Abstract
Intestinal neuronal dysplasia type B (IND) denotes an increased proportion of hyperplastic submucosal ganglia, as resolved histochemically in 15 µm-thick frozen sections. IND has been reported proximal to the aganglionic segment in patients with Hirschsprung disease (HSCR) and is putatively associated with a higher rate of post-surgical dysmotility. We have developed and validated histological criteria to diagnose IND-like submucosal ganglion cell hyperplasia (IND-SH) in paraffin sections, and used the approach to study the incidence and clinical/genetic associations of IND-SH at the proximal margins of HSCR pull-through resection specimens. Full-circumference paraffin sections from the proximal margins of 64 HSCR colonic pull-through specimens and 24 autopsy controls were immunostained for the neuron-specific Hu antigen and nucleated ganglion cells in each submucosal ganglion were counted. In controls, an age-related decline in the relative abundance of “giant” ganglia (≥7 nucleated Hu+ ganglion cells) was observed. A conservative diagnostic threshold for IND-SH (control mean + 3 times the standard deviation) was derived from 15 controls less than 25 weeks of age. No control exceeded this threshold, whereas in the same age range, IND-SH was observed at the proximal margins in 15% (7/46) of HSCR resections, up to 15 cm proximal to the aganglionic segment. No significant correlation was observed between IND-SH and length of or distance from the aganglionic segment, gender, trisomy 21, RET or SEMA3C/D polymorphisms, or clinical outcome, but analysis of more patients with better long-term follow-up will be required to clarify the significance of this histological phenotype.
Keywords: Hirschsprung, neuronal dysplasia, hyperganglionosis, transition zone, observer bias
INTRODUCTION
Hirschsprung disease (HSCR) is a congenital malformation characterized by absent myenteric and submucosal ganglion cells in the distal rectum and a variable length of contiguous bowel. It most commonly presents with intestinal obstruction in the newborn. Surgical treatment consists of resection of the aganglionic segment of bowel followed by a pull-through procedure in which anatomically normally innervated (“normoganglionic”) bowel is anastomosed to the distal rectum. Between the aganglionic and normoganglionic segments is a variable length of neuroanatomically abnormal bowel termed the “transition zone” (TZ). Accurate delineation and resection of the TZ is considered important because anastomosis within the TZ (“TZ pull-through”) has been associated with persistent postoperative obstructive symptoms, and used to justify a redo pull-through surgery [1–4]. Reported histopathologic features of the TZ include partial circumferential aganglionosis [5–7], myenteric hypoganglionosis [8], hypertrophic submucosal innervation [9], and intestinal neuronal dysplasia type B (IND) [10].
IND is a histological phenotype of the submucosal enteric neural plexus. It has been described both as a feature of the TZ and as an isolated disorder clinically resembling HSCR, but without aganglionosis. Since the first description by Meier-Ruge in 1971 [11], the diagnostic criteria for IND have continued to evolve. The most consistently invoked finding has been a disproportionately high percentage of “giant ganglia” within the submucosal plexuses of the colon. A more recent diagnostic algorithm, described by Meier-Ruge [12], employs the following: 1) a “giant ganglion” is a ganglion containing 9 or more nerve cell bodies in a single tissue section, 2) more than 20% of submucosal ganglia must be giant ganglia, 3) a minimum of 25 submucosal ganglia must be evaluated, and 4) the patient must be older than 1 year. However, in the context of HSCR, most reported patients with transition zone IND have been under a year of age, often neonates [3, 4, 13–16].
The diagnosis of IND has been controversial for many reasons, including inconsistencies in study methodology and lack of appropriate controls. The published diagnostic criteria derive entirely from analyses of 15 µm-thick, frozen cryostat sections, which are enzyme histochemically stained to highlight neuronal cell bodies [12]. This method of tissue processing, section thickness, and section staining are not routine, and have not been adopted by many laboratories. Instead, some pathologists have unjustifiably applied the same diagnostic criteria to conventional 4–5 µm-thick, H&E-stained paraffin sections [3, 14, 17–19]. While establishing the prevalence of giant ganglia is required to diagnose IND, identification of individual ganglion cells in tissue sections is subjective and very few studies specify how they were discriminated and counted [18, 20]. Not surprisingly, Koletzko and colleagues demonstrated a high rate of inter-observer variability among pathologists with regard to diagnosis of IND [21].
The original descriptions of IND were based on subjective interpretation of an increased fraction of giant ganglia [22, 23]. Later, giant ganglia in histochemically stained frozen sections were defined as 7 or more ganglion cells/ganglion [24, 25] and then as ≥ 8 ganglion cells/ganglion [26]. These definitions of giant ganglia seem arbitrary, as none of the studies reported the ranges of ganglion cells per ganglion or the percentages of giant ganglia observed within their control populations. Kobayashi and colleagues used rectal suction biopsies obtained from symptomatic patients to exclude HSCR, but which contained ganglion cells, as “controls” [3]. As discussed by Lumb and Moore [20], biopsies from symptomatic patients with “low” percentages of giant ganglia are not appropriate controls and represent a form of selection bias that is prone to misrepresent the association between large numbers of giant ganglia with the disease phenotype. A variety of other “controls” have been applied to establish normal data for the distribution of ganglion cells per submucosal ganglion, each with significant flaws that compromise their validity (Table 1). Arguably, the best reported control data to date are from the series of autopsied patients without any history of intestinal dysmotility reported by Coerdt et al [28]. They divided patients into 4 age groups ranging from premature infants to adults and used the Meier-Ruge sectioning and staining protocols. However, they pooled data for each age group and failed to report normal ranges for the percentage of giant ganglia observed, which is necessary to validate the diagnostic criteria of >10% [25] or >20% giant ganglia [12] for IND. Coerdt and colleagues did demonstrate that the mean number of giant ganglia decreases with age, which appears to be the basis for contemporary recommendation that isolated IND should not be diagnosed before age 1 year [29].
Table 1.
Controls used to define intestinal neuronal dysplasia in published studies
| Source | Controls (age range) | Specimen | Fixation, section thickness |
Type of staining |
Histological criteria described1 |
>1 anatomic location within bowel sampled |
% giant ganglia reported2 |
|---|---|---|---|---|---|---|---|
| [27] | 10 patients being evaluated for HSCR or allergic proctitis (3d to 12wks) |
Rectal suction biopsy |
Formalin, section thickness not stated |
H&E | No | Not stated | No |
| [28] | 36 autopsy specimens from patients with no history of intestinal dysfunction (<35wks gestational age to >70yrs) |
Not Stated | Frozen, 15µm |
AChE, LDH, SDH, and NADPH |
No | Yes | No |
| [3] | 24 patients being evaluated for constipation with HSCR excluded (15d – 9yrs) |
Rectal suction biopsy |
Frozen, 15µm |
AChE | No | No | No |
| [3] | 1) 4 patients having colostomy closure for imperforate anus, 2) 6 patients undergoing bladder augmentation, 3) Autopsy specimens from 13 patients with no history of intestinal disease (1d – 12yrs) |
Full thickness biopsy |
Frozen, 10µm |
AChE | No | Yes | No |
| [24] | 20 patients; no details stated (3mo – 6.3yrs) |
Rectal mucosal biopsy |
Frozen, 15µm |
AChE, LDH, and SDH |
No | No (rectum up to 15 cm from pectinate line) |
No |
Clear description of histological features used to identify and count individual ganglion cells;
Statement of range or upper limit of normal for percentage of giant ganglia in control population;
Abbreviations: H&E, hematoxylin and eosin; AChE, acetylcholinesterase histochemistry; LDH, lactate dehydrogenase histochemistry; SDH, succinate dehydrogenase histochemistry; NADPH, nicotinamide-adenine-dinucleotide-phosphate diphosphorase histochemistry; d, days; wks, weeks; mo, months; yrs, years.
