Abstract
Conversational storytelling integrates diverse cognitive and socio-emotional abilities that critically differ across neurodegenerative disease groups and may have diagnostic relevance and predict anatomic changes. The present study employed mixed methods discourse and quantitative analyses to delineate patterns of storytelling across focal neurodegenerative disease groups, and to clarify the neuroanatomical contributions to common storytelling characteristics in these patients. Transcripts of spontaneous social interactions of 46 participants (15 behavioral variant frontotemporal dementia (bvFTD), 7 semantic variant primary progressive aphasia (svPPA), 12 Alzheimer's disease (AD), and 12 healthy older normal controls) were analysed for storytelling characteristics and frequency, and videos of the interactions were rated for patients' social attentiveness. Compared to controls, svPPAs also told more stories and autobiographical stories, and perseverated on aspects of self during storytelling. ADs told fewer autobiographical stories than NCs, and svPPAs and bvFTDs failed to attend to social cues. Storytelling characteristics were associated with a processing speed and mental flexibility, and voxel-based anatomic analysis of structural magnetic resonance imaging revealed that temporal organization, evaluations, and social attention correlated with atrophy corresponding to known intrinsic connectivity networks, including the default mode, limbic, salience, and stable task control networks. Differences in spontaneous storytelling among neurodegenerative groups elucidated diverse cognitive, socio-emotional, and neural contributions to narrative production, with implications for diagnostic screening and therapeutic intervention.
Keywords: Narrative, Autobiographical memory, Social functioning, Focal neurodegenerative disease, Neural Networks
1. Introduction
Everyday storytelling is a complex social practice that distills and integrates diverse cognitive and socio-emotional processes (Hirsh, Mar, & Peterson, 2013). It reflects and supports memory encoding and retrieval (Mattarella-Micke & Beilock, 2010; Schank & Abelson, 1995; Wyer, Adaval, & Colcombe, 2002), theory of mind (Guajardo & Watson, 2002), and emotional regulation (Cappeliez, Guindon, & Robitaille, 2008). Storytelling also functions to foster rapport and intimacy with others (Bluck, 2003), and facilitate a sense of self by imposing a temporal structure on personal experiences (Bamberg, 2004; Bruner, 1991; McLean, Pasupathi, & Pals, 2007). Thus, storytelling can be a dynamic social tool that reflects and supports aspects of identity and social functioning. While much work has been done on psychological mechanisms and outcomes of storytelling (e.g., McAdams, Josselsonm and Lieblich, 2006; McLean et al., 2007; Pennebaker, & Seagal, 1999), the neural systems underlying everyday storytelling have not been clearly delineated, thus the impact of neurologic injury and disease on the capacity to produce meaningful narratives is unknown. For example, while differences in naturalistic storytelling might be expected to occur between neurodegenerative disease groups, and might be relevant to syndromic diagnosis or localization of neural dysfunction, spontaneous storytelling has not been examined in these patients.
While there is no single way to operationalize stories, narrative theorists and socio-linguist converge on two key ingredients: temporal organization and narrative evaluations (Bruner, 1990; Labov & Waletzky, 1967; Ochs & Capps, 1996). Though story plots hang on the temporal organization of past events (Mandler; 1984; Pennington & Hastie, 1992; Shank & Abelson, 1977), evaluations convey the speaker's interpretation of events and are cited as the raison d'etre behind personal storytelling (Bruner, 1990; Labov & Waletzky, 1967). In addition to conveying personal meaning, evaluations function as a social adhesive by inviting listeners to collaboratively interpret a set of events (Alea & Bluck, 2003; Losh, Bellugi, Reilly, & Anderson, 2000). While storytelling is complex, operationalization into these two primary components allows more direct linking of narrative processes with neural mechanisms.
1.1 Temporal Organization
The default mode network (DMN) is identified as central to story comprehension and production (D'Argembeau, Cassol, Phillips et al., 2013; Mar, 2011; Spreng, Mar, & Kim, 2008; Yang, Bossmann, Schiffhauer et al., 2012), although this network is likely involved primarily in the memory and temporal organization, or event sequencing, components of narrative. Comprehending and simulating narrative fiction has been shown to recruit the entire DMN (Mar, 2011; Wilson, Molnar-Szakacs, & Iacoboni, 2008) as well as regions within the DMN, such as the medial prefrontal cortex (mPFC) and medial temporal lobe (MTL) which have been linked to event sequencing (Krueger et al., 2009; Mar, 2004) and episodic memory processes (Eichenbaum, Sauvage, Fortin et al., 2012), respectively. While self-referential processes are often attributed to this network, specifically to the dorsal subsystem of the DMN (comprised of the anterior-dorsal mPFC and posterior cingulate regions; Andrews-Hanna et al., 2010; Yeo et al., 2011, 2014), emerging research suggests that a more precise characterization of the DMN and its subsystems involves the top-down memory indexing, elaboration and control (Kragel & Polyn, 2013; Robin et al., 2014; St. Jacques et al., 2011). While self-generated memories are inherently personal, more ventral mPFC regions, belonging to a separate network (discussed in detail below) mediate personalized evaluations. Thus, the DMN likely contributes to memory processes involving the temporal organization and content of conversational stories.
1.2. Narrative Evaluations
Unlike the basis of personal memories and story structure, very little is known about neural circuitry of personal stories and the processes involved in memory evaluations. Evaluations interrupt a story's action and often reference the speaker's ‘frame of mind’, or subjective appraisal of events (Bamberg & Damrad-Frye, 1991). Emerging understanding of social valuation and decision-making processes shows that ventromedial cortico-limbic structures are involved in dynamic hedonic appraisal (Amodio, 2014; Hare, O'Doherty, Camerer et al., 2008; Plassmann, O'Doherty, & Rangel, 2010), thus the intrinsically connected “limbic network”, which includes parts of the amygdala, caudate head, ventral striatum, ventromedial / medial orbitofrontal prefrontal cortex (vmPFC/mOFC), and temporal poles (Choi, Yeo, & Buckner, 2012; Seeley et al., 2011; Yeo et al., 2011, 2014), is likely associated with narrative evaluations. Regions within this network are linked to processing personally meaningful social information by applying socio-emotional knowledge to social event contexts (Ross & Olson, 2010; Olson, McCoy, Klobusicky, & Ross, 2012) and by identifying the emotions and beliefs of oneself and others (Burin, Acion, Kurczek et al., 2014; Lieberman, Eisenberger, Crockett et al., 2007; Ochner et al., 2004). In narrative comprehension tasks, regions within this network are specifically linked to processing the emotional content, rather than the action or orientation components of stories (Chow et al., 2013).
1.3. Social Attention
Two tightly linked networks appear to contribute to whether one is likely to initiate storytelling during a social interaction. Specifically, the salience network and cingulo-opercular (or “task control”) networks mediate awareness of- and attention to- important socio-emotional cues, respectively1. These networks provide motivational signals that are revealed as individual differences in level of apathy or anxiety displayed during social interactions (Seeley 2007; Seeley et al., 2011). In addition to mediating social awareness and cuing, the salience network also modulates activity of the DMN (Chiong et al., 2013; Jilka et al., 2014; Sridharan, Levitin, & Menon, 2008), suggesting that injury dysfunction of the salience / task control networks may not only lead to failure to attend to social cues (which we hereon refer to “social attention deficits”), but may indirectly lead to deficits in spontaneous narrative organization and production.
Direct measurement of the neural underpinnings of storytelling in social interactions is hindered by the restricted degree to which genuine social interactions can occur during a neuroimaging experiment. Thus, behavioral observations of focal neurodegenerative disease patients who have selective neural network damage may currently provide the best model for understanding the ways these networks mediate spontaneous storytelling. Prior studies examining aspects of narrative in focal neurodegenerative groups have found that the ability to detect coherent event sequences (or “scripts”) is particularly impaired in bvFTD patients compared to AD and svPPA patients (Farag et al., 2010). Further, asked to narrate events depicted in a wordless picture book, bvFTDs also show deficits in the ability to produce coherent narratives and deduce the point of the story (or its evaluative meaning) compared to svPP A patients and normal controls (NCs; Ash et al., 2006); however, much less is known about spontaneous narrative behaviors and narrative evaluation patterns across patient groups. While inaccurate self-appraisal is a hallmark feature of bvFTD (Neary et al., 1998; Rankin et al., 2005; Rosen et al., 2010), the tendency and frequency with which patients offer personal evaluations of past events and circumstances is unknown.
In sum, temporal organization of personal experiences during storytelling involves structures in the DMN, while evaluations arguably rely on structures in the limbic network and attention of social cues relies on the salience and task control networks. These three major aspects of spontaneous personal storytelling described above – temporal organization, narrative evaluations, and social attention – are differentially compromised in focal neurodegenerative disease groups. Specifically, Alzheimer's disease (AD) patients have memory deficits and dysfunction of the DMN. Thus we hypothesize these patients will have difficulty remembering and organizing past events. Semantic variant primary progressive aphasia patients (svPPA) have semantic and sometimes social deficits and limbic network dysfunction. Thus they are likely to show narrative evaluation deficits. Finally, behavioral variant frontotemporal dementia patients (bvFTD), who show task and salience network dysfunction and exhibit severe socio-emotional deficits such as apathy and social withdrawal, are likely to fail to respond to important social cues and tell fewer stories in casual conversation. To examine our hypotheses, we analyzed transcript and video data of 46 neurodegenerative disease patients and healthy controls engaged in a 4-minute spontaneous interaction with a confederate. Transcripts were analyzed for the presence and frequency of storytelling. Employing a thematic analysis rubric (Braun & Clark, 2006), story characteristics were also analyzed. Story characteristics included temporal organization of past events, the frequency and pattern of evaluations, as well as other common narrative features. Neuropsychological correlates of story characteristics were examined and vox el-based morphometry analyses of participant-groups who demonstrated particular storytelling characteristics were conducted.
2 Methods
2.1. Participants
A sample of 46 participants (15 bvFTD, 7 svPPA, 12 probable AD, and 12 older normal controls) were selected for analysis because they had undergone the confederate-based social interaction component of a larger study on social behavior in dementia patients, and the transcriptions of these interactions were available for analysis. Patients met consensus research criteria for the diagnosis of their syndrome: bvFTD patients were diagnosed according to FTDC criteria (Rascovsky et al., 2011), svPPA patient met new International PPA criteria (Gorno-Tempini et al., 2011), and the AD patients met the National Institute on Ageing-Alzheimer's Association criteria (McKhann et al., 2011). Finally, 93 healthy controls, who were not part of the larger social behavior study, were added to our group-based VBM analyses. These controls were selected based on matching age and scan type. Please see Table 1 for demographic characteristics of the sample.
