voordouw MJ. Co-feeding transmission in Lyme disease pathogens. Parasitology. 2015;142:290–302. doi: 10.1017/S0031182014001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B, Rikihisa Y, Mott J, Fuerst PA, et al. Ehrlichia muris sp. nov identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics. Int J Syst Bacteriol. 1995;45:250–254. doi: 10.1099/00207713-45-2-250. [DOI] [PubMed] [Google Scholar]