The presence of IND proximal to the aganglionic segment is putatively associated with an increased incidence of post-pull-through dysmotility [2, 3, 10, 15, 30, 31]. Using the diagnostic criteria for isolated IND discussed above, some investigators have reported transition zone IND (HSCR-associated IND) in up to 75% of HSCR patients [16, 32, 33]. Using a different method to identify giant ganglia, based on 9 autopsy controls, our recent study of TZ in resections from 15 patients with short-segment HSCR found IND-like submucosal ganglion cell hyperplasia (IND-SH) in 8 patients [7]. Among all the neuroanatomical features of TZ, IND-SH was the most common to extend more than 5 cm proximal to the aganglionic segment, and the most frequent to involve the proximal surgical margin.
In this study, we refine our method to identify IND-SH using an expanded series of controls and various validation measures. We apply the approach to large series of full-circumference, proximal margin sections from surgical resections for HSCR, because anatomy of the proximal surgical margin is considered a good representation of adjacent bowel incorporated into the patient’s anastomosis and affords the best opportunity to diagnose neuroanatomical changes that might lead to a TZ pull-through. The histopathological results are correlated with available pre- and post-operative clinical information and the presence/absence of 3 genetic polymorphisms known to contribute to the heterogeneous genetic basis of HSCR.
METHODS
Study population
Paraffin-embedded full-circumference sections and corresponding surgical pathology reports from 70 HSCR patients were retrieved from the surgical pathology archives of two institutions. All of the patients had pull-through procedures between 2006 and 2014. Only full-circumference sections from the proximal margin of the most proximal resection specimen (primary pull-through or ostomy takedown for two-stage procedure) were used. The HSCR study population was limited to patients with resection margins in the colon, which excluded 5 patients with total colonic aganglionosis, as well as 1 patient who had a separate segment of ganglionic ileum resected (two anastomoses). Full-circumference sections of large intestine, most commonly from proximal rectum, were obtained from 24 autopsy controls with no history of intestinal dysmotility. The clinical features of the study patients and controls are presented in Table 2. Multiple sites at different distances from the anus, along the entire length of the colon, were analyzed from 6 controls, 2 of which were accrued after most of the data analysis was complete and were only used to study the percentage of giant ganglia at different points along the colon (see Results). All of the samples and clinical records were de-identified in accordance with approvals from review boards at the participating institutions.
Table 2.
Clinical features of autopsy controls and HSCR patients
| Gender | Distance from Anus (cm)* | Trisomy 21 |
Other Anomalies |
Pull-Through Procedure |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | 1–5 | 6–10 | 11–20 | >20 | +ve | −ve | +ve | −ve | 1- stage |
2- stage |
|
| Controls | ||||||||||||
| ≤25 wks | 7 | 8 | 14 | 7 | 5 | 12 | 1 | 14 | 4 | 11 | ||
| >25 wks | 3 | 6 | 7 | 2 | 0 | 0 | 1 | 8 | 6 | 3 | ||
| Total | 10 | 14 | 21 | 9 | 5 | 12 | 2 | 22 | 10 | 14 | ||
| HSCR | ||||||||||||
| ≤25 wks | 12 | 34 | 1 | 9 | 28 | 7 | 14 | 32 | 13 | 33 | 37 | 9 |
| >25 wks | 5 | 13 | 0 | 4 | 7 | 6 | 4 | 14 | 5 | 13 | 4 | 14 |
| Total | 17 | 47 | 1 | 13 | 35 | 13 | 18 | 46 | 18 | 36 | 41 | 23 |
Distances include multiple sites from 2 additionalnon-aneuploidcontrols (1d M with total anomalous venous return and 10 d F with hypoplastic left heart); distance (length of resection) not available for 2 HSCR patients;
Abbreviations: F, female; M, male; d, day; wks, weeks; +ve, positive; −ve, negative.
Hu immunohistochemical (IHC) staining
Full-circumference, 4-µm thick paraffin sections from the proximal surgical margins of each of the autopsy controls and 64 HSCR specimens were immunostained with an antibody that recognizes the pan-neuronal marker, Hu C/D (Molecular Probes, Eugene, OR) [34]. An automated immunostainer (Benchmark XT; Ventana Medical Systems, Tucson, AZ) was used with the immunostainer’s “standard” conditions (60 minutes, cell conditioner 1) and a 32-minute incubation period with a 1:1250 dilution of the primary antibody. Slides were counterstained with hematoxylin.
Quantification of submucosal ganglion cells
Formal criteria to identify submucosal ganglia, discriminate individual ganglion cells, and determine the number of ganglion cells per ganglion were developed cooperatively by two observers (RK and MS; (Supplemental Table 1). Each observer independently applied these criteria to determine the number of ganglion cells in every submucosal ganglion in full-circumference sections from 15 controls and 14 HSCR patients. Ganglion cell counts per ganglion were recorded sequentially as the observer shifted the microscopic field along the full-circumference of the tissue section, such that the total number of submucosal ganglia, number of ganglion cells in each ganglia, and relative distribution of smaller and larger ganglia around the circumference were recorded.
Intra-observer variability was tested by having a single observer count submucosal ganglia and nucleated ganglion cells within each ganglion for 5 randomly selected Hu immunohistochemistry slides on day 1 and again 300 or more days later. To minimize recognition, the slide labels were masked. Similarly, inter-observer variability was tested by having two observers independently score the same set of 29 Hu immunostained slides. Relative percentage difference (absolute difference between counts divided by mean of two counts) for ganglion cell counts obtained by two observers or the same observer on two occasions were calculated to assess inter-observer and intra-observer agreement, respectively.
Subsequently, a single observer (M.S.) recorded submucosal ganglion cell counts in the Hu-immunostained full-circumference sections from 24 autopsy controls and 64 HSCR patients. Slides were coded and shuffled to eliminate potential bias. The control data were used to establish ≥7 Hu-positive ganglion cells per ganglion in an individual tissue section as the definition of a “giant ganglion” for infants less than age 25 weeks (n = 15; see RESULTS) and to establish an upper limit of normal for the percentage of giant ganglia in a full-circumference section, as follows. Percentages of giant ganglia from the control population under 25 weeks of age were used to calculate an upper limit of normal for the percentage of giant ganglia based on the mean value + 3 × standard deviation (SD). Data available for this calculation included percentages from a single site in the colons of 11 controls and multiple sites (1.5 – 45 cm from the anus) in 4 controls. For controls that were sampled at multiple sites, a randomly selected section obtained between 10 and 30 cm from the anus was used (one site per patient) because the length of most of the HSCR specimens was in this range. IND-SH was defined as a percentage of giant submucosal ganglia above the upper limit of normal (7.7%; see RESULTS).
Correlations between proximal margin IND-SH and other anatomic (length of aganglionic segment and distance from proximal margin to aganglionic segment), age, gender, clinical (post-operative enterocolitis, soiling, chronic constipation), or genetic (trisomy 21, 3 common HSCR-associated polymorphisms in or near the RET and SEMA3C/SEMA3D genes) variables were investigated. The 3 common polymorphisms were identified in genome-wide association study of HSCR patients of European ancestry and replicated independently [35, 36] The prevalence of each of the variables listed was compared between patients with and without proximal IND-SH using Welch Two Sample T-test, Fisher’s Exact test, or Permutation Chi-Squared test.
SNP Genotyping Assays
DNA was extracted from ribbons of formalin fixed paraffin embedded (FFPE) tissue using QIAmp DNA FFPE Tissue kit (Qiagen, Valencia, CA) as per manufacturer’s protocol. 20ng genomic DNA was used for genotyping each SNP using TaqMan Human Pre-Designed genotyping assays following the manufacturer’s protocol (Applied Biosystems, Grand Island, NY). The assays IDs are as follows: C__16017524_10 (rs2435357), C__26742714_10 (rs2506030), C__30936238_10 (rs12707682). The end-point fluorescence measurements were performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Grand Island, NY) and analyzed using Sequence Detection System Software v.2.1 (Applied Biosystems, Grand Island, NY).