Table 1. Least square means, standard deviations, and 95% confidence intervals for general and neuropsychiatric characteristics classified by diagnostic group. (n=46).
| Mean ± SD [95% CI] |
bvFTD (N = 15) |
svPPA (N = 7) |
AD (N = 12) |
NC (N= 12) |
F | P-value | η2 |
|---|---|---|---|---|---|---|---|
| Gender: (M/F) |
12/3 | 5/2 | 5/7 | 6/6 | χ2 = 5.1 | .17 | V=.19 |
| Age: | 61.5±8.3 [57.3, 65.7] |
62.4±8.2 [56.3, 68.6] |
57.8±6.4 [53.1,62.5] |
65.7±9.2 [59.5, 69.7] |
1.89 | .27 | .09 |
| Education: | 17.1±2.4 [15.8, 18.5] |
17±3.3 [15, 19] |
14.9±2.8 [13.4, 16.4] |
17.8±2.1 [16.3, 19.5] |
2.83 | .05 | .18 |
| MMSE Total (max 30): | 24.5±5 [21.9,27.0] * |
19.4±7 [15.7,23.2] * |
21.4±5.7 [18.6,24.3] * |
29.6±0.8 [26.7, 32.5] |
26.11 | <.001 | .37 |
| CDR Total: | 1.1±0.5 [0.9, 1.4] * |
0.7±0.6 [0.5, 1.2] * |
0.8±0.5 [0.6, 1.1] * |
0±0 [-0.3, 0.3] |
8.29 | <.001 | .50 |
| Boxscore: | 6.8±2.2 [5.8, 7.9] * |
4.7±2.3 [3.2, 6.3] * |
4.7±2.3 [3.5,5.9] * |
0±0 [1.4, 1.4] |
7.91 | <.001 | .62 |
| NPI: | 41.9±21.5 [34, 49.8] * |
36.3±20.9 [24.7, 47.8] * |
9.1±6.4 [-0.5, 17.1] * |
0±0 [-8.82, 8.8] |
18.56 | <.001 | .46 |
Group effects were analyzed using glms with post-hoc Dunett-Hsu tests;
p < 0.05 compared to normal controls.
Abbreviations: SD=standard deviation; CI=confidence interval; AD = Alzheimer's dementia, bvFTD = behavioral variant frontotemporal dementia, svPPA = semantic variant primary progressive aphasia, NC = Normal Controls; MMSE= Mini-Mental State Examination; CRD = Clinical Dementia Rating; NPI = Neuropsychiatric Inventory
Patients were recruited from a dementia specialty clinic and were excluded if they had a Clinical Dementia Rating (CDR) score greater than 2 or were not fluent in English. Normal controls (NCs) were recruited through newspaper advertisements and from a local senior community center. Inclusion criteria for normal controls included a normal neurological exam, CDR score = 0, Mini-mental state exam score > 28/30, and delayed memory performance > 25* percentile in both verbal and visual-spatial domains.
The research was approved by the University of California, San Francisco Committee for Human Resource Independent Review Board. All participants signed a consent form to participate in the study.
2.2 Procedure
Participants presented at the Memory and Aging Center at the University of California, San Francisco (UCSF) for an assessment of their social functioning abilities, and gave their written consent to be videotaped during that assessment. At a fixed time during the testing session the examiner introduced the participant to a confederate whom they had not met before and then left the room. In an effort to keep their behavior as spontaneous as possible, patients were told that the confederate was there to participate in a test with them in a few minutes, but were not told specifically that the four-minute interaction with the confederate would be evaluated later. Confederates were instructed to engage with participants according to Tse and Bond (2001)'s protocol, which instructs the confederate to engage in normal levels of eye contact, respond to the participants' conversation with quick and relevant topics and to initiate topics relevant to the participant (i.e., the “active-participate” manner). The confederate remained in the testing room for four minutes, until the main examiner returned. During that time a video of the spontaneous social interaction between the participant and the confederate was recorded and transcribed using the Elan program (http://tla.mpi.nl/tools/tla-tools/elan/) for later analysis.
2.3 Social Attention
Social attention captured the participant's level of interpersonal engagement with the confederate during the interview. Deficits in social attention were measured according to four selected items on the 20-item Two-Dimensional Social Interaction Scale (2DSIS; Tse & Bond, 2001). The 2DSIS is an observer rating scale that measures the quality of an individual's social interaction with a confederate (e.g., self-focus, other-focus, response latency, smiling, etc.). Two independent raters who were blind to participants' diagnoses analyzed participant videos and rated participants' levels of interpersonal “indifference”,“attentiveness”,“reservedness” and “detachment” on a 7-point where high scores indicate severe social attention deficits (“attentiveness” was reverse scored). A composite score for social attention was calculated by averaging scores across the four items (ICC=0.80±0.15).
2.4 Neuropsychological Assessment
Participants were administered a neuropsychological battery, described elsewhere (Kramer et al., 2003), assessing various aspects of cognition, including memory, language, processing speed, and executive functioning. Depression and affect naming were also assessed. Tests included the California Verbal Learning Test (CVLT; patients were administered an abbreviated form with 9 items), Benson Figure copying and delayed recall and recognition, Fluency (semantic, category and design), abbreviated Boston Naming Test (15 items), Stroop color naming test, abbreviated Trail Making Test (numbers and days of the week), serial m's and n's (graphomotor perseverations), abstract reasoning (including three similarity items and three proverbs), the Geriatric Depression Scale (GDS) and the Comprehensive Affect Testing System (CATS).
2.5 Storytelling Analyses
Story coding schemes were developed by the first author and coding was performed by two reliability coders. The coders were blind to the diagnoses of the participants. For all coding categories a subset of 10 transcripts from a separate set of patients (excluded from this study on the basis of diagnosis) were coded, and reliability statistics were derived.
Story coding occurred in four phases in order to identify stories and story characteristics. In the first phase, two trained coders read through the transcripts independently to identify two kinds of story clauses: action and evaluation clauses. An action clause was defined as a clause containing - at minimum - a subject and an active verb in the past or historical present tense (e.g., I ran…; So I'm running…; Coates, 2003). An evaluation clause also consisted of a minimum of a subject and predicate, and reflected the speaker's perspective on the events described. Evaluations referenced introspective cognitive or affective states (e.g., “I hate not working”, “I thought I had if”) or evaluations of others' internal states or affective behaviors (e.g., “he was angry”, “he was yelling”; Labov & Waletsky, 1967). In the second phase they coded for the presence of stories (described below). Once stories were identified and isolated, the first author inductively developed coding schemes for the following story categories: evaluation patterns, story content, and autobiographical stories. Coding schemes for other story categories (temporal organization, story complexity, and episodic stories) were informed by prior narrative studies.
2.5.1 Stories
Due to the cognitive impairments among the participants in our study and the relatively small subsamples, our operationalization of stories aimed to be as inclusive as possible. Story coding was based on Labov and Waletzky's (1967) framework for coding oral narratives. A story was defined as three or more contiguous evaluation and/or past tense action clauses. The following example was considered a story with 3 action clauses and 1 evaluation (lines 1-3 were considered action clauses, while line 4 was considered an evaluation):
I fell yesterday.
My doctors has checked it out.
They said it wasn't broken or anything,
so I'm sure it's OK.
Reliability for stories and action and evaluation clauses was excellent: 83%, 87%, and 84%,2 respectively.
2.5.2 Temporal Organization
Narrative organization was measured according to levels of local and global coherence developed by Glosser and Deser (1992). Local coherence measured the degree to which adjoining clauses were conceptually linked, whereas global coherence measured the degree to which clauses were hierarchically organized and related to the story's overall theme. Both local and global coherence were rated on a 5-point scale where high scores indicated greater coherence and interrater reliability was excellent (ICC=0.81 and 0.88, respectively).
2.5.3 Evaluation Patterns
In addition to analyzing the overall frequency of evaluations, we also assessed the heterogeneity, alternatively the perseveration, of narrative evaluations. Coders read through the entire transcript to determine whether the evaluations were attributed to one of three common, inductively identified topics: the self, another person, or environmental conditions (e.g., “It was beautiful out”). Evaluation subtopics were also identified. For instance, the participant may have evaluated the self in diverse context (“I felt really tired all the time”, “I really loved gardening”) or perseveratively (“my memory is not as good as it used to be”, “I have trouble finding words”). A metric for evaluation perseverance for each topic was achieved by dividing the total number of evaluations by the sum of topics and subtopics. Reliability for evaluation topics and subtopics was excellent (k= .82 and ICC = .80, respectively).
2.5.4 Autobiographical stories
Once stories were identified and isolated, coders then determined whether the stories were autobiographical. Stories were considered autobiographical if they were about the participant's own actions and experiences as opposed to the actions and/or experiences of another. Coding for autobiographical stories was dichotomous, i.e., either present (1) or absent (0). (k =.90).
2.5.6 Episodic Story
Stories were considered episodic if the events occurred at a specific place and/or time. Episodic stories were identified by at least one time marker (e.g., yesterday, once, last week, etc.) in connection with at least one simple past or historical present tense verb, such as “I spilled coffee on my computer last Wednesday”. Major life events such as weddings, births, relocations, and deaths were not considered episodic stories unless they were relatively rich in information. Following Conway and Pleydell-Pearce (2000), in order for such major life events to be considered episodic, they needed to describe the specific place, characters, mood, and/or conditions of the event. Coding for episodic stories was dichotomous, i.e., either present (1) or absent (0). (k =.92).
2.5.7 Complexity
Complexity was operationalized as the degree to which the participants negotiated two or more perspectives when telling the story (adapted from Suedfeld, Tetlock, & Streufert, 1992). A 5-point scale was used to rate the degree to which the story presented any of the following four characteristics: two or more points of view (e.g., My husband thinks my memory is bad, but I think it's fine”) two or more aspects of the self (e.g., “I have always been able to remember people's names, but lately I'm having a hard time”), multiple motivations (e.g., “I came to get tested for dementia because it runs in my family and I've been having memory problems”), and/or complex emotional experiences (e.g., “I lost my mother to Alzheimer's which was sad, but it was also a relief). A story received a default score of 1 if it did not present any of the above characteristics. An additional point was added for each complexity characteristic that appeared in the story and there was no limit to how often each complexity characteristic could be counted within a story. For instance, if a participant negotiated two or more personal points of view (e.g., “I used to think X, but now I think Y”) as well as two or more points of view of another (e.g., “M used to also think X, but now thinks Y”), complexity points for points of view could be counted twice (ICC = 0.83).
2.6 MRI scanning and VBM preprocessing
All structural MRI studies were performed using a 1.5T Magnetom VISION system (SiemensInc., Iselin, NJ, USA) equipped with a standard quadrature head coil. Anatomic MR imaging sequences were obtained for each patient and included a volumetric magnetization prepared rapid gradient echo (MPRAGE, TR/TE/TI = 10/4/300 ms) in order to obtain T1-weighted images, 15° flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0-mm squared in-plane resolution and 1.5-mm slice thickness. All imaging was done within three months of clinical assessment.
The voxel-based morphometry (VBM) technique utilizes an image preprocessing step (spatial normalization, segmentation, modulation, and smoothing) followed by a statistical analysis. Both stages were performed using the SPM8 software package, using the intrinsic DARTEL toolbox for warping (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm/ running on Matlab 7.0.1 (MathWorks, Natick, MA), using a template derived from 320 healthy older control participants. For preprocessing, default settings and tissue probability maps were maintained, with the exception that light cleanup of partitions was performed. Spatially normalized, segmented, and modulated grey matter images were then smoothed with an 8-mm FWHM isotropic Gaussian Kernel.
