Abstract
Testosterone acts though the androgen receptor in Sertoli cells to support germ cell development (spermatogenesis) and male fertility, but the molecular and cellular mechanisms by which testosterone acts are not well understood. Previously, we found that in addition to acting through androgen receptor to directly regulate gene expression (classical testosterone signaling pathway), testosterone acts through a nonclassical pathway via the androgen receptor to rapidly activate kinases that are known to regulate spermatogenesis. In this study, we provide the first evidence that nonclassical testosterone signaling occurs in vivo as the MAP kinase cascade is rapidly activated in Sertoli cells within the testis by increasing testosterone levels in the rat. We find that either classical or nonclassical signaling regulates testosterone-mediated Rhox5 gene expression in Sertoli cells within testis explants. The selective activation of classical or nonclassical signaling pathways in Sertoli cells within testis explants also resulted in the differential activation of the Zbtb16 and c-Kit genes in adjacent spermatogonia germ cells. Delivery of an inhibitor of either pathway to Sertoli cells of mouse testes disrupted the blood-testis barrier that is essential for spermatogenesis. Furthermore, an inhibitor of nonclassical testosterone signaling blocked meiosis in pubertal mice and caused the loss of meiotic and postmeiotic germ cells in adult mouse testes. An inhibitor of the classical pathway caused the premature release of immature germ cells. Collectively, these observations indicate that classical and nonclassical testosterone signaling regulate overlapping and distinct functions that are required for the maintenance of spermatogenesis and male fertility.
Keywords: androgen receptor, fertility, nongenomic, Sertoli cell, testis
INTRODUCTION
Testosterone (T) is essential for the maintenance of spermatogenesis (reviewed in [1, 2]). T is maintained at high levels in the testis, 25- to 125-fold greater than in plasma [3–5], and acts through the androgen receptor (AR, also denoted NR3C4) in Sertoli cells within the seminiferous tubules of the testis to support germ cell development. In the absence of T or a functional AR, males are infertile because spermatogenesis rarely proceeds beyond the diplotene or pachytene stages of meiosis [6–8]. T signaling contributes to maintaining tight junctions between adjacent Sertoli cells that are required to maintain the blood-testis barrier (BTB) and a specialized environment for germ cells [7, 9–11]. The absence of T or AR also results in the premature detachment of developing spermatid germ cells from Sertoli cells [12, 13] and blocks the release of mature spermatids/spermatozoa from Sertoli cells [14–17].
Developing germ cells are not thought to respond directly to T because they do not express functional AR [18–21]. The major target for T in the seminiferous tubules is the Sertoli cell [22, 23]. AR expression changes dramatically in Sertoli cells in a manner that is correlated with the stages of the seminiferous epithelial cycle. In the rat, the expression of AR protein in Sertoli cells is low, except during stages VI–VIII, when levels increase dramatically [19, 24]. In the mouse and rat, it is during stages VI–IX that the lack of T or AR interrupts spermatogenesis [1, 8, 10, 14, 25].
T has been shown to act via classical and nonclassical pathways [26, 27]. In the classical pathway, T diffuses through the plasma membrane and interacts with AR that then binds to androgen response elements (AREs) in gene promoter regions, recruits coregulator proteins, and regulates gene transcription. Activation of the classical pathway requires at least 30–45 min to detect the first increases in gene transcription, with protein-mediated effects occurring even later [28]. In the nonclassical pathway, androgen stimulation triggers the direct association of the proline-rich region of AR (amino acids 352–359) and the SH3 domain of SRC kinase [29] that results in the activation of a series of downstream kinases. Our studies in Sertoli cells demonstrated that T-mediated activation of Src stimulated the EGF receptor (EGFR) and subsequently the MAP kinase cascade (RAF, MEK, ERK) and the CREB transcription factor [30]. T-mediated nonclassical signaling is rapid, such that phosphorylation of SRC, ERK, and CREB is elevated within 1 min. Moreover, the pathway has prolonged effects through increased protein phosphorylation that is sustained for at least 12 h as well as via long-term gene expression changes that are mediated by increased kinase activity [31].