Effect of sample size on diagnostic accuracy
The bowel circumference from every control or HSCR section was divided into 8 equal sectors. The percentage of giant ganglia was calculated for each of the 8 contiguous sectors, and the results compared with that obtained from the entire circumference (“gold standard”). Similarly, percentages of giant ganglia in subsets of sequentially acquired ganglia were analyzed to identify the largest possible sample with a percentage of giant ganglia sufficient to produce an erroneous diagnosis. In both of these analyses, diagnosis of IND-SH required >7.7% giant ganglia (see RESULTS) and was considered inaccurate if a false-positive or false-negative diagnosis of IND-SH was obtained, as compared with the diagnosis obtained from all ganglia in the full-circumference.
Measurement of internuclear distances between submucosal ganglion cells
For each of the Hu C/D-immunostained control sections, distances between nuclei in adjacent ganglion cells in 25 randomly selected submucosal ganglia were measured and averaged. Digital images of 400× fields were captured with a Nikon DS camera and measurements made with digital calipers using Nikon NIS Elements BR 3-2 software. For each ganglion cell one end of the caliper was placed on its nucleus and distance measured to the nearest nucleus of an adjacent ganglion cell.
RESULTS
Reproducibility of submucosal ganglion cell counts
An initial study of submucosal ganglion cell counts in 5 Hu-immunostained full-circumference sections, chosen at random from the controls and HSCR samples, demonstrated that ganglion cell counts of Hu-immunostained slides, performed by the same observer, were reproducible. The mean relative percentage difference (RPD) in the number of ganglion cells per circumference was 9.6+/− 3.5% for observer 1 (MS) and 7.9 +/− 2.9% for observer 2 (RPK) for counts performed on the same slide set by the same observer more than 300 days apart (Supplemental Table 2).
Inter-observer reproducibility (mean RPD ± se = 26.7 ± 3.81%; n = 29 Hu-immunostained sections) was poorer than counts obtained by a single observer (Supplemental Table 3). For 27/29 of the samples (93%; sign-test p-value <0.001), observer 1’s total ganglion cell count was less than that of observer 2 (mean difference = 148.3 ± 23.2 ganglion cells; p-value <0.001). Retrospective co-review suggested that the greatest discrepancies resulted from different thresholds for nuclear staining and discrimination of individual cell and individual ganglia boundaries in the Hu-immunostained sections. Except as otherwise stated, all of the remaining data were collected by observer 1.
Defining a giant ganglion using Hu IHC sections
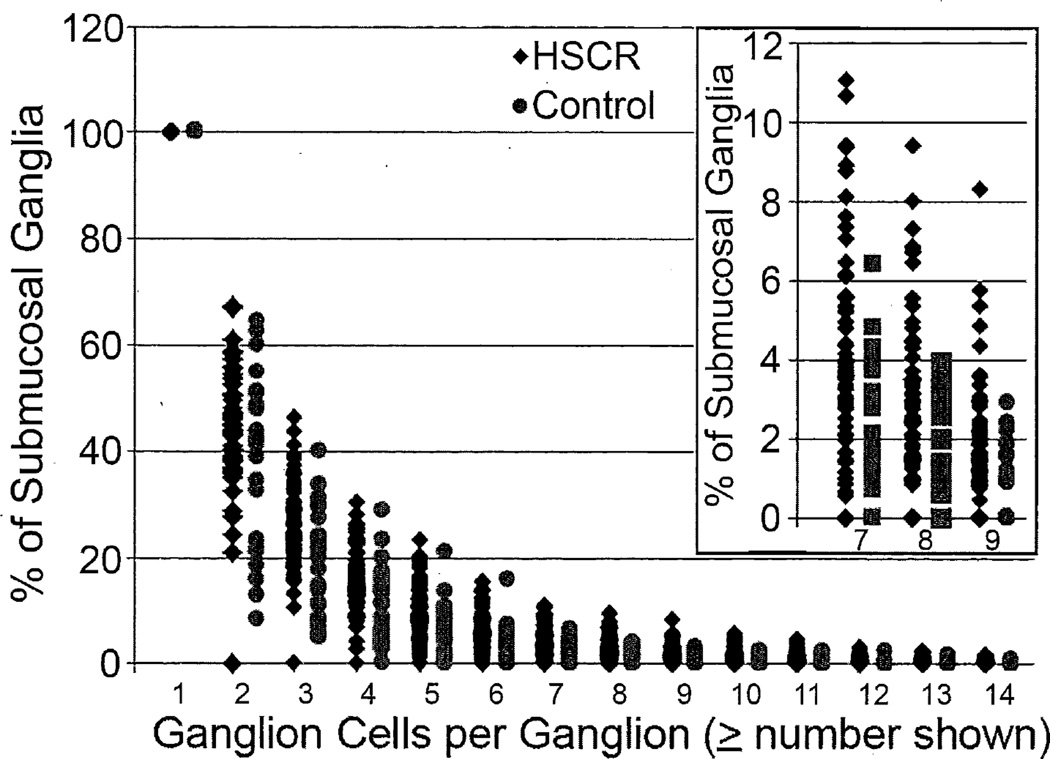
The number of Hu+ ganglion cell bodies per submucosal ganglion was recorded for every ganglion in full circumference colon sections from 24 control patients and the proximal surgical margins of 64 HSCR patients. In each population, a non-linear inverse relationship was observed between the prevalence of ganglia and number of neurons / ganglion (ganglion size), with the majority of ganglia (>90%) containing 6 or fewer ganglion cells in any section (Figure 1). The average percentage of ganglia of given size was fairly similar in HSCR patients and controls. However, for larger ganglia (i.e., ≥7, ≥8, or ≥9 ganglion cells), a subset of up to 14 HSCR cases emerged in which the percentage of affected ganglia exceeded the control range (Figure 1, inset). Based in part on this empirical distribution, we defined giant ganglia as those with greater than or equal to 7 ganglion cells. The decision was influenced by considerations of statistical sensitivity and inter-rater variability, which increased with higher thresholds (data not shown) because the percentage of giant ganglia in any given specimen is small and including more ganglia in the definition of giant ganglia reduces the impact of intra-observer variability in individual ganglion cell counts.
Figure 1.
Relative abundance of submucosal ganglia of various sizes (ganglion cells per ganglion) in controls and HSCR patients. The inset shows expansion of the Y-axis for ganglia with ≥7, ≥8, and ≥9 ganglion cells.
Defining IND-SH based on density of giant ganglia
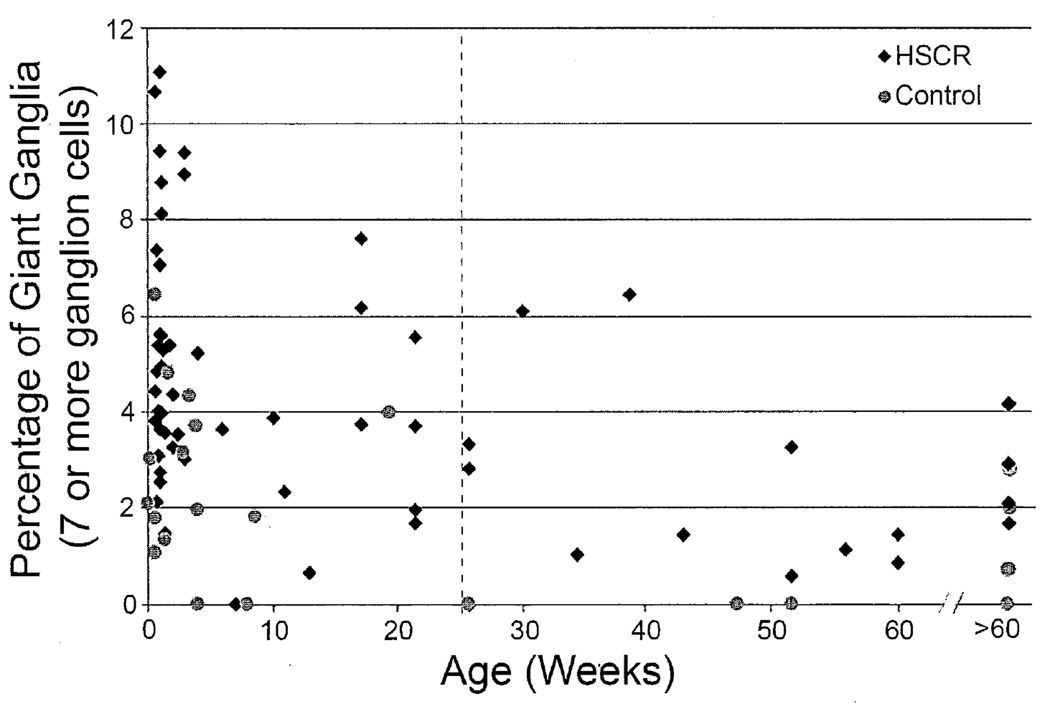
In order to develop diagnostic criteria for IND-SH, an upper limit of normal for the percentage of giant ganglia was established using the control data. Two variables – age and distance from the anus - and their effects on the density of giant ganglia in control patients were considered in this process. Figure 2 shows the control patients graphed according to age and % giant ganglia. The percentage of giant submucosal ganglion cells declined with age, as reported by others [28]. The mean percentage of giant ganglia of control patients ≤25 weeks (2.36 ± 1.85) was significantly greater than that of patients >25 weeks (0.69 ± 1.10; T-test, p = 0.03).