2.7 Statistical Analysis
Statistical analyses of behavioral data were carried out in SAS (9.4) and R (3.1.3). Due to the exploratory nature and low power of this study, as well as violations to parametric modeling in our story data, Kruskal-Wallis and Wilcoxon rank-sum tests were used to compare across groups for all storytelling variables. For post-hoc comparisons, Mann-Whitney U tests were applied. Hodges-Lehmann estimates and Moses 95% confidence intervals were chosen to compare the difference between the post-hoc parameters. Story variables contained a substantial number of ties (i.e., equal ranking; 18-32%). To control for ties, permutation-based Goodman-Kruskal gamma rank correlation coefficients were used to test the strength of the associations between storytelling characteristics and neuropsychological features. The Goodman-Kruskal Gamma test is a modification of Kendall's Tau-a for use in contingency tables which adjusts for tied cases (for a greater description of this method see Goodman & Kruskal, 1954).
Due violation of statistical assumptions and lack of power, a standard whole-brain VBM analysis correlating brain volume with storytelling scores could not be performed. However, we still wished to identify any neuroanatomic correlates of storytelling variables, so a group-based approach was utilized to improve the statistical sensitivity of the analysis. Patients' storytelling scores were converted into z-scores using the healthy control group as the standardization sample. Participants were then grouped by whether they demonstrated extreme storytelling patterns, with a cutoff for extreme patterns at z < -1.3 (i.e., patients were considered extreme if they performed at less than the 20th percentile compared to healthy older controls). Statistical models were used to show group differences in voxel-wise gray matter volume between participants who demonstrated extreme storytelling patterns, derived in reference to the MRIs of a set of 93 healthy NCs. Separate design matrixes were created for each storytelling pattern group-control set, and age, sex, and total intracranial volume (TIV) were included as confounding covariates. Regionally specific differences in grey matter volumes at each voxel were assessed using a general linear model, and the significance of group effects was determined using the theory of Gaussian fields.
3. Results
3.1 Demographic characteristics of the sample
The demographic characteristics are presented in Table 1. Age of the sample ranged from 47 to 83, they were predominantly right-handed and well-educated, with a greater percentage of males (61%). The mean Mini-Mental State Examination (MMSE) score was 24.2 (SD = 5.9) and the mean Clinical Dementia Rating (CDR) was 0.8 (SD = 0.6) with a mean sum of boxes of 4.4 (SD = 2.9). The Neuropsychiatric Inventory (NPI) had a mean of 25.0 (SD = 22.5) with a range of 0 to 88. Dementia diagnosis was not significantly related to gender χ2(3) = 5.1, p = .17, or age F(3, 34) = 1.89, p = .27, and although diagnoses did approach significance with regard to education F(3, 34) = 2.83, p = .05, the difference was not significant. Therefore age, gender, and education were not included in storytelling analyses. Storytelling frequency was also not related to disease severity CDR (rs= .00, p = .93) or MMSE (rs=.00, p=.95).
3.2 Behavioral Results
3.2.1 Preliminary Findings
Stories emerged in 69.6% of the conversations (n = 84 stories). Overall, the 46 participants told between 0-6 stories (Mdn = 1.5) in conversation. 90% of the stories were autobiographical. Preliminary analysis revealed that amount of conversational speech (i.e., the number of words) was related to story frequency γ =.62, p < 0.001; however, diagnostic groups did not differ in the amount of speech they produced during the conversation, H=2.62, p =.37, nor did they differ in the amount of speech they produced per story, H=1.29, p =.73.
3.2.2 Social attention and storytelling frequency
Storytelling findings are presented in Table 2. Patients with bvFTD and svPPA were rated as less socially attentive by observers compared to NCs (median group differences: -2.5: 95% CI -5 to -1, p < 0.01 and 3: 95% CI 0 to 4,p = 0.04, respectively); however social attention deficits were not related to storytelling frequency γ =.01, p = 0.98. Contrary to our hypothesis, bvFTDs did not differ from NCs with regard to the number of stories they told. Unexpectedly, svPPA patients told significantly more stories than NCs during the conversation (median group differences: 1.5: 95% CI 0 to 3,p = 0.04).
Table 2. Descriptive statistics and median group difference compared to controls for storytelling characteristics classified by diagnostic group (n=46).
| bvFTD | svPPA | AD | NC | P value | |
|---|---|---|---|---|---|
| n=15 | n = 7 | n = 12 | n = 12 | ||
| Conversational speech produced3 | |||||
| Mean± s.d. | 126.8±78. | 241.6±187. | 167.1±123. | 133.3±54. | 0.31 |
| Range | 5 | 9 | 8 | 9 | |
| Median | 85-504 | 221-558 | 100-5.25 | 115-405 | |
| 284 | 366 | 354 | 288.5 | ||
| Median group difference with controls (95% CI) | 11 (-87, 103) | 102.5 (-39, 226) | 59 (-51, 162) | ||
| Social Attention | |||||
| Mean± s.d. | 3±1.4 | 2.7±1.3 | 2.6±1.5 | 1.6±0.8 | 0.03 |
| Range | 1-5.5 | 1-4.5 | 1-6 | 1-3 | |
| Median | 2.5 a | 2.5 a | 2 | 1 | |
| Median group difference with controls (95% CI) | -2.5 (-5,-1)** | 3 (0,4)* | -2 (-3,0) | ||
| Story frequency | |||||
| Mean± s.d. | 1.2±1.6 | 3.2±1.7 | 1.5±1.5 | 1.8±1.4 | 0.02 |
| Range | 0-5 | 2-6 | 0-4 | 0-4 | |
| Median | 1 | 3 | 1 | 2 | |
| Median group difference with controls (95% CI) | 1(0, 2) | 1.5(0,3)* | 0(-1,2) | ||
|
| |||||
| n = 8 | n = 7 | n = 8 | n = 9 | ||
|
| |||||
| Story speech produced | |||||
| Mean± s.d. | 126.8±78.5 | 241.6±187.9 | 167.1±123.8 | 133.3±54.9 | 0.65 |
| Range | 27-230 | 50-558 | 33-316 | 15-193 | |
| Median | 127.5 | 177 | 117 | 152 | |
| Median group difference with controls (95% CI) | 3 (-103, 70) | 55 (-80, 307) | 18 (-97, 157) | ||
|
| |||||
| bvFTD | svPPA | AD | NC | P value | |
| n = 8 | n = 7 | n = 8 | n = 9 | ||
|
| |||||
| Evaluation clauses | |||||
| Mean ± s.d. | 1.1±0.5 | 2.2±1.8 | 1.9±1.1 | 1.2±0.6 | 0.16 |
| Range | 0.6-2 | 0.7-5.5 | 1-3 | 0.5-2.33 | |
| Median | 1 | 1.5 | 2.34 | 1 | |
| Median group difference with controls (95% CI) | 0 (-0.7, 0.5) | 0.5 (0.3-3) | 0.7 (-0.3, 1.8) | ||
| Action clauses | |||||
| Mean± s.d. | 5.7±2.6 | 5.4±2.3 | 5.3±1.9 | 5.7±1.9 | 0.99 |
| Range | 2-10.5 | 1.33-8 | 2-7.5 | 3-10 | |
| Median | 5.5 | 5 | 5.38 | 5.33 | |
| Median group difference with controls (95% CI) | 0.13 (-2.3, 2.2) | 0.3 (-2.8, 2.5) | -0.17 (-2.7, 2) | ||
| Self-evaluation perseveration | |||||
| Mean± s.d. | 1.5±0.8 | 4.1±3.7 | 2±0.7 | 1.4±0.3 | 0.04 |
| Range | 1-3.33 | 1-12 | 1.4-3.67 | 1-2 | |
| Median | 1.14 | 3 | 1.84 | 1.4 | |
| Median group difference with controls (95% CI) | -0.19 (-0.5, 0.2) | 1.6(0.5, 3.1)* | 0.37 (0, 0.9) | ||
| Autobiographical stories | |||||
| Mean± s.d. | 1.8±1.4 | 3.1±1.4 | 0.9±1 | 1.8±0.8 | 0.01 |
| Range | 1-5 | 1-5 | 0-3 | 1-3 | |
| Median | 1 | 3 | 1 | 2 | |
| Median group difference with controls (95% CI) | 0(-1,1) | 1 (0, 3)* | -1 (-2, 0)* | ||
| Event specific | 0.77 | ||||
| Mean± s.d. | 1±1.9 | 0.7±0.5 | 1.3±1.2 | 1.5±1.1 | |
| Range | 0-3 | 0-1 | 0-2 | 0-3 | |
| Median | 0.5 | 1 | 0.5 | 1 | |
| Median group difference with controls (95% CI) | -1 (-2, 0) | 0 (-1, 0) | -0.5 (-1, 0) | ||
| Temporal Organization (Local) | |||||
| Mean± s.d. | 3.16±0.4 | 3.65±0.3 | 2.9±1 | 3.16±0.6 | 0.19 |
|
| |||||
| bvFTD | svPPA | AD | NC | P value | |
| n = 8 | n = 1 | n = 8 | n = 9 | ||
|
| |||||
| Range | 2.67-4 | 3.25-4 | 2-4 | 2-4.25 | |
| Median | 3 | 3.67 | 2.63 | 3 | |
| Median group difference with controls (95% CI) | -0.05 (-0.5, 4) | -0.5 (-0.0, 1) | 0(-1,1) | ||
| Temporal Organization (Global) | |||||
| Mean± s.d. | 4.3±0.42 | 4.6±0.40 | 4.1±0.9 | 4.8±0.3 | 0.16 |
| Range | 3-5 | 3.5-5 | 2.5-5 | 4-5 | |
| Median | 4.33 | 4.67 | 4.16 | 5 | |
| Median group difference with controls (95% CI) | -0.63 (-1, 0) | 0 (-0.7, 0) | -0.67 (-1.3, 0) | ||
| Complexity | 0.11 | ||||
| Mean± s.d. | 2.8±0.8 | 3.2±1 | 4.5±1.8 | 3.2±0.8 | |
| Range | 1.67-4 | 2-4.75 | 2-7 | 2-5 | |
| Median | 3 | 3.5 | 4.13 | 3 | |
| Median group difference with controls (95% CI) | -.3 (-1, 0.25) | 0(-1, 1) | 1 (0, 3) | ||
Group effects on storytelling variables were analyzed using SAS 9.4
The first four rows represent the entire sample, the remaining six rows represent only the storytelling sample. NC = Normal Controls, AD = Alzheimer's dementia, bvFTD = behavioral variant frontotemporal dementia, svPPA = semantic variant primary progressive aphasia.
P values in the farthest right column are based on the Kruskal Wallis H test.
p<0.05 compared to Controls,
p<0.01 compared to Controls.
3.2.3 Storytelling characteristics
There were no significant between-group differences in the mean number of episodic stories participants told, nor the number of action or evaluation clauses produced. There were also no differences in the degree of complexity or temporal organization (either local or global coherence) of participants' stories. AD patients did tell significantly fewer autobiographical stories than NCs (median group differences: -1: 95% CI -2 to 0, p = 0.04). Unexpectedly, svPPA patients told more autobiographical stories than controls (median group differences: 1: 95% CI 0 to 3, p = 0.04). Finally, svPPA patients perseverated on aspects of self more often than controls (median group differences: 1.6: 95% CI 0.5 to 3.1, p = 0.02).