In the current studies, we perform the first investigation of whether nonclassical T signaling occurs in vivo. We test whether Sertoli cell-specific gene expression mediated by T can be activated in testis explants using selective activators of classical or nonclassical signaling. We also determine whether activation of either the classical or nonclassical T pathway in Sertoli cells alters gene expression in germ cells. Finally, we deliver selective inhibitors of the classical or nonclassical pathway to Sertoli cells within mouse testes in vivo to determine whether spermatogenesis can be inhibited.
MATERIALS AND METHODS
Animal Care and Use
Male Sprague Dawley rats were obtained from Charles River Laboratories (Boston, MA). Male Tfm rats that expressed a ligand binding-defective AR [32] and male C57BL/6 mice were obtained from our breeding colony maintained at the University of Pittsburgh [31]. Animals used in these studies were maintained and euthanized according to the principles and procedures described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. These studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and conducted in accordance with the specific guidelines and standards of the Society for the Study of Reproduction.
Antibodies
Rabbit antiserum against ERK phosphorylated on Thr202/Tyr204 (1:1000 dilution, catalog number 9101) and total ERK (1:10 000 dilution, catalog number 06-182) were purchased from Cell Signaling Technology (Danvers, MA) and EMD Millipore (Billerica, MA), respectively. Rabbit antisera against AR (1:1000 dilution, catalog number sc-815) and N-cadherin (1:1000 dilution, catalog number sc-7939) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Plasmid and Adenovirus Constructs
Plasmids and adenoviral constructs plus a summary of their functions are listed in Table 1. PSALuc (PSAEnh E4TATA-luc) containing a 496-bp enhancer region from the PSA gene in pGL3Basic was described previously [33]. To construct pDC315mARΔexon3, exons I and II were amplified from mouse AR cDNA using the primers 5′-GGGGCTAGCATGGAGGTGCAGTTAGGGCTGGGAA-3′ and 5′-GGGCTGCAGCGGCTCTTTTGAAGAAGACCTTG-3′. The region including exon IV through the stop codon was amplified using the primers 5′-GGGCTGCAGCTCGTAAGCTGAAGAAACTTGGAAATCTA-3′ and 5′-GGGGTCGACTCACTGTGTGTGGAAATAGATGGGCTTGA-3′. The amplicon including exons I–II was digested with NheI and PstI. The amplicon extending from exon IV to the stop codon was digested with PstI and SalI. The digested amplicons were ligated into pDC315 digested with NheI and SalI to create pCD315mARΔexon3. The plasmid pSG5HA-AR encoding wild-type human AR was described previously [34]. PCR3.1HDAC1-1KRAB-AR122 obtained from Guido Jenster (Erasmus University, Rotterdam, Netherlands) was digested with NheI and BamHI and a 3.8-kb fragment was inserted into pDC315 digested with the same enzymes to produce the pDC315-H-K-AR122 shuttle vector. To construct adenovirus vectors expressing the human S1 and S3 peptides as well as the control S1s and SS peptides, the oligonucleotide pairs listed in Table 2 were hybridized and the NheI and SalI overhangs were used for ligation into the pDC315 shuttle vector digested with NheI and SalI. Adenoviral constructs were produced from the shuttle vectors using the AdMax system (Microbix Biosystems, Inc., Toronto, ON, Canada). The Adβ-Gal, AdGFP, AdARC562G, and AdARΔ372-385 adenovirus constructs were described previously [35]. Each adenovirus was amplified in 293T cells and the virus concentrations were determined by measuring the optical density at 260 nm using a ratio of 1.1 × 1012 virus particles per 1 optical density unit [36]. The pCMV5ARtfm expressing the rat testicular feminization (tfm) AR mutant was previously named p5ARD [32] and was obtained from Elizabeth Wilson (University of North Carolina, Chapel Hill, NC).
TABLE 1.