Figure 2.
Relative abundance of giant submucosal ganglia (≥7 ganglion cells) versus postnatal age for controls and HSCR patients. Dashed line indicates boundary at 25 weeks, which was used to exclude older patients for further analysis as described in the text.
The percentage of giant ganglia is based on counts of nucleated ganglion cells in individual ganglia in the plane of section, which will decrease as cell soma enlargement and added neuropil drive neuronal nuclei apart. Therefore, age-related growth of submucosal ganglion cell bodies and accumulation of intervening neuropil are factors that might reduce calculated percentages of giant ganglia. To investigate this possibility, mean interganglion cell distances were compared across different age groups and were found to be significantly smaller in control patients ≤25 weeks (11.17 ± 2.71 µm) compared to controls >25 weeks (14.26 ± 1.45 µm; T-test, p = 0.002).
The percentage of giant ganglia in our control population declined with age, probably at least in part due to cell growth and neuropil accumulation. This suggests that lower threshold values to define IND-SH are likely appropriate for older age groups, for which the percentage of submucosal ganglia with ≥7 ganglion cells is likely much lower. Unfortunately insufficient older controls were present in our series to establish normal ranges for patients older than 25 weeks. Because of the high likelihood of misclassifying older patients with proximal margin IND-SH as normal, we excluded controls over 25 weeks when developing our diagnostic criteria for IND-SH and limited our investigation to only those HSCR patients in the same age group (i.e., 0 – 25 weeks). Thus, 15 controls and 46 HSCR patients constitute the remainder of the study (Table 1).
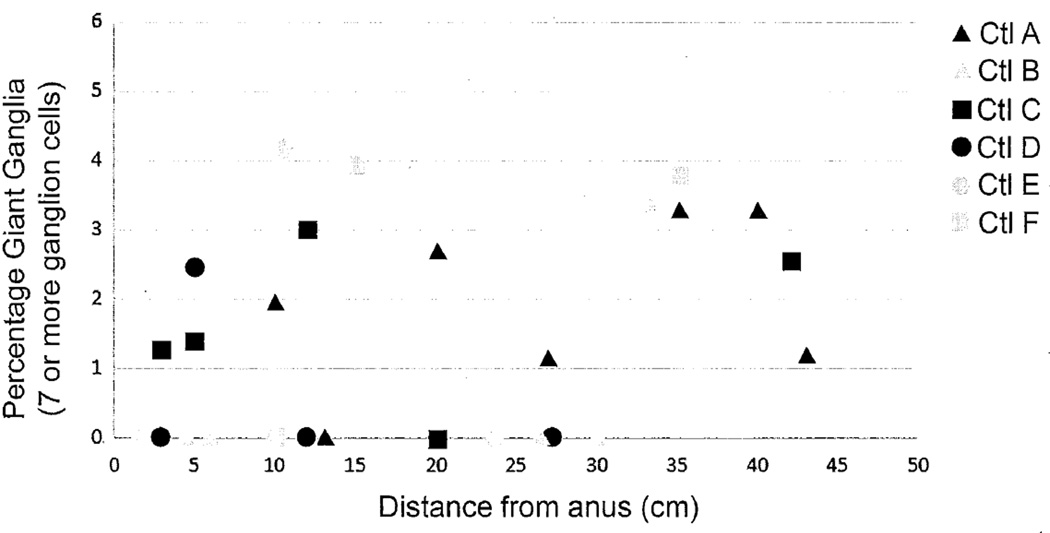
The percentages of giant ganglia observed in full-circumference sections from the 15 controls under 25 weeks of age ranged from 0 to 6.4% (mean ± se = 2.6 ± 0.5). The majority of the control sections were obtained from the terminal 10 cm of the large intestine. However, examination of sections taken at multiple sites from 6 controls suggested only a minor increase in the percentage of giant ganglia with distance from the anus, which equated linearly to a very gradual increase of 0.06%/cm, p=0.05 (Figure 3). Over the entire length of the colon, the effect of this trend on the total percentage of giant ganglia was minimal and the latter never exceeded 6.4% in any control section. An upper limit of normal was defined as 7.7% based on the percentages of giant ganglia observed in the controls. The normal limit was established conservatively using the mean + 3SD (see Methods). This value was based on control counts by a observer 1 and identical calculations using independent counts of the same sections by a observer 2 yielded a higher value (mean + 3SD = 8.3%).
Figure 3.
Relative abundance of giant submucosal ganglia (≥7 ganglion cells) versus distance from the anus for full-circumference sections of large intestine from 6 autopsy controls, all less than 25 weeks of age. Despite a slight trend toward more giant ganglia in more proximal sections, the upper range does not change significantly.