3.2.4 Neuropsychological correlates of storytelling and story characteristics
Greater conversational story frequency was related to greater mental flexibility (modified trails; p<0.001) and higher depression scores (p=0.02). It was also related to working memory (digits backwards), though this was a non-significant trend (p=0.05). Autobiographical and episodic stories were related to mental flexibility (p=0.01 and p=0.04, respectively). Autobiographical stories were also associated with cognitive processing speed (number of correct Stroop word colors; p=0.01) and working memory (p=0.02). There were no significant relationships between evaluations and neuropsychological functions, though self-evaluation perseverance was associated with graphomotor perseveration (p<0.01). Local coherence was linked to a number of neuropsychological functions including delayed recall (p<0.001), processing speed (p=0.02), verbal attention (digits forward; p=0.05), non-verbal generativity (design fluency; p=0.02), graphomotor perseverations (p<0.01), and depression scores (p<0.0). Finally, global coherence and complexity were related to depression scores (p=0.04 and p<0.001, respectively), and complexity was associated with delayed recall (p=0.04). Findings are presented in Table 3.
Table 3.
| Story frequency | Event specific | Autobiographical | Evaluations | Self-evaluation perseverance | Local coherence | Global coherence | Compleity | |
|---|---|---|---|---|---|---|---|---|
| Language | ||||||||
| BNT | -0.14 [-0.4, 0.2] |
-0.03 [-0.4,0.4] |
-0.01 [-0.4, 0.3] |
-0.11 [-0.5, 0.2] |
-0.13 [-0.4,0.1] |
0.08 [-0.3, 0.5] |
-0.18 [-0.6, 0.2] |
-0.07 [-0.4, 0.3] |
| Semantic fluency | -0.08 [-0.3, 0.2] |
0.09 [-0.2, 0.4] |
-0.1 [-0.4, 0.2] |
-0.08 [-0.4, 0.2] |
-0.02 [-0.3, 0.3] |
0.26 [-0.0, 0.6] |
-0.09 [-0.4, 0.2] |
-0.02, [-0.4, 0.3] |
| Phonemic fluency | 0.01 [-0.3, 0.3] |
-0.09 [-0.4, 0.2] |
0.05 [-0.2, 0.4] |
0.01 [-0.3, 0.3] |
0.03 [-0.2, 0.3] |
0.17 [-0.2, 0.5] |
0.01 [-0.3, 0.3] |
0.05 [-0.2, 0.3] |
| Abstract reasoning | 0.00 [-0.3, 0.3] |
0.19 [-0.2, 0.6] |
0.00 [-0.3, 0.3] |
0.14 [-0.3, 0.6] |
-0.05 [-0.4, 0.3] |
0.19 [-0.2, 0.6] |
-0.23 [-0.5, 0.1] |
-0.13 [-0.5, 0.2] |
| Verbal memory | ||||||||
| CVLT | 0.08 [-0.2, 0.4] |
0.02 [-0.3. 0.4] |
0.18 [-0.2, 0.5] |
-0.19 [-0.5, 0.1] |
-0.32 [-0.6, -0.0] |
0.02 [-0.3, 0.3] |
-0.15 [-0.5, 0.2] |
-0.24 [-0.6,0.1] |
| Visuospatial Memory | ||||||||
| Benson Figure delayed recall | 0.13 [-0.1, 0.4] |
0.02 [-0.4, 0.4] |
0.10 [-0.2, 0.4] |
0.07 [-0.3, 0.4] |
-0.04 [-0.4, 0.3] |
-0.27 [-0.04, 0.6] |
0.08 [-0.2, 0.4] |
0.42* [0.1, 0.8] |
| Benson Figure delayed recognition | 0.16 [-0.3, 0.6] |
-0.38 [-1, 0.2] |
0.44 [-0.0, 0.9] |
0.03 [-0.6, 0.6] |
-0.43 [-0.9, -0.0] |
0.51*** [3,8] |
0.29 [-0.3, 0.9] |
-0.10 [-0.6, 0.4] |
| Executive Functioning | ||||||||
| Processing speed (Stroop color/min) | 0.25 [-0.0, 0.5] |
0.01 [-0.3, 0.3] |
0.40** [0.2, 0.6] |
-.28 [-.6, 0] |
-0.11 [-0.3, 2] |
0.39* [0.1, 0.7] |
0.22 [-0.0, 0.5] |
-0.11 [-0.4, 0.2] |
| Story frequency | Event specific | Autobiographi cal | Evaluat ions | Self-evaluation perseverance | Local coherence | Global coherence | Complexity | |
| Stroop interference (words/min) | 0.07 [-0.3, 0.4] |
0.00 [-0.5, 0.5] |
0.13 [-0.3, 0.5] |
-0.24 [-0.6, 0.1] |
-0.25 [-0.6,0.1] |
0.29 [-0.0, 0.6] |
0.10 [-0.3, 0.5] |
-0.19 [-0.5, 0.2] |
| Digit span forwards | 0.14 [-0.2, 0.48] |
-0.03 [-0.48, 0.41] |
0.22 [-0.11, 0.55] |
0.01 -0.35, 0.4] |
-0.18 [-0.5,0.1] |
0.39* [0.0, 0.8] |
0.25 [-0.1,0.6] |
-0.23 [-0.6,0.1] |
| Digit span backwards |
0.29* [0.1, 0.5] |
0.08 [-0.4, 0.5] |
0.40* [0.1, 0.7] |
0.11 [-0.2, 0.4] |
0.01 [-0.3, 0.3] |
0.15 [-0.3, 0.6] |
0.03 [-0.4, 0.4] |
0.15 [-0.2, 0.5] |
| Modified trails speed (lines/min) |
0.40*** [0.2, 0.6] |
0.35* [0.1, 0.6] |
0.39* [0.2, 0.6] |
0.06 [-0.3, 0.4] |
0.06 [-0.3, 0.3] |
0.15 [-0.2, 0.5] |
-0.12 [-0.4, 0.2] |
0.09 [-0.2, 0.4] |
| Design fluency | 0.24 [-0.1, 0.5] |
0.06 [-0.3, 0.4] |
0.23 [-0.1, 0.6] |
0.22 [-0.1, 0.6] |
-0.08 [-0.4, 0.2] |
0.40* [0.1, 0.7] |
0.2 [-0.0, 0.4] |
0.05 [-0.3, 0.4] |
| m-n | -0.41 [-0.8, 0.0] |
-0.21 [-0.9, 0.4] |
-0.46 [-0.9, 0.0] |
0.09 [-0.5, 0.7] |
0.38** [.1, .6] |
-0.42** [-7,-1] |
-0.37 [-0.9, 0.2] |
-0.03 [-0.8, 0.8] |
| GDS |
0.30* [0.0, 0.6] |
0.13 [-0.3, 0.5] |
-0.22 [-0.7, 0.2] |
0.09 [-0.2, 0.4] |
0.06 [-0.2, 0.3] |
-0.53** [-0.8, -0.3] |
-0.30* [-0.6, -0.0] |
0.44** [0.2, 0.7] |
| CATS | -0.07 [-0.5, 0.3] |
-0.39 [-0.8, 0.0] |
0.02 [-0.4, 0.4] |
0.19 [-0.2, 0.6] |
0.01 [-0.33, 0.35] |
0.38 [-0.0, 0.8] |
0.07 [-0.3, 0.4] |
0.33 [0.0, 0.7] |
p<05,
p<01,
p<001
Scores presented are permutation-based (1,000 permutations) Goodman-Kruskal Gamma coefficients; BNT = Boston Naming Test; CVLT = California Verbal Learning Test; GDS = Geriatric Depression Scale; CATS = Comprehensive Affective Testing System
3.3. VBM Group Comparisons
3.3.1 Temporal organization (local + global coherence) deficit group vs. NCs
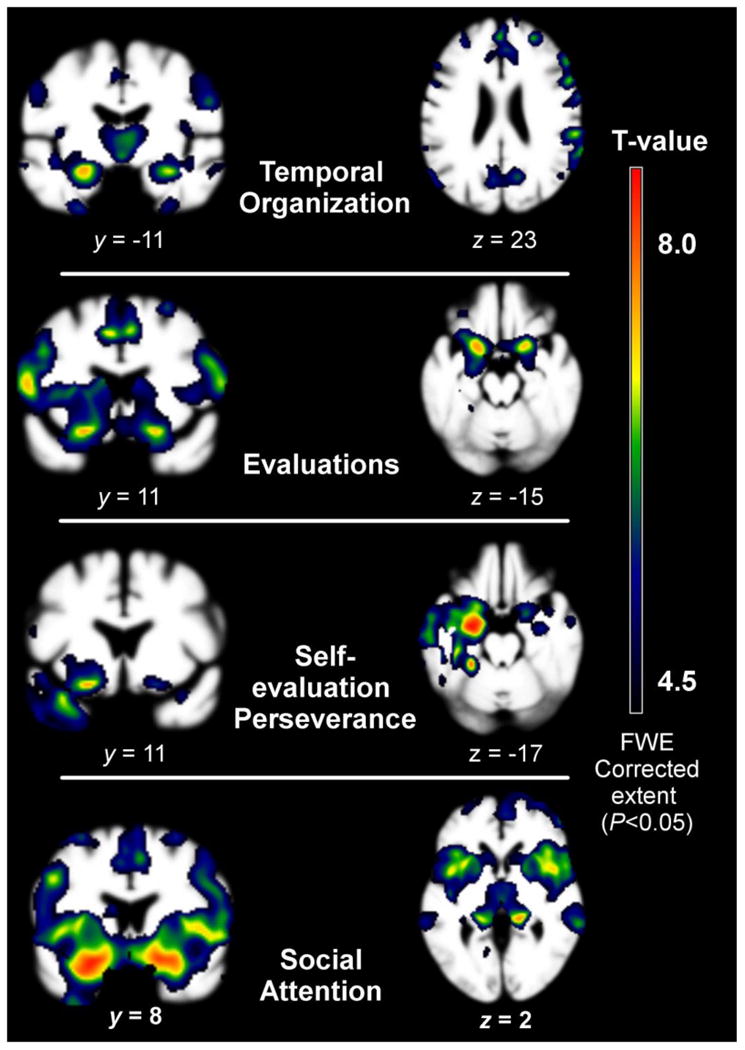
The storytelling group comprised of participants with temporal organization deficits (27% bvFTD, 0% svPPAs, 63% ADs, 9% NCs) showed peak atrophy patterns overlapping with the default mode and, to lesser degree, the salience/task and limbic networks. Significant regions of DMN degeneration included the left medial temporal lobe (MTL), right MTL, middle temporal gyrus (MTG), the temporoparietal junction (TPJ), as well as the bilateral posterior cingulate cortex (PCC) and precuneus. The frontal pole was also atrophied. Areas of considerable salience network degeneration included the ACC and bilateral putamen. Limbic network degeneration included the accumbens area and left amygdala. Finally, significant atrophy was seen in regions consistent with the adaptive executive control network including the right dorsolateral prefrontal cortex (dlPFC) and left intraparietal sulcus (Dosenbach et al., 2007) (for additional regions see Table 4; Figure 1).