Descriptions of plasmids, adenovirus constructs, peptides, and peptidomimetics used for this study.
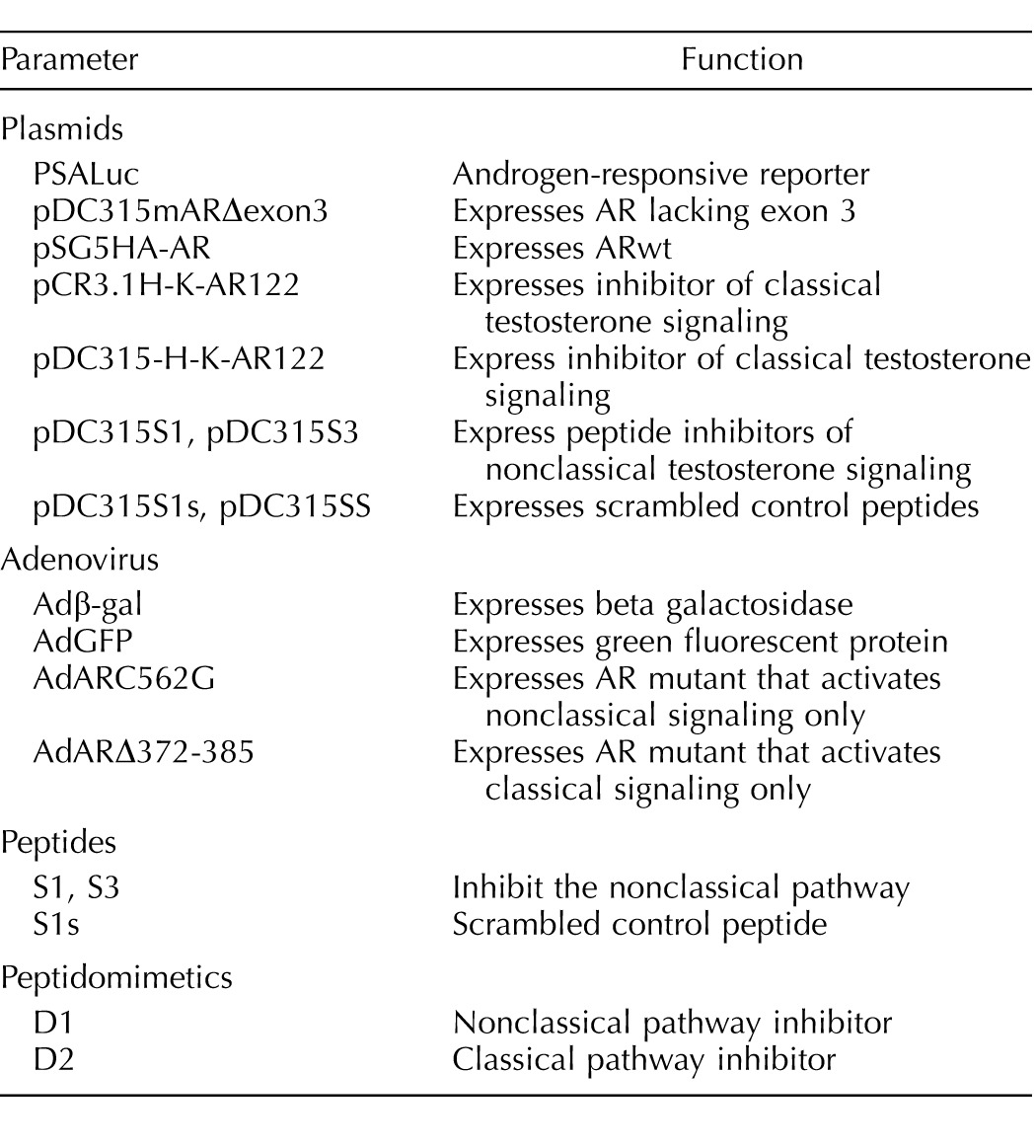
TABLE 2.
Oligonucleotide