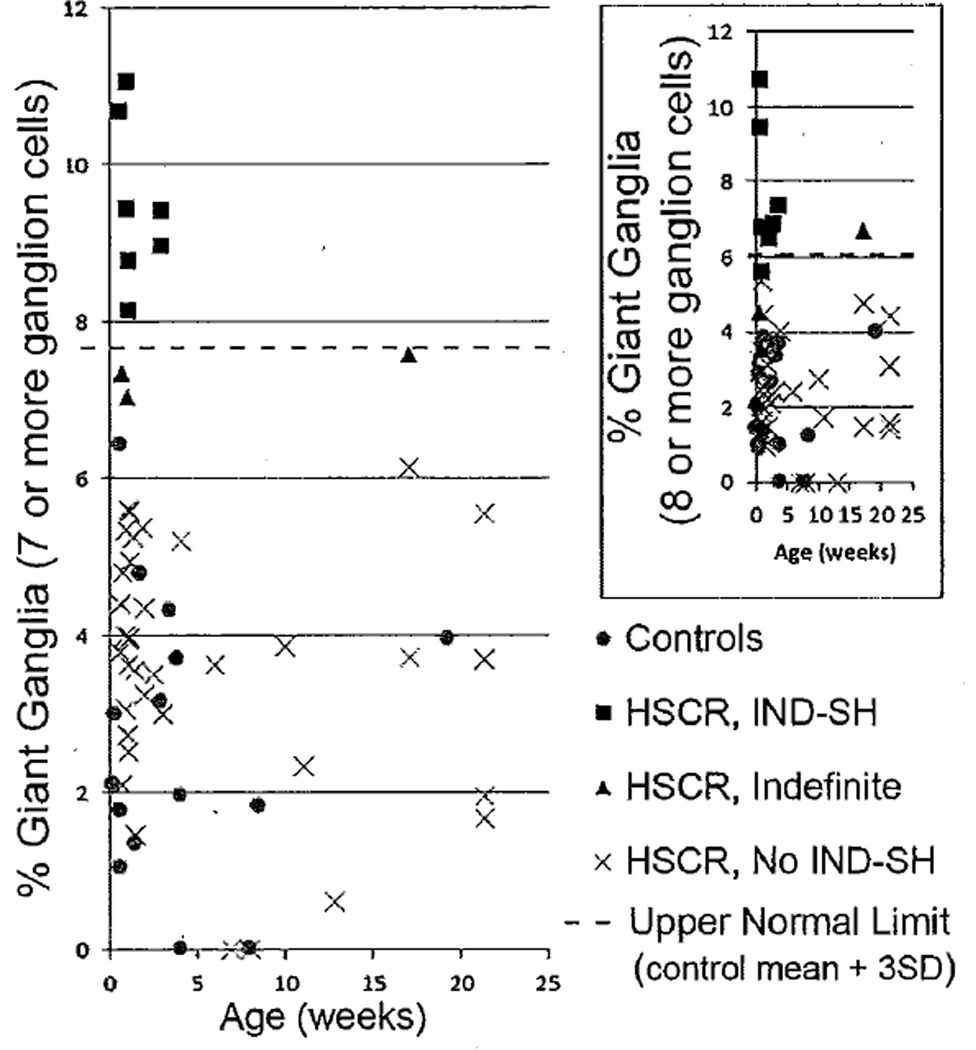
Using this approach, the 46 HSCR patients can be segregated into three groups based on the percentage of giant submucosal ganglia observed at their proximal margins (Figure 4). A subset of 7 patients (15%, black diamonds in Figure 4) with percentages of giant ganglia above 7.7% is considered to have IND-SH at their proximal surgical margins. Another group of 36 (78%, “X” in Figure 4) were within the range of control values and are not considered to have IND-SH. Percentages of giant ganglia for the remaining 3 (7%, black triangles in Figure 4) fell in between the highest value observed in controls and 7.7%, and might be considered “indefinite” for IND-SH. Despite the somewhat arbitrary definition of a giant ganglion used in this classification scheme, 6 of the 7 IND-SH patients are classified identically when similar analysis is performed using “mean + 3SD” and ≥8 ganglion cells per ganglion as an alternative definition of IND-SH (Figure 4, inset). Furthermore, the same 7 patients were classified as IND-SH by observer 2, using his counts from their proximal margins and a threshold (mean + 3SD = 8.3%) from his independent analysis of the controls (Supplemental Figure 1).
Figure 4.
Relative abundance of giant submucosal ganglia (≥7 ganglion cells) versus age for subset of controls and HSCR cases ≤25 weeks of age. The dashed horizontal line denotes the upper limit of normal (control population mean + 3SD). Patients with ganglion cell counts above the line (black squares) are considered IND-SH, as opposed to those within the normal range (gray circles). Patients with values in between the normal range and upper limit of normal (black triangles) are considered “indefinite”. The inset shows analysis of the same populations (symbols correspond to classification based on ≥7 ganglion cells per giant ganglion) using ≥8 ganglion cells per ganglion as the definition for giant ganglia.
Sample size needed to diagnose or exclude IND-SH
Given that our definition of IND-SH is based entirely on the percentage of giant submucosal ganglia, analysis of an adequate sample of ganglia to reliably diagnose or exclude IND-SH is important. Full-circumference section counts yielded a generous assessment of submucosal ganglia [121 (mean) ± 5.53 (SD) ganglia] and were regarded as the diagnostic gold standard in this study. However, such counts are time-consuming and tedious, particularly when large numbers of cases have to be evaluated. In order to investigate the reliability of smaller samples, quantitation of giant ganglia from portions of the circumferences of control tissue sections were analyzed. In more than a third of control samples (8/21), at least 50% of the circumference needed to be counted in order to avoid any possible “false-positive” diagnosis of IND-SH (>7.7% giant ganglia).
An alternative approach is to consider the fewest number of ganglia necessary to count in order to reliably exclude IND-SH in controls or correctly diagnose IND-SH in HSCR patients. Again, diagnosis of IND-SH based on all submucosal ganglia in the entire circumference was regarded as the “gold-standard”. From the recorded counts of the entire circumference of each section, subsets of sequentially counted ganglia were evaluated to find the maximum number of ganglia, with a percentage of giant ganglia that produced an opposite diagnosis (false-negative or false-positive) to that obtained from the entire circumference. For the control samples, HSCR patients within the control range (“not IND-SH” group) and those considered “indefinite for HSCR”, the mean (±SD) number of ganglia needed to avoid a false-positive diagnosis was 37+/−35 (n=15), 55+/−44 (n=36) and 178+/−22 (n=3) ganglia, respectively. For the 7 patients considered “definite” IND-SH, an average count of 184+/−95 ganglia prevented a false-negative diagnosis.
Potential associations with proximal margin IND-SH
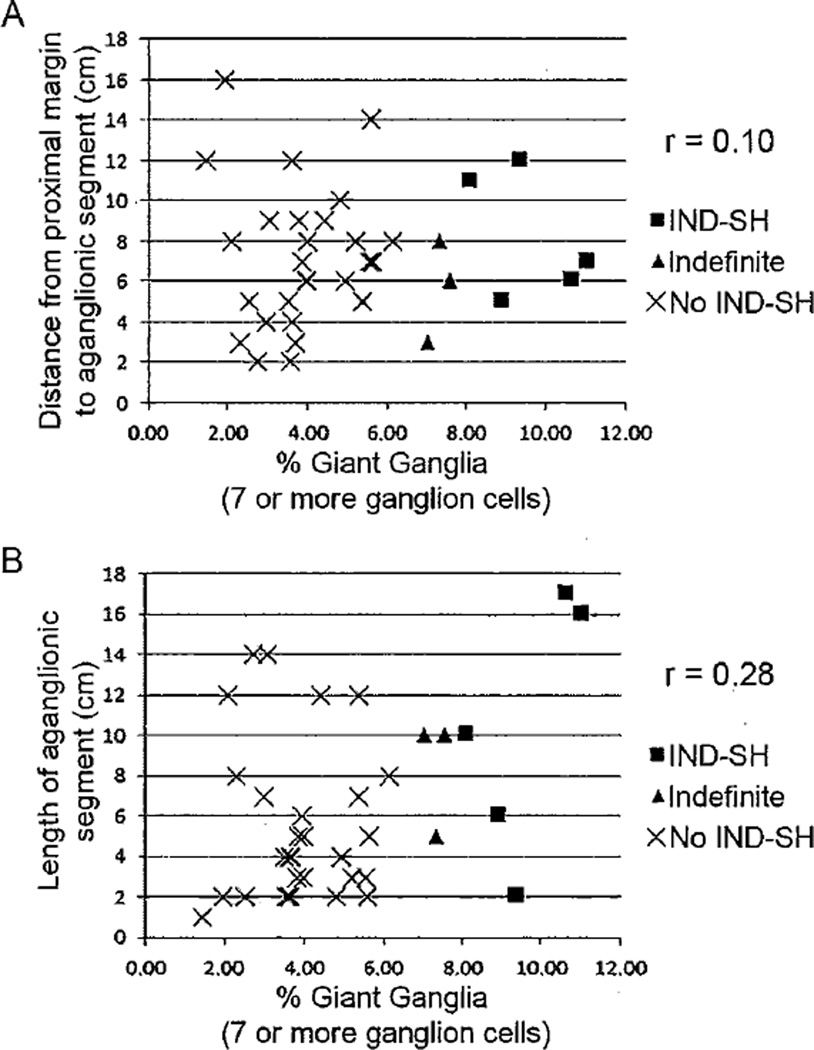
More detailed clinical information regarding the 7 patients with IND-SH is provided in Table 3. 80% of HSCR is short-segment aganglionosis, limited to the rectosigmoid colon [37]. In our entire series of patients, which excluded total colonic aganglionosis, 55/61 (90%) of patients had aganglionic segments ≤15 cm (Table 1). Short-segment HSCR has an increased prevalence in males compared with females, with a ratio of 4:1. Our study population reflected this as 47/64 (73%) of HSCR patients were males. Among those patients less than 6 months, no correlation was found with IND-SH and either the length of the aganglionic segment (Figure 5A) or the distance from the proximal margin to the aganglionic segment (Figure 5B). Similarly, although all but one of the 7 HSCR patients with IND-SH at their proximal margins were male, this gender bias was not significantly different from those without IND-SH (26 males / 13 females; Fisher’s Exact test, p = 0.41). Surprisingly, the mean distance from the proximal margin to the aganglionic segment was significantly longer in the IND-SH group (9.6 cm) versus other HSCR patients (6.1 cm) (p = 0.04; Table 4). In one patient, IND-SH was present at the proximal margin despite resection of 15 cm of ganglionic bowel.