Table 4. Regions of atrophy in the patient group with temporal organization scores less than the 20th percentile of healthy older controls.
| Brain Regions and Networks | Peak MNI Coordinates | T-value (PFWE<0.05) |
||
|---|---|---|---|---|
|
| ||||
| X | Y | Z | ||
| DMN Structures† | ||||
| L MTL | -22 | 1 | -21 | 8.04 |
| RMTL | 30 | -13 | -15 | 7.36 |
| RTPJ | 63 | -43 | 20 | 6.06 |
| RMTG | 63 | -22 | -22 | 6.00 |
| LMTG | -61 | -23 | -7 | 5.51 |
| LPCC | -13 | -37 | 0 | 7.04 |
| RPCC | 15 | -35 | -1 | 6.82 |
| R precuneus | 15 | -64 | 25 | 6.01 |
| L precuneus | -4 | -64 | 19 | 5.84 |
| Frontal Pole | 2 | 47 | 23 | 6.02 |
| Limbic Structures† | ||||
| L Amygdala | -24 | -8 | -15 | 7.9 |
| R Amygdala | 18 | -1 | -16 | 6.86 |
| L Accumbens area | -15 | 10 | -12 | 6.65 |
| R Accumbens area | 12 | 7 | -9 | 6.36 |
| R Caudate | 16 | 16 | 1 | 6.25 |
| L Caudate | -9 | 10 | 13 | 6.10 |
| Salience§/ Task Control Network‡ | ||||
| ACC | 4 | 24 | 31 | 6.56 |
| RAI | 36 | 4 | 4 | 5.74 |
| RFO | 49 | 18 | 0 | 5.77 |
| R Putamen | 18 | 12 | -12 | 7.10 |
| L Putamen | -18 | 9 | -13 | 6.98 |
| Additional regions belonging to separate networks | ||||
| Adaptive executive contror‡† | ||||
| R dlPFC | 51 | 13 | 29 | 7.40 |
| L Intraparietal sulcus | -25 | -73 | 34 | 6.09 |
| R Thalamus | 18 | -32 | -1 | 7.34 |
| L Thalamus | -15 | -34 | -1 | 7.26 |
| R Precentral gyrus | 51 | 12 | 29 | 6.93 |
R = right; L = left; MTL=medial temporal lobe; MTG = middle temporal gyrus; TPJ = temporoparietal junction; PCC = posterior cingulate cortex; ACC = anterior cingulate cortex; AI = anterior insula; FO=frontal operculum; dlPFC = dorsolateral prefrontal cortex
=Regions based on Yeo et al. (2011);
=regions based on Seeley et al., (2007);
based on Dosenbachet al. (2007)
Figure 1.
Patterns of grey matter loss in storytelling group analyses compared to controls, adjusting for age, gender, and total intracranial volume (TIV). All results are presented at a threshold of pFWE P<0.001 uncorrected. Images were overlaid with MRIcron (http://http://www.mccauslandcenter.sc.edu/CRNL/) on an average brain based on the healthy older control gray matter template used for DARTEL warping.
3.3.2 Evaluation deficit group vs. NCs
Contrary to our hypothesis, the group comprised of participants who offered the fewest evaluations (30% bvFTD, 10% svPPAs, 60% ADs, 0% NCs) showed peak atrophy in salience and task control network regions, followed by limbic network regions. Salience and task control regions included the ACC, mid cingulate, as well as the bilateral inferior frontal gyrus, putamen, insula, and frontal operculum. Areas of prominent atrophy in the limbic network included the L>R medial orbitofrontal cortex (mOFC) and bilateral accumbens area. Additional regions with considerable atrophy were the bilateral dlPFC and precentral gyrus (Table 5).
Table 5. Regions of atrophy in the patient group with evaluation scores less than the 20th percentile of healthy older controls.
| Brain Region | Peak MNI Coordinates | T-value (PFWE<0.05) |
||
|---|---|---|---|---|
|
| ||||
| x | Y | Z | ||
| Default Mode Network | ||||
| R MTL | 24 | 4 | -19 | 6.14 |
| L MTL | -21 | -11 | -15 | 5.70 |
| L MTG | -58 | -58 | 16 | 4.98 |
| L Precuneus | -7 | -37 | 49 | 5.16 |
| Frontal pole | -22 | 61 | -7 | 6.22 |
| dmPFC | 27 | 15 | 53 | 6.54 |
| Limbic Network | ||||
| L Caudate | -9 | 10 | 13 | 6.34 |
| R Caudate | 10 | 12 | 10 | 5.71 |
| L Accumbens area | -13 | 6 | -13 | 7.05 |
| R Accumbens area | 10 | 7 | -13 | 5.96 |
| L Amygdala | -19 | -2 | -16 | 6.17 |
| R Amygdala | 21 | -2 | -16 | 5.18 |
| L mOFC | -19 | 9 | -18 | 7.49 |
| R mOFC | 16 | 7 | -19 | 6.95 |
| Salience / Task Control Network | ||||
| Mid Cingulate | -3 | -13 | 44 | 8.82 |
| ACC | -1 | 27 | 32 | 8.04 |
| L AI | -36 | 19 | 2 | 7.29 |
| R AI | 40 | 3 | 7 | 7.19 |
| L FO | -37 | 19 | 2 | 7.36 |
| R FO | 48 | 18 | 1 | 6.82 |
| L Inferior frontal gyrus | -48 | 21 | 1 | 7.19 |
| R Inferior frontal gyrus | 52 | 28 | 19 | 6.53 |
| L Putamen | -16 | 6 | -13 | 7.43 |
| R Putamen | 15 | 7 | -13 | 7.05 |
| Additional regions | ||||
| R dlPFC | 57 | 13 | 13 | 7.66 |
| L dlPFC | -43 | 6 | 34 | 7.28 |
| L Fusiform | -24 | -41 | -17 | 4.93 |
| R Precentral Gyrus | 58 | 12 | 11 | 7.47 |
| L Precentral gyrus | -52 | 7 | 13 | 7.45 |
| R Thalamus Proper | 1 | -8 | 4 | 6.11 |
| L Thalamus Proper | 0 | -8 | 4 | 6.05 |
R = right; L = left; MTL=medial temporal lobe; MTG = middle temporal gyrus; dmPFC = dorsomedial prefrontal cortex; ACC = anterior cingulate cortex; OFC = orbitofrontal cortex; mOFC = medial orbitofrontal cortex AI = anterior insula; FO=frontal operculum;; dlPFC = dorsolateral prefrontal cortex;
3.3.3 Evaluation perseveration group vs. NCs
The storytelling group comprised of participants whose self-evaluations were highly perseverative (9% bvFTD, 46% svPPA, 36% ADs, 9% NCs) showed peak atrophy in left lateralized DMN and limbic network. DMN structures included the L>R MTL, the L>R TPJ / supramarginal gyrus (SMG), the mPFC and the left PCC. Limbic structures included the L>R amygdala, accumbens area, anterior temporal lobe, and medial orbitofrontal (mOFC)/ventral medial prefrontal cortex (vmPFC). Additional regions included the L>R fusiform area, bilateral thalami, and R>L insula and supplementary motor area (SMA) (Table 6).
Table 6. Regions of atrophy in the patient group with self-evaluation perseveration scores greater than the 80th percentile of healthy older controls.
| Brain Region | Peak MNI Coordinates | T-value (PFWE <0.05) |
||
|---|---|---|---|---|
|
| ||||
| x | Y | z | ||
| Default Mode Area | ||||
| L MTL | -22 | 0 | -19 | 8.64 |
| R MTL | 21 | 1 | -16 | 6.24 |
| L MTG | -58 | -13 | -12 | 7.83 |
| R MTG | 55 | -1 | -18 | 5.33 |
| L TPJ/SMG | -57 | -5 | -13 | 7.11 |
| R TPJ/SMG | 55 | -2 | -16 | 5.32 |
| L PCC | -12 | -37 | 0 | 5.38 |
| Limbic Network | ||||
| L Amygdala | -22 | -8 | -15 | 8.92 |
| R Amygdala | 18 | -1 | -16 | 5.81 |
| Accumbens area | -15 | 10 | -12 | 6.17 |
| L mOFC | -22 | 7 | -18 | 6.69 |
| R mOFC | 22 | 7 | -18 | 5.72 |
| L aTL | -25 | 4 | -27 | 7.32 |
| R aTL | 31 | 7 | -22 | 5.41 |
| Salience Network | ||||
| L AI | -34 | 6 | -16 | 6.14 |
| R AI | 41 | 3 | 6 | 7.05 |
| L posterior insula | -40 | -7 | -10 | 7.36 |
| L Putamen | -18 | 9 | -13 | 6.64 |
| R Putamen | 15 | 7 | -13 | 5.53 |
| Addition Regions | ||||
| L Fusiform | -25 | -37 | -18 | 8.20 |
| L Lingual | -12 | 56 | 0 | 4.82 |
| L Thalamus | -12 | -34 | -1 | 5.69 |
| R Thalamus | 15 | -32 | 1 | 4.88 |
| R SMA | 63 | -44 | 20 | 5.71 |
| L SMA | -61 | -43 | 19 | 4.78 |
R = right; L = left; MTL = medial temporal lobe; MTG = middle temporal gyrus; TPJ = temporoparital junction; SMG = supramarginal gyrus; PCC = posterior cingulate cortex; mOFC = medial orbitofrontal cortex; aTL = anterior temporal lobe; AI = anterior insula; SMA= supplementary motor area
3.3.4 Social attention deficit group vs. NCs
The group comprised of participants who showed severe deficits in attention to social cues (43% bvFTD, 14% svPPA, 43% ADs, 0% NCs) showed extreme loss across the salience/task control network. DMN structures, including the bilateral MTL and dmPFC, and limbic structures, including the L>R OFC and amygdala, were also severely atrophied. Additional regions of atrophy included the bilateral dlPFC, precentral gyri, and thalami (Table 7).