Table 3.
Possible clinical and genetic covariates with IND-SH1
| IND-SH | No IND SH | Statistical Test / p value |
|
|---|---|---|---|
|
Mean Distance between Proximal Margin and Aganglionic Segment |
9.6 | 6.1 | Welch Two-sample T test, p = 0.04 |
|
Mean Length of Aganglionic Segment |
9.8 | 6.7 | Welch Two-sample T test, p = 0.27 |
| Gender | Male: 6; Female: 1 | Male: 26; Female: 13 | Fisher’s Exact test, p=0.41 |
| Trisomy 21 | 3 Yes / 4 No | 11 Yes / 28 No | Fisher’s Exact test, p=0.39 |
| Post-Op Complications | 1 Yes / 6 No | 11 Yes / 28 No | Fisher’s Exact test, p=0.66 |
| SNPs3 | |||
| RET (rs2435357) | CC 2 / CT 2 / TT 1 | CC 10 / CT 19 / TT 5 | Permutation Chi- Squared test, p=0.82 |
| RET (rs2500603) | AA 1 / AG 2 / GG 1 | AA 10 / AG 18 / GG 4 | Permutation Chi- Squared test, p=1.00 |
| SEMA3D (rs12707682) | CC 0 / CT 4 / TT2 | CC 4 / CT 19 / TT13 | Permutation Chi- Squared test, p=0.85 |
For the 46 patients under 6 months of age;
Complications include soiling, constipation, enterocolitis, incontinence, or other signs of dysmotility as noted in clinical records;
Underlined nucleotide indicates allele associated with HSCR susceptibility.
Figure 5.
Relationships between relative abundance of giant ganglia (≥7 ganglion cells per ganglion) and (A) distance from the proximal margin to the aganglionic segment or (B) length of the aganglionic segment. No significant correlation was observed.
Table 4.
Clinical features of IND-SH patients
| Age1 / Sex | Gest. Age (wks) |
T21 | Other Malformations |
Family History of HSCR |
Procedure | Resection Length (cm) |
Agang. Segment (cm) |
Pre-op Compli- cations |
Follow- up (yrs) |
Post-op Compli- cations |
|---|---|---|---|---|---|---|---|---|---|---|
| 17 d / M | 41 | N | N | N | So | 14 | 2 | N | 3 | Soiling |
| 8 d / M | 37 | Y | Undescended testes |
N | Sw | 21 | 10 | N | 1.83 | Mild constipation |
| 4 d / F | 37 | Y | N | N | Sw | 23 | 17 | HAEC | 1.17 | N |
| 7 d / M | nr | Y | Patent ductus arteriosus |
N | Sw | 14.5 | 2.5 | N | 5 | Soiling |
| 21 d / M | nr | N | N | N | Sw | 11 | 6 | N | 1.08 | N |
| 6 d / M | "term" | N | N | N | So | 31 | 21 | N | 3.5 | Soiling |
| 6 d / M | 38 | N | N | N | Sw | 23 | 16 | N | 0.33 | N |
Age at time of pull-through procedure;
Abbreviations: Gest, gestational; T21, trisomy 21; So, Soave; Sw, Swenson; Agang, aganglionic; wks, weeks; yrs, years; d, days; N, “no” or “none”; Y, “yes” or “present”.
Possible correlations between proximal margin IND-SH and 4 of the most common genetic alterations observed in HSCR were explored. Trisomy 21 is the most common chromosomal abnormality associated with HSCR and is reported in 2 – 10 percent of cases in most series [38]. A higher prevalence of trisomy 21 in this study (18 of 64 patients, 28%) probable reflects referral patterns to the tertiary care centers involved. Regardless, no difference in the prevalence of trisomy 21 among the patients under 6 months with IND-SH was found compared to the similarly aged patients without it (Table 4).
In the context of the complex genetics of HSCR, specific single nucleotide variants of non-coding sequences in or near RET (rs2435357, rs2506030) and SEMA3D (rs12707682) appear to be relatively common HSCR susceptibility factors, albeit with incomplete penetrance [36]. Genotype data was available for 42/46 HSCR patients under age 6 months. Among 3 single nucleotide polymorphisms associated with HSCR, no obvious correlation was identified between IND-SH and the prevalence of any individual polymorphism (Table 4) or collective pattern of high-risk alleles (not shown).
DISCUSSION
The incidence of IND is highly discordant across different centers due to significant variations in laboratory methodology, ganglion cell sampling technique, and interpretation of histologic features. The diagnostic criteria for IND are based on frozen-section histochemistry, a specialized approach with limited general utility and inapplicable to routine formalin-fixed paraffin-based tissue processing. A goal of our investigation was to develop an efficient and reproducible method for identifying IND-like submucosal ganglion cell hyperplasia (IND-SH) using methods that are routine in most clinical laboratories. Paraffin sections, immunostained for the neuron-specific antigen, Hu C/D, and counter-stained with hematoxylin, satisfied our requirements, in that ganglion cells were easily identified and quantified with good reproducibility by a single observer. However, interobserver variability was an issue, as despite the immunostain and formal written criteria for neuron identification, submucosal ganglion cell counts from the same sections by two observers differed significantly. The finding that Hu-based ganglion cell counts by individual observers are internally consistent, but different from each other, was also observed in our prior study of myenteric ganglia [8]. This phenomenon almost certainly reflects observer bias, which we were only able to eliminate by using a single observer to analyze all of the control and HSCR sections, and basing diagnosis on calculations of an observer-specific norm for the density of giant ganglia. It is reassuring that although counts of control sections by a second observer shifted the normal range slightly, the proportion of giant ganglia in HSCR patients also increased and perfect inter-observer diagnostic concordance was reached for the subset of HSCR sections that underwent dual analysis. As a consequence, we are confident that 7 of the 46 HSCR patients less than 25 weeks of age have an abnormally high percentage of submucosal giant ganglia (IND-SH), but caution that the quantitative “cut-offs” employed in this research study (7.7% giant ganglia for observer 1, 8.3% giant ganglia for observer 2) must be regarded as observer-specific.
The distinction between IND-SH, as defined in this study, and IND, as defined (and redefined) by others is important. Although a disproportionately high percentage of giant submucosal ganglia is the primary diagnostic feature for both IND-SH and IND, the approaches used to delineate each “entity” differ markedly. Lack of appropriate control data is one reason for skepticism about IND [20, 39–41]. The current study established a normal range for the prevalence of giant submucosal ganglia based on controls with no history of intestinal dysmotility. This is important because the percentage of giant ganglia is the most important variable for the diagnosis of IND. Surprisingly, prior studies of IND either did not use appropriately selected controls and/or only reported mean number of ganglion cells per submucosal ganglion, rather than the percentage of giant ganglia [27, 28, 42, 43]. Collection of robust control data allowed us to confidently establish observer-specific conservative upper limits of normal (control mean + 3SD) for the percentage of ganglia, show that the limit is valid at any site along the length of the colon, and objectively define IND-SH as values above this limit. Coerdt et al. also showed that the mean number of submucosal ganglion cells per ganglion was stable at different colonic sites [28], but to the best of our knowledge, no prior study has addressed the proportion of giant ganglia in this manner. Since resections for HSCR encompass the terminal rectum and a variable length of contiguous bowel, diagnostic criteria of IND-SH must be relevant to multiple colonic sites, if they are to be applied to the proximal margins of HSCR resections.