Table 7. Regions of atrophy in the patient group with social attention scores less than the 20th percentile of healthy older controls.
| Brain Region | Peak MNI Coordinates | T-value (PFWE <0.05) |
||
|---|---|---|---|---|
|
| ||||
| x | y | z | ||
| Default Mode Area | ||||
| L MTL | -25 | 3 | -21 | 9.51 |
| R MTL | 25 | 4 | -18 | 8.78 |
| L PCC | -13 | -37 | 0 | 7.43 |
| dmPFC | 1 | 30 | 33 | 8.23 |
| Limbic Network | ||||
| L Amygdala | -22 | -11 | -13 | 9.07 |
| L Accumbens | -13 | 9 | -13 | 8.35 |
| R Accumbens | 10 | 7 | -13 | 8.04 |
| L mOFC | -22 | 7 | -18 | 9.48 |
| R vmPFC/mOFC | 10 | 9 | -18 | 8.55 |
| L aTL | -30 | 7 | -25 | 7.80 |
| R aTL | 27 | 7 | -24 | 7.79 |
| Salience / Task Control Network | ||||
| R inferior frontal gyrus | 24 | 9 | -16 | 9.01 |
| L AI | -27 | 9 | -16 | 8.82 |
| R AI | 25 | 9 | -16 | 8.78 |
| ACC | 1 | 28 | 31 | 8.21 |
| Mid Cingulate | 4 | 21 | 35 | 7.97 |
| RFO | 45 | 9 | -1 | 7.86 |
| R Putamen | 15 | 9 | -13 | 9.07 |
| L Putamen | -16 | 9 | -13 | 8.53 |
| Addition Regions | ||||
| Right Thalamus Proper | 15 | -32 | 1 | 8.51 |
| Left Thalamus Proper | -15 | -34 | -1 | 8.12 |
| L dlPFC | 49 | 15 | 28 | 7.89 |
| R dlPFC | -42 | 15 | 29 | 7.88 |
| L Precentral Gyrus | -43 | 6 | 31 | 7.66 |
| R Precentral Gyrus | 55 | -4 | 29 | 7.46 |
R = right; L = left; MTL=medial temporal lobe; PCC=posterior cingulate cortex; dmPFC=dorsomedial prefrontal cortex; mOFC=medial orbitofrontal cortex; vmPFC=ventromendial prefrontal cortex; aTL=anterior temporal lobe; AI=anterior insula; 10FC=lateral orbitofrontal cortex; ACC = anterior cingulate cortex; FO=frontal operculum; dlPFC = dorsolateral prefrontal cortex
4. Discussion
The narrative abilities of focal neurodegenerative disease patients have previously been compared in controlled, experimental studies (Ash et al., 2011; Ash et al., 2006; Chapman et al., 2005; Farag et al., 2010); however, this is the first study to quantify narrative production of neurodegenerative disease groups in the form of storytelling in a naturalistic social interaction and to compare patterns of neurodegeneration with specific narrative devices such as narrative evaluations, oft-cited as the most important aspect of storytelling and which reflect key inferential and valuation processes inherent to personal narratives.
4.1. Group differences in storytelling characteristics
The most distinct group of conversational storytellers among our participants was svPPA. svPPA is associated with limbic network dysfunction, which performs evaluative functions that we hypothesized to be related to the number of evaluations and type of evaluative choices in storytelling. While we had expected that their stories might have fewer overall evaluations due to limbic network damage, we found that instead, the breadth of their evaluations had become stereotyped, demonstrated in their abnormally perseverative self-evaluations. These patients also showed deficits in social attention during the social interaction, though contrary to our expectations this did not affect storytelling frequency. In fact, they told significantly more stories controls.
Consistent with prior studies, AD patients did not differ from controls in attention to dynamic social cues (for a review see Shany-Ur & Rankin, 2011). While prior studies have found that these patients have difficulty reading emotions in static images, these patients do not differ from controls in their ability to read and respond to others' emotions when tested under ecologically valid conditions (Henry et al., 2008). Contrary to our hypothesis, ADs did not tell fewer stories than NCs, although they did tell significantly fewer autobiographical stories than NCs, despite being engaged in personally relevant topics. AD is characterized by episodic memory loss and DMN dysfunction. In autobiographical memory tasks, these patients have significant difficulty with episodic detail and tend to produce overly general and semanticized autobiographical memories (Donix, Brons, Jurjanz et al., 2010; Meulenbroek, Rijpkema, Kessels et al., 2010). Episodic stories are relatively rare in conversations between strangers (Thorne, 1987), which may explain why there was no difference between ADs and controls in this regard. Though it may be the case that during unstructured social interactions, rather than produce overly general personal memories mediated by the DMN (Meulenbroek, et al., 2010), ADs more often produce non-personal memories. Memory for non-personal past events has been shown to recruit the right inferior frontal cortex (Abraham, Schubotz, & von Cramon, 2008), which is relatively preserved in AD patients.
As predicted, bvFTD patients demonstrated social attention deficits compared to NCs, though they did not differ from controls with regard to the number of stories they produced. Further, bvFTD patients did not differ from NCs on any story variable. The present study was an exploratory study done naturalistically and assessed only a very short duration of interaction. Prior studies have shown narrative deficits in these patients (Ash et al., 2011; Ash et al., 2006; Chapman et al., 2005; Farag et al., 2010) and it is possible that pressing for a story, or examining a longer duration of social interaction, might yield different results.
4.2. Neuropsychological Correlates of Storytelling
Consistent with past studies on narrative processing, storytelling was associated with a number of neuropsychological functions such as processing speed, working memory, and mental flexibility (Coelho, Liles, & Duffy, 1995; Mar, 2004; Marini, Zettin, & Galetto, 2014). These studies have primarily examined the relationship of neuropsychological functions with narrative comprehension or depersonalized narrative production under prescribed experimental conditions, whereas this study is among few studies to examine the role of cognition in spontaneous personal story production (see also Rogalski et al., 2010). We found that processing speed and mental flexibility (which in itself involves a combination of processing speed, working memory and task-switching abilities; Sanchez-Cubillo et al., 2009) were associated with the greatest number of storytelling characteristics. We also found that local coherence (thematic unity between story clauses) was associated with a number of memory and attentional indices, including verbal attention, recognition, and graphomotor perseveration, as well as processing speed and mental flexibility.
Personal narratives are argued to reflect the highest degree of cognitive and socio-emotional integration (Hirsh et al., 2013), as such processing speed, i.e., the maximum number of cognitive operations one can perform within a given time unit, predictably plays a crucial role. In order to tell a coherent and meaningful story, speakers must quickly toggle among long term memory, short term and working memory, and the current socio-emotional context. While these functions have very rarely been studied in the context of personal storytelling, a study examining discourse coherence in stroke patients showed that global coherence (i.e., the ability to maintain thematic unity during storytelling), was more strongly predictive of neuropsychological functioning than local coherence. Our study did not replicate these findings, though rather than indicating the absence of a true relationship, this non-replication may have occurred because the nonparametric nature of our data made it difficult to control for known coviariates, such as age and disease severity, and the small number of participants our study may not have afforded adequate power to detect a relationship. Compared to younger adults, healthy older adults have more difficulty maintaining global coherence than local coherence (Glosser & Deser, 1992), thus failure to control for age may have resulted in a failure to detect true relationships between global coherence and neuropsychological functions.
Storytelling was also associated with socio-emotional functioning. Specifically, greater depression scores were linked to greater storytelling frequency, greater complexity, and poor temporal organization (both coherence scores). Prior studies have also found that depression is associated with violations to temporal structuring of memories (Fromholt et al., 1995; Habermas, Ott, Schubert et al., 2008). This may be mediated by working memory deficits characteristic of depression or alternatively by distortions in time perception (also characteristic of depression; Gil & Droit-Volet, 2009) which could influence narrative linearity and coherence.
Complexity was a composite measure derived from expressions of complex emotions (e.g., “bittersweet”), the ability to identify multiple points of view during storytelling (including past and present points of view), and presence of contrasting points of view between self and other. It is possible that greater depression scores reflecting increased feelings of isolation and loss may result from patients' propensity to reflect on past abilities in the context of current deficits, or from a greater awareness of the division between their self-image and how they are perceived by others, which may result in greater storytelling frequency as storytelling functions to resolve internal conflicts (McAdams, 1993). Alternatively, it is also possible that the propensity to dwell in the past among depressed adults (Habermas et al., 2008) leads to greater storytelling frequency during a spontaneous social interaction.
4.3 Anatomic Correlates of Storytelling
Because our study was underpowered to perform a direct brain-behavior correlation analysis due to the nonparametric nature of the storytelling variables, we instead looked at atrophy patterns in the subgroups of patients demonstrating particular storytelling characteristics. While this approach does provide reliable information about regional atrophy directly related to the behavior in question, this method can yield results that are overinclusive, i.e., pointing to other regions that are affected by the degenerative disease, but are not necessarily directly related to the target behavior. Also, storytelling is multidimensional, without clear one-to-one correspondence in brain-behavior relationships. For instance, in order for a speech act to be considered a story, both evaluations, which we hypothesized would be linked to the limbic network, and past tense action sequences, which we argued would be linked to the DMN, had to be present, which may explain why regions belonging to multiple networks were observed in each storytelling group analysis. Thus, to provide a balanced interpretation of the results, we identify where atrophy patterns support or disprove our hypotheses, but also note other regions that were significant in the analysis that could be related to storytelling.
4.3.1. Temporal organization
We hypothesized that because of its memory retrieval and organization functions, structural integrity of the DMN would impact story structure. Consistent with our hypothesis, temporal organization was associated with DMN structures, predominantly the bilateral MTL and PCC/precuneus, though the frontal pole was also significantly atrophied. While event sequencing is typically attributed to prefrontal integrity and functioning (Krueger et al., 2009; Wood & Grafman, 2003), event sequencing studies have primarily been based on experimental paradigms in which subjects are asked to temporally order sets of commonly occurring non-autobiographical events. More recent temporal sequencing studies in mice suggest that the hippocampus, and specifically specialized cells within the hippocampus (“time cells”), may play a crucial role in organizing elements of personal experiences across time (Eichenbaum, 2014; MacDonald et al., 2014). Translating this finding to humans, Lehn et al. (2009) found selective response in the hippocampus during personal event sequencing compared to sequencing non-autobiographical events.
In addition to the DMN, regional atrophy in other networks was also observed in our patients with diminished temporal organization. A number of salience network structures, including the putamen, ACC, and insula, were highly correlated with temporal organization of patients' narratives. Examining narrative organization abilities in Dementia with Lewy Body patients, Ash et al. (2010) also found subcortical atrophy to be related to narrative structuring, including damage to the putamen, as well as cortical areas including the ACC, and ventrolateral prefrontal region. Prior studies have found that the putamen is involved in aspects of sequential processing, such as organizing motor and event sequences (Albouy et al., 2008; Melrose et al., 2008; Tinaz et al., 2008; Wymbs et al., 2012; for a review see Kotz et al., 2009), which supports our finding that it is involved in the organizational sequencing during spontaneous narrative production. Salience network involvement in temporal organization may suggest that level of engagement with the storytelling task might also influence the quality of the story's temporal organization. Other regions and networks also appeared to be contributory, including the dorsolateral PFC/intraparietal sulcus of the adaptive executive control network (Dosenbach et al., 2007), which may mediate the working memory processes involved in narrative organization.
4.3.2. Evaluations
We had hypothesized that the tendency to spontaneously interject personalized evaluations into a narrative would correspond to the limbic network, which mediates personalized hedonic valuations and learned semantic distinctions. While limbic regions were compromised in our low evaluation patient group, correlations with salience and task network integrity were stronger. Narrative evaluations involve value-based references to the internal states of self and others. Whereas the limbic network likely corresponds to the affective judgments one makes about circumstances and people, basic attention to internal and external states is likely mediated by the salience and task control networks. Damage to these networks may result in failure to attend to one's own internal state during storytelling, as well as to the perceived interest of the listener.