Patient age is another important variable, and a previous study suggested that mean submucosal ganglion cell counts decline during the first year of age [28]. This observation led Meier-Ruge and others to conclude that IND should not be diagnosed in patients less than 1 year of age, or more conservatively less than 4 years of age [29]. In our series, the younger controls (≤25 weeks) had a significantly higher percentage of giant ganglia compared with older individuals (>25 weeks), with a fairly sharp “drop-off” between birth and 1 year of age. In part, we found the change probably relates to enlargement of ganglion cell bodies and intervening neuropil, which increase the distance between ganglion cell nuclei and reduce the likelihood of encountering as many nucleated ganglion cells in the same section of a single ganglion. Age-related neuronal degeneration may be another mechanism, for which we have no direct evidence at present. Regardless, this age-related reduction in percentage of giant ganglia indicates that age-matched controls are necessary to diagnose IND-SH, and the empirically defined thresholds for percent giant ganglia is probably insensitive beyond 25 weeks. Analysis of large numbers of controls at various ages may be necessary to devise age-appropriate criteria for IND-SH.
Based on empirically derived and validated criteria for excessive submucosal ganglia, IND-SH was diagnosed in the proximal margins of 7/46 HSCR patients (15%) in our under 6 month-old cohort. The data probably underestimate the overall prevalence of HSCR-associated IND-SH, because we examined only proximal resection margins and would not identify IND-SH confined to more distal ganglionic bowel between the margin and aganglionic segment. Regardless, our findings substantiate that IND-SH is present in a significant subset of HSCR resections and exists at the proximal margin. In our series of patients, the average distance between the proximal margin and the aganglionic segment was longer on average in patients with IND-SH, up to 15 cm proximal to the aganglionic segment in one case. In this respect, IND-SH differs from other neuropathologic alterations (e.g. hypoganglionosis, hypertrophic submucosal nerves) used to define the transition zone in HSCR, which are often restricted to a distance of ≤5 cm proximal to the aganglionic segment [7].
The results independently appear to confirm the existence of HSCR-associated IND, put forth by others, and raise the question as to whether IND / IND-SH has any genetic or prognostic significance. The significance of IND at proximal margins is unclear. Some studies have suggested a negative correlation between IND and clinical outcome [3, 10, 30, 31], while others have reported no significant correlations [16, 44, 45]. We chose to investigate proximal resection margins because they provide a reasonable representation of the post-surgical bowel “upstream” to the patient’s anastomosis. However, limited available outcome data and the relatively small size of our patient cohort, coupled with the genetic, surgical, and pathological (i.e., length of aganglionic segment) heterogeneity imposed significant limitations on our ability to identify meaningful associations between proximal margin IND-SH and other findings. We examined multiple adverse outcomes including soiling, enterocolitis, need for additional surgery, and chronic constipation, but did not find any significant correlation. However, long-term follow-up was incomplete for many patients. While this study is insufficiently powered to detect many potentially relevant covariates of IND-SH, the diagnostic criteria we’ve developed form a foundation for a larger multi-institutional investigation of such relationships in the future.
Even if diagnosis of IND-SH were shown to be important, inter-observer discordance poses a major impediment to widespread use of the approach describe here. Not surprisingly, similar problems have hampered acceptance of IND diagnosis by the traditional histochemical approach [45]. Poor agreement of submucosal ganglion cell counts between observers suggest that either each observer must analyze their own control data and establish observer-specific criteria to discriminate IND-SH or alternative observer-independent methods to reliably identify IND-SH must be developed. Sample size is another important consideration, given the quantitative nature of IND-SH. We found that analysis of small or medium-sized portions of the circumference or subsets of ganglia were unreliable, in comparison to analysis of all ganglia around the entire circumference. Unfortunately, analysis of the entire circumference is time-consuming. Until a less laborious approach with no significant observer bias is developed, and clinical significance is demonstrated, diagnosis of IND-SH should probably remain a research activity, not a routine part of HSCR surgical pathology.
Supplementary Material
Acknowledgments
This study was partially supported by the Hirschsprung Disease Research Collaborative (www.hdrcstudy.org).
REFERENCES
- 1.Kapur RP, Kennedy AJ. Transitional zone pull through: surgical pathology considerations. Semin Pediatr Surg. 2012;21:291–301. doi: 10.1053/j.sempedsurg.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Ure BM, Holschneider AM, Meier-Ruge WA. Neuronal intestinal malformations: a retro- and prospective study on 203 patients. Eur J Pediatr Surg. 1994;4:279–286. doi: 10.1055/s-2008-1066118. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Hirakawa H, Surana R, O'Briain DS, Puri P. Intestinal neuronal dysplasia is a possible cause of persistent bowel symptoms after pull-through operation for Hirschsprung disease. J Pediatr Surg. 1995;30:253–259. doi: 10.1016/0022-3468(95)90570-7. [DOI] [PubMed] [Google Scholar]
- 4.Schmittenbecher PP, Gluck M, Wiebecke B, Meier-Ruge W. Clinical long-term follow-up results in intestinal neuronal dysplasia (IND) Eur J Pediatr Surg. 2000;10:17–22. doi: 10.1055/s-2008-1072317. [DOI] [PubMed] [Google Scholar]
- 5.White FV, Langer JC. Circumferential distribution of ganglion cells in the transition zone of children with Hirschsprung disease. Pediatr Dev Pathol. 2000;3:216–222. doi: 10.1007/s100249910028. [DOI] [PubMed] [Google Scholar]
- 6.Gherardi GJ. Pathology of the ganglionic-aganglionic junction in congenital megacolon. Arch Pathol. 1960;69:520–528. [PubMed] [Google Scholar]
- 7.Kapur RP, Kennedy AJ. Histopathologic delineation of the transition zone in short-segment Hirschsprung disease. Pediatr Dev Pathol. 2013;16:252–266. doi: 10.2350/12-12-1282-OA.1. [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan M, Kapur RP. Counting myenteric ganglion cells in histologic sections: an empirical approach. Hum Pathol. 2010;41:1097–1108. doi: 10.1016/j.humpath.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Coe A, Collins MH, Lawal T, Louden E, Levitt MA, Pena A. Reoperation for Hirschsprung disease: pathology of the resected problematic distal pull-through. Pediatr Dev Pathol. 2012;15:30–38. doi: 10.2350/11-02-0977-OA.1. [DOI] [PubMed] [Google Scholar]
- 10.Schmittenbecher PP, Sacher P, Cholewa D, et al. Hirschsprung's disease and intestinal neuronal dysplasia - a frequent association with implications for the postoperative course. Pediatr Surg Int. 1999;15:553–558. doi: 10.1007/s003830050669. [DOI] [PubMed] [Google Scholar]
- 11.Meier-Ruge W. Ueber ein Erkarakungsbild des Kolons mit Hirschsprung Symptomatik. Verh Dtsch Ges Pathol. 1971;55:506–511. [PubMed] [Google Scholar]
- 12.Meier-Ruge WA, Ammann K, Bruder E, et al. Updated results on intestinal neuronal dysplasia (IND B) Eur J Pediatr Surg. 2004;14:384–391. doi: 10.1055/s-2004-821120. [DOI] [PubMed] [Google Scholar]
- 13.Estevao-Costa J, Fragoso AC, Campos M, Soares-Oliveira M, Carvalho JL. An approach to minimize postoperative enterocolitis in Hirschsprung's disease. J Pediatr Surg. 2006;41:1704–1707. doi: 10.1016/j.jpedsurg.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 14.Meyrat BJ, Laurini RN. Plasticity of the enteric nervous system in patients with intestinal neuronal dysplasia associated with Hirschsprung's disease: a report of three patients. Pediatr Surg Int. 2003;19:715–720. doi: 10.1007/s00383-003-1035-0. [DOI] [PubMed] [Google Scholar]
- 15.Schulten D, Holschneider AM, Meier-Ruge W. Proximal segment histology of resected bowel in Hirschsprung's disease predicts postoperative bowel function. Eur J Pediatr Surg. 2000;10:378–381. doi: 10.1055/s-2008-1072395. [DOI] [PubMed] [Google Scholar]
- 16.Hanimann B, Inderbitzin D, Briner J, Sacher P. Clinical relevance of Hirschsprung-associated neuronal intestinal dysplasia (HANID) J Pediatr Surg. 1992;27:344–348. doi: 10.1055/s-2008-1063425. [DOI] [PubMed] [Google Scholar]
- 17.Cord-Udy CL, Smith VV, Ahmed S, Risdon RA, Milla PJ. An evaluation of the role of suction rectal biopsy in the diagnosis of intestinal neuronal dysplasia. J Pediatr Gastroenterol Nutr. 1997;24:1–6. doi: 10.1097/00005176-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lumb PD, Moore L. Are giant ganglia a reliable marker of intestinal neuronal dysplasia type B (IND B)? Virchows Arch. 1998;432:103–106. doi: 10.1007/s004280050141. [DOI] [PubMed] [Google Scholar]
- 19.Banani SA, Forootan HR, Kumar PV. Intestinal neuronal dysplasia as a cause of surgical failure in Hirschsprung's disease: a new modality for surgical management. J Pediatr Surg. 1996;31:572–574. doi: 10.1016/s0022-3468(96)90499-6. [DOI] [PubMed] [Google Scholar]
- 20.Lumb PD, Moore L. Back to the drawing board. Intestinal neuronal dysplasia type B: not a histological entity yet. Virchows Arch. 1998;432:99–102. doi: 10.1007/s004280050140. [DOI] [PubMed] [Google Scholar]
- 21.Koletzko S, Jesch I, Faus-Kebetaler T, et al. Rectal biopsy for diagnosis of intestinal neuronal dysplasia in children: a prospective multicentre study on interobserver variation and clinical outcome. Gut. 1999;44:853–861. doi: 10.1136/gut.44.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fadda B, Maier WA, Meier-Ruge W, Scharli A, Daum R. Neuronal intestinal dysplasia. Critical 10-years' analysis of clinical and biopsy diagnosis. Z Kinderchir. 1983;38:305–311. doi: 10.1055/s-2008-1059994. [DOI] [PubMed] [Google Scholar]
- 23.Meier RW. Ultrashort segment Hirschsprung disease. An objective picture of the disease substantiated by biopsy. Z Kinderchir. 1985;40:146–150. doi: 10.1055/s-2008-1059734. [DOI] [PubMed] [Google Scholar]
- 24.Meier-Ruge WA, Schmidt PC, Stoss FS. Intestinal neuronal dysplasia and its morphometric evidences. Pediatr Surg Int. 1995;10:447–453. [Google Scholar]
- 25.Meier-Ruge WA, Bronnimann PB, Gambassi F, Schmid PC, Schmidt CP, Stoss F. Histopathological criteria for intestinal neuronal dysplasia of the submucosal plexus (type B) Virchows Arch. 1995;426:549–556. doi: 10.1007/BF00192108. [DOI] [PubMed] [Google Scholar]
- 26.Meier-Ruge WA, Longo-Bauer CH. Morphometric determination of the methodological criteria for the diagnosis of intestinal neuronal dysplasia (IND B) Pathol Res Pract. 1997;193:465–469. doi: 10.1016/s0344-0338(97)80098-2. [DOI] [PubMed] [Google Scholar]
- 27.Simpser E, Kahn E, Kenigsberg K, Duffy L, Markowitz J, Daum F. Neuronal intestinal dysplasia: quantitative diagnostic criteria and clinical management. J Pediatr Gastroenterol Nutr. 1991;12:61–64. [PubMed] [Google Scholar]
- 28.Coerdt W, Michel JS, Rippin G, et al. Quantitative morphometric analysis of the submucous plexus in age-related control groups. Virchows Arch. 2004;444:239–246. doi: 10.1007/s00428-003-0951-7. [DOI] [PubMed] [Google Scholar]
- 29.Meier-Ruge WA, Bruder E, Kapur RP. Intestinal neuronal dysplasia type B: one giant ganglion is not good enough. Pediatr Dev Pathol. 2006;9:444–452. doi: 10.2350/06-06-0109.1. [DOI] [PubMed] [Google Scholar]
- 30.Meyrat BJ, Lesbros Y, Laurini RN. Assessment of the colon innervation with serial biopsies above the aganglionic zone before the pull-through procedure in Hirschsprung's disease. Pediatr Surg Int. 2001;17:129–135. doi: 10.1007/s003830000507. [DOI] [PubMed] [Google Scholar]
- 31.Moore SW, Laing D, Kaschula ROC, Cywes S. A histological grading system for the evaluation of co-existing NID with Hirschsprung's disease. Eur J Pediatr Surg. 1994;4:293–297. doi: 10.1055/s-2008-1066120. [DOI] [PubMed] [Google Scholar]
- 32.Hirobe S, Doody DP, Ryan DP, Kim SH, Donahoe PK. Ectopic class II major histocompatability antigens in Hirschsprung's disease and neuronal intestinal dysplasia. J Pediatr Surg. 1992;27:357–363. doi: 10.1016/0022-3468(92)90861-z. [DOI] [PubMed] [Google Scholar]
- 33.Briner J, Oswald HW, Hirsig J. Neuronal intestinal dysplasia - clinical and histochemical findings and its association with Hirschsprung's disease. Z. Kinderchir. 1986;41:282–286. doi: 10.1055/s-2008-1043360. [DOI] [PubMed] [Google Scholar]
- 34.Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor A, Jiang Q, Chatterjee S, et al. Population variation in total genetic risk of Hirschsprung disease from common RET, SEMA3 and NRG1 susceptibility polymorphisms. Hum Mol Genet. 2015;24:2997–3003. doi: 10.1093/hmg/ddv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Q, Arnold S, Heanue T, et al. Functional loss of semaphorin 3C and/or semaphorin 3D and their epistatic interaction with ret are critical to Hirschsprung disease liability. Am J Hum Genet. 2015;96:581–596. doi: 10.1016/j.ajhg.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda K, Goto S. Diagnosis of Hirschsprung's disease in Japan. An analysis of 1628 patients. Ann Surg. 1984;199:400–405. doi: 10.1097/00000658-198404000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakravarti A, Lyonnet S. Hirschsprung disease. In: Scriver CR, et al., editors. The Metabolic & Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 931–942. [Google Scholar]
- 39.Sacher P, Briner J, Hanimann B. Is neuronal intestinal dysplasia (NID) a primary disease or a secondary phenomenon. Eur J Pediatr Surg. 1993;3:228–230. doi: 10.1055/s-2008-1063549. [DOI] [PubMed] [Google Scholar]
- 40.Schofield DE, Yunis EJ. What is intestinal neuronal dysplasia? Pathol Ann. 1992;27:249–262. [PubMed] [Google Scholar]
- 41.Berry CL. Intestinal neuronal dysplasia: does it exist or has it been invented? Virchows Arch [A] 1993;422:183–184. doi: 10.1007/BF01621800. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi H, Hirakawa H, Puri P. What are the diagnostic criteria for intestinal neuronal dysplasia? Pediatr Surg Int. 1995;10:459–464. [Google Scholar]
- 43.Meier-Ruge WA, Scharli AF, Stoss F. How to improve histopathological results in the biopsy diagnosis of gut dysganglionosis. A methodological review. Pediatr Surg Int. 1995;10:454–458. [Google Scholar]
- 44.Haricharan RN, Seo JM, Kelly DR, et al. Older age at diagnosis of Hirschsprung disease decreases risk of postoperative enterocolitis, but resection of additional ganglionated bowel does not. J Pediatr Surg. 2008;43:1115–1123. doi: 10.1016/j.jpedsurg.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 45.Koletzko S, Ballauff A, Hadziselimovic F, Enck P. Is histological diagnosis of neuronal intestinal dysplasia related to clinical and manometric findings in constipated children? Results of a pilot study. J Pediatr Gastroenterol Nutr. 1993;17:59–65. doi: 10.1097/00005176-199307000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.