4.3.4. Evaluation perseverations
Whereas the frequency of narrative evaluations was linked to integrity of the salience network, integrity of the limbic network and DMN were associated with a particular subcategory seen in our patients: repetitive, rigidly stereotypical. i.e. perseverative, self-evaluations. The subgroup of patients who repeatedly made observations about the same personal characteristic were more likely to show atrophy in predominantly left temporal regions, including the amygdala, MTL, anterior temporal lobe (aTL), and medial temporal gyrus. This is consistent with the pattern of atrophy seen in svPPA, thus these results may reflect the overrepresentation of svPPAs' in this group (9% bvFTD, 46% svPPA, 36% ADs, 9% NCs). However, correlations in the vmPFC/mOFC, right amygdala, MTL, and aTL were more bilateral than the classic left-sided pattern of svPPA atrophy, but remains consistent with limbic network connectivity. In animal and human studies, the amydala, hippocampus, ventral striatum and vmPFC have been implicated in emotion extinction (Quirk & Mueller, 2008; Sotres-Bayon et al., 2004). The amygdala functions to tag emotionally arousing events which are encoded by the hippocampus, and imbalance between these regions, as well as dysfunction of ventral striatum (responsible for habit learning), results in the kind of emotional memory distortions seen in post-traumatic stress disorder such as memory intrusions and inability to suppress emotional responses (Goodman et al., 2012; Tsoory et al., 2007).
Whereas PTSD patients have difficulty suppressing emotional memories of a single event, the perseverators in our study tended to fixate on single aspects of self, which in some patients corresponded with increased drive to generate stories, reflected in increased story frequency. For instance, one patient offered six different stories during the conversation, all of which pivoted around a single evaluation: his difficulty with word finding. The limbic network is implicated in personally meaningful evaluations of contextualized socio-emotional information (Amodio et al., 2014; Seeley et al., 2012).The reciprocal relationships between the limbic network and the DMN are still being defined; however, if the limbic network and the DMN function in a balanced relationship to support the evaluation of autobiographical memories, dysfunction of the limbic network may result, not just in perseverant self-evaluations, but in disinhibition of the DMN and subsequently an increase in autobiographical storytelling.
4.3.4. Social attention
As predicted, the social attention deficit group had severe atrophy across a number of salience and task control network regions, including the bilateral insula, putamen and ACC, as well as the right fronto-operculum. Additional regions that were severely atrophied in this group included the left MTL, R>L OFC, and left amygdala. Right OFC volumes have been linked to tracking dynamic emotion (Goodkind et al. 2012) and the salience network is implicated in a number of neuropsychiatric disorders distinguished by severe socio-cognitive and motivational deficits. For instance, Williams Syndrome and ASD both evidence altered SN functioning (Jabbi et al., 2012; Uddin, 2014; Vega, Hohman, Prywelle et al., 2015) and share symptoms such as reduced joint attention and unusual levels of attention to social stimuli (Lincoln, Searcy, Jones, & Lord, 2007; Riby & Hancock, 2009). For our study, we measured social attention via scores on observer rating scales of features such as degree of observed social detachment, indifference, attentiveness, and reservedness. Operationalized this way, social attention was not related to the number of stories a participant told, suggesting that these ratings might have derived more from observers' sense that the participant was engaging in specific paralinguistic communication behaviors that indicated indifference to the confederate's emotions and goals, rather than that they were failing to engage in a conversation.
4.4. Clinical and Therapeutic Implications
In addition to providing information about patterns of brain network functioning that can inform differential diagnostic decisions, examining storytelling practices in patients with neurodegenerative diseases has important implications with regard to patients' mental and physical health. Story-based interventions such as reminiscence and life review therapies are the most widely used therapeutic interventions for dementia symptoms, although evidence supporting the effectiveness of these therapies is underdeveloped (Woods et al., 2009). Storytelling not only requires the coordination of a diverse range of cognitive abilities, but has been shown to strengthen these abilities as well, an attribute relevant to any clinical program attempting to perform cognitive rehabilitation with dementia patients. Specifically, theory of mind (Guajardo & Watson, 2002), emotional regulation (Cappeliez, Guindon, & Robitaille, 2008), and both long term (Bower & Clark, 1969; Wyer et al., 2002) and working memory (Mattarella-Micke & Beilock, 2010) appear to be supported and strengthened through storytelling practices. Further, structured storytelling practices have been shown to improve physical health and psychological well-being in healthy young adults (Pennebaker, & Seagal, 1999; Petrie, Booth, Pennebaker et al., 1995), and can predict cognitive decline later life (Snowdon et al., 1996), suggesting that there may be clinical relevance for aging dementia patients as well.
Similarly, stories are social tools that can both emerge from and strengthen social interactions. Individuals suffering from neurodegenerative disease can become socially isolated as a result of their cognitive and sensorimotor symptoms, thus any mechanism shown to counter or at least moderate this progressive social separation can become an important clinical tool.
4.5. Limitations
Our analyses and interpretations were designed to investigate patterns of storytelling as a hermeneutic for understanding the role of network functioning in spontaneous social behavior. However, one obvious limitation of the study design was that as a small, exploratory study, it was powered to detect only medium to large effects. Further, the small sample size and violations to assumptions of parametric analyses in our story variables inhibited our ability perform a direct brain-behavior correlation analyses. To address this, we looked at group atrophy patterns in patients who demonstrated particular storytelling behaviors. As mentioned above, this may have resulted in imaging findings that were artifacts of degenerative disease (i.e. patterns resulting from disease related atrophy). However, the fact that the groups exemplifying particular storytelling characteristics were diagnostically heterogeneous reduces the likelihood that this was a major factor. Another caveat is that the interactions we studied as the basis for patients' storytelling were brief, thus may not have captured patients' full range of abilities or deficits. A more controlled examination of aspects of storytelling with larger samples, and a longer assessment time may provide better insight into the narrative habits of these groups.
4.6. Conclusions
We found that semantic variant patients had the most unusual patterns of storytelling across neurodegenerative disease groups, demonstrating rigid, perseverative, and self-oriented evaluation patterns and increased autobiographical storytelling. We also found that common characteristics of storytelling mapped onto patterns of atrophy consistent with known intrinsic connectivity networks, providing insight into how these networks subserve realistic social behavior. Evaluations are rarely considered in studies of narrative behaviors, and may have particular clinical relevance in neurodegenerative disease populations.
Highlights.
Focal neurodegenerative groups differ in the frequency and quality of spontaneous story production.
Semantic variant primary progressive patients had the most unique narrative signature.
Spontaneous storytelling was related to diverse neuropsychological functions.
Particular storytelling characteristics were linked to patterns of atrophy consistent with known intrinsic connectivity networks.
Acknowledgments
This research was supported by grants from NIH (5 K23 AG021606, 1R01AG029577, P01AG019724, P50AG023501) and The Larry L. Hillblom Foundation (#2002/2J, 2007/2I) in affiliation with UCSF.
Footnotes
These networks are often regarded as a single network, however, accruing clinical and imaging evidence suggest otherwise (Dosenbach et al., 2007; Powers et al., 2011).
Percentage agreement was computed rather than a k because k cannot account for the absence of a story in an open-ended conversation.
“Speech” was measured by the number of words produced by the participant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A, Schubotz RL, von Cramon DY. Thinking about the future versus the past in personal and non-personal contexts. Brain research. 2008;1233:106–119. doi: 10.1016/j.brainres.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Alea N, Bluck S. Why are you telling me that? A conceptual model of the social function of autobiographical memory. Memory. 2003;11(2):165–178. doi: 10.1080/741938207. [DOI] [PubMed] [Google Scholar]
- Amodio DM. The neuroscience of prejudice and stereotyping. Nature Reviews Neuroscience. 2014:15670–682. doi: 10.1038/nrn3800. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65(A):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gross RG, Cook P, Morgan B, Boiler A, Grossman M. The organization of narrative discourse in Lewy body spectrum disorder. Brain and Language. 2011;119:30–41. doi: 10.1016/j.bandl.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, Mccawley G, Work M, Grossman M. Trying to tell a tale: Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Bamberg M. Talk, small stories, and adolescent identities. Human development. 2004;47(6):366–369. [Google Scholar]
- Bamberg M, Damrad-Frye R. On the ability to provide evaluative comments: Further explorations of children's narrative competencies. Journal of Child Language. 1991;75(03):689–710. doi: 10.1017/s0305000900011314. [DOI] [PubMed] [Google Scholar]
- Bluck S. Autobiographical memory: Explaining its functions in everyday life. Memory. 2003;11:113–123. doi: 10.1080/741938206. [DOI] [PubMed] [Google Scholar]
- Borella E, de Ribaupierre A. The role of working memory, inhibition, and processing speed in text comprehension in children. Learning and Individual Differences. 2014;34:86–92. [Google Scholar]
- Bower GH, Clark MC. Narrative stories as mediators for serial learning. Psychonomic Science. 1969;14:181–182. [Google Scholar]
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative research in psychology. 2006;3(2):77–101. [Google Scholar]
- Bruner JS. Acts of meaning. Cambridge, Massachusetts: Harvard University Press; 1990. [Google Scholar]
- Bruner J. The narrative construction of reality. Critical inquiry. 1991:1–21. [Google Scholar]
- Burin DI, Acion L, Kurczek J, Duff MC, Tranel D, Jorge RE. The role of ventromedial prefrontal cortex in text comprehension inferences: Semantic coherence or socio-emotional perspective? Brain and language. 2014;129:58–64. doi: 10.1016/j.bandl.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappeliez P, Guindon M, Robitaille A. Functions of reminiscence and emotional regulation among older adults. Journal of Aging Studies. 2008;22:266–272. doi: 10.1016/j.aging.2007.06.003. [DOI] [Google Scholar]
- Chapman SB, Bonte FJ, Wong SBC, Zientz JN, Hynan LS, Harris TS, Lipton AM. Convergence of connected language and SPECT in variants of frontotemporal lobar degeneration. Alzheimer Disease and Associated Disorders. 2005;19:202–13. doi: 10.1097/01.wad.0000189050.41064.03. [DOI] [PubMed] [Google Scholar]
- Chiong W, Wilson SM, D'Esposito M, Kayser AS, Grossman SN, Poorzand P, Rankin KP. The salience network causally influences default mode network activity during moral reasoning. Brain. 2013;136:1929–1941. doi: 10.1093/brain/awt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. Journal of neurophysiology. 2012;705(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Mar RA, Xu Y, Liu S, Wagage S, Braun AR. Embodied Comprehension of Stories : Interactions between Language Regions and Modality-specific Neural Systems. Journal of Cognitive Neuroscience. 2013:279–295. doi: 10.1162/jocn. [DOI] [PubMed] [Google Scholar]
- Coates J. Men talk: Stories in the making of masculinities. Maiden, MA: Blackwell; 2003. [Google Scholar]
- Coelho CA, Liles BZ, Duffy RJ. Impairments of discourse abilities and executive functions in traumatically brain-injured adults. Brain injury. 1995;9(5):471–477. doi: 10.3109/02699059509008206. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological review. 2000;107(2):261. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Cassol H, Phillips C, Balteau E, Salmon E, Van der Linden M. Brains creating stories of selves: the neural basis of autobiographical reasoning. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Brons C, Jurjanz L, Poettrich K, Winiecki P, Holthoff VA. Overgenerality of autobiographical memory in people with amnestic mild cognitive impairment and early Alzheimer's disease. Archives of Clinical Neuropsychology. 2010;25(1):22–27. doi: 10.1093/arclin/acp098. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neuroscience & Biobehavioral Reviews. 2012;36(7):1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nature Reviews Neuroscience. 2014;15(11):732–744. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag C, Troiani V, Bonner M, Powers C, Avants B, Gee J, Grossman M. Hierarchical organization of scripts: Converging evidence from FMRI and frontotemporal degeneration. Cerebral Cortex. 2010;20:2453–63. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil S, Droit-Volet S. Time perception, depression and sadness. Behavioural Processes. 2009;80(2):169–176. doi: 10.1016/j.beproc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Glosser G, Deser T. A comparison of changes in macrolinguistic and microlinguistic aspects of discourse production in normal aging. Journal of Gerontology. 1992;47(4):P266–P272. doi: 10.1093/geronj/47.4.p266. [DOI] [PubMed] [Google Scholar]
- Goodman J, Leong KC, Packard MG. Emotional modulation of multiple memory systems: implications for the neurobiology of post-traumatic stress disorder. 2012 doi: 10.1515/revneuro-2012-0049. [DOI] [PubMed] [Google Scholar]
- Goodman LA, Kruskal WH. Measures of association for cross classifications*. Journal of the American Statistical Association. 1954;49(268):732–764. [Google Scholar]
- Goodkind MS, Sollberger M, Gyurak A, Rosen HJ, Rankin KP, Miller B, Levenson R. Tracking emotional valence: the role of the orbitofrontal cortex. Human Brain Mapping. 2012;33(4):753–762. doi: 10.1002/hbm.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guajardo NR, Watson AC. Narrative discourse and theory of mind development. The Journal of Genetic Psychology. 2002;163:305–325. doi: 10.1080/00221320209598686. [DOI] [PubMed] [Google Scholar]
- Habermas T, Ott LM, Schubert M, Schneider B, Pate A. Stuck in the past: Negative bias, explanatory style, temporal order, and evaluative perspectives in life narratives of clinically depressed individuals. Depression and Anxiety. 2008;25(11):E121–E132. doi: 10.1002/da.20389. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. The Journal of Neuroscience. 2008;28(2) doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, J. D., Ruffman, T., McDonald, S., O'Leary, M. A. P., Phillips, L. H., Brodaty, H., 2), 5623-5630.
- Henry JD, Rendell PG. Recognition of disgust is selectively preserved in Alzheimer's disease. Neuropsychologia. 2008;46(5):1363–1370. doi: 10.1016/j.neuropsychologia.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Hirsh JB, Mar RA, Peterson JB. Personal narratives as the highest level of cognitive integration. Behavioral and Brain Sciences. 2013;36:216–217. doi: 10.1017/S0140525X12002269. [DOI] [PubMed] [Google Scholar]
- Jilka SR, Scott G, Ham T, Pickering A, Bonnelle V, Braga RM, Sharp DJ. Damage to the salience network and interactions with the default mode network. The Journal of Neuroscience. 2014;34(33):10798–10807. doi: 10.1523/JNEUROSCI.0518-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel JE, Polyn SM. Functional interactions between large-scale networks during memory search. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht258. bht258. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sharon JS, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends in Cognitive Sciences. 2009;13:103–109. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Labov W, Waletzky J. Narrative analysis. In: Helm J, editor. Essays on the Verbaland Visual Arts. Seattle: U of Washington Press; 1967. pp. 12–44. [Google Scholar]
- Lehn H, Steffenach HA, van Strien NM, Veltman DJ, Witter MP, Haberg AK. A specific role of the human hippocampus in recall of temporal sequences. The Journal of Neuroscience. 2009;29(11):3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words affect labeling disrupts amygdala activity in response to affective stimuli. Psychological science. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Searcy YM, Jones W, Lord C. Social interaction behaviors discriminate young children with autism and Williams syndrome. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(3):323–331. doi: 10.1097/chi.0b013e31802b9522. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Mind and body: Psychology of emotion and stress. New York: Norton; 1984. [Google Scholar]
- Mar RA. The neuropsychology of narrative: Story comprehension, story production and their interrelation. Neuropsychologia. 2004;42:1414–1434. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annual review of psychology. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Marini A, Zettin M, Galetto V. Cognitive correlates of narrative impairment in moderate traumatic brain injury. Neuropsychologia. 2014;64:282–288. doi: 10.1016/j.neuropsychologia.2014.09.042. [DOI] [PubMed] [Google Scholar]
- Mattarella-Micke A, Beilock S. Situating math word problems: The story matters. Psychonomic Bulletin and Review. 2010;17:106–111. doi: 10.3758/PBR.17.1.106. [DOI] [PubMed] [Google Scholar]
- McAdams DP, Josselson RE, Lieblich AE. Identity and story: Creating self in narrative. American Psychological Association; 2006. [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean KC, Pasupathi M, Pals JL. Selves creating stories creating selves: A process model of self-development. Personality and Social Psychology Review. 2007;11:262–278. doi: 10.1177/1088868307301034. [DOI] [PubMed] [Google Scholar]
- McLean KC, Thorne A. Identity light: Entertainment stories as a vehicle for self-development. In: McAdams DP, Josselson R, Lieblich A, editors. Identity and story: Creating self in narrative Narrative study of lives. Vol. 4. American Psychological Association; 2006. pp. 111–127. [Google Scholar]
- Meulenbroek O, Rijpkema M, Kessels RP, Rikkert MGO, Fernandez G. Autobiographical memory retrieval in patients with Alzheimer's disease. Neuroimage. 2010;53(1):331–340. doi: 10.1016/j.neuroimage.2010.05.082. [DOI] [PubMed] [Google Scholar]
- Ochs E, Capps L. Narrating the self. Annual Review of Anthropology. 1996;25:19–43. doi: 10.1146/annurev.anthro.25.1.19. [DOI] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Social cognitive and affective neuroscience. 2012;2:123–133. doi: 10.1093/scan/nss119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennebaker JW, Seagal JD. Forming a story: The health benefits of narrative. Journal of clinical psychology. 1999;55(10):1243–1254. doi: 10.1002/(SICI)1097-4679(199910)55:10<1243::AID-JCLP6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Pennington N, Hastie R. Explaining the evidence: Tests of the story model for juror decision making. Journal of Personality and Social Psychology. 1992;62:189–206. doi: 10.1037/0022-3514.62.2.189. [DOI] [Google Scholar]
- Petrie KJ, Booth RJ, Pennebaker JW, Davison KP, Thomas MG. Disclosure of trauma and immune response to a hepatitis B vaccination program. Journal of consulting and clinical psychology. 1995;63(5):787. doi: 10.1037//0022-006x.63.5.787. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. The Journal of neuroscience. 2010;30(32):10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Miller BL. Sensitivity of revised diagnostic criteria for the behavioral variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awrl79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riby DM, Hancock PJ. Do faces capture the attention of individuals with Williams syndrome or autism? Evidence from tracking eye movements. Journal of autism and developmental disorders. 2009;39(3):421–431. doi: 10.1007/s10803-008-0641-z. [DOI] [PubMed] [Google Scholar]
- Rogalski Y, Altmann LJ, Plummer-D'Amato P, Behrman AL, Marsiske M. Discourse coherence and cognition after stroke: A dual task study. Journal of communication disorders. 2010;43(3):212–224. doi: 10.1016/j.jcomdis.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Alcantar O, Rothlind J, Sturm V, Kramer JH, Weiner M, Miller BL. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage. 2010;49(4):3358–3364. doi: 10.1016/j.neuroimage.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. Neuroimage. 2010;49(4):3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu JEEA, Barcelo F. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society. 2009;75(03):438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, Mackenzie IR, Miller BL. Unravelling Bolero: progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131(1):39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Zhou J, Kim E. Frontotemporal dementia: What can the behavioral variant teach us about human brain organization? Neuroscientist. 2012;18:373–385. doi: 10.1177/1073858411410354. [DOI] [PubMed] [Google Scholar]
- Shank RC, Abelson RP. Scripts, goals, plans and understanding: An inquiry into human knowledge structures. New York: Wiley; 1977. [Google Scholar]
- Shany-Ur T, Rankin KP. Personality and social cognition in neurodegenerative disease. Current opinion in neurology. 2011;24(6) doi: 10.1097/WCO.0b013e32834cd42a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life: findings from the Nun Study. Jama. 1996;275(7):528–532. [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extmction. Learning & Memory. 2004;11(5):525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suedfeld P, Tetlock PE, Streufert S. Conceptual/integrative complexity 1992 [Google Scholar]
- Thorne A. The press of personality: A study of conversations between introverts and xtraverts. Journal of Personality and Social Psychology. 1987;53(4):718. [Google Scholar]
- Tse WS, Bond AJ. Development and validation of the Two-Dimensional Social Interaction Scale. Psychiatry Research. 2001;203:249–260. doi: 10.1016/S0165-1781(01)00271-2. [DOI] [PubMed] [Google Scholar]
- Tsoory MM, Vouimba RM, Akirav I, Kavushansky A, Avital A, Richter-Levin G. Amygdala modulation of memory-related processes in the hippocampus: potential relevance to PTSD. Progress in brain research. 2007;167:35–51. doi: 10.1016/S0079-6123(07)67003-4. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neuroscience & Biobehavioral Reviews. 2009;33(8):1198–1203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Molnar-Szakacs I, Iacoboni M. Beyond superior temporal cortex: intersubject correlations in narrative speech comprehension. Cerebral Cortex. 2008;75(1):230–242. doi: 10.1093/cercor/bhm049. [DOI] [PubMed] [Google Scholar]
- Vega JN, Hohman TJ, Pryweller JR, Dykens EM, Thornton-Wells TA. Resting-State Functional Connectivity in Individuals with Down Syndrome and Williams Syndrome Compared with Typically Developing Controls. Brain connectivity. 2015 doi: 10.1089/brain.2014.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 2003;4(2):139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Woods RT, Bruce E, Edwards RT, Hounsome B, Keady J, Moniz-Cook, Russell IA. Reminiscence groups for people with dementia and their family carers: Pragmatic eight-centre randomized trial of joint reminiscence and maintenance versus usual treatment: a protocol. Trials. 2009;10:64. doi: 10.1186/1745-6215-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyer RS, Adaval R, Colcombe SJ. Narrative-based representations of social knowledge: Their construction and use in comprehension, memory and judgment. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 34. San Diego, CA: Academic Press; 2002. pp. 131–197. [Google Scholar]
- Yang XF, Bossmann J, Schiffhauer B, Jordan M, Immordino-Yang MH. Intrinsic default mode network connectivity predicts spontaneous verbal descriptions of autobiographical memories during social processing. Frontiers in psychology. 2012;3:1–10. doi: 10.3389/fpsyg.2012.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–65. doi: 10.1152/jn.00338.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Chee MW, Buckner RL. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage. 2014;88:212–227. doi: 10.1016/j.neuroimage.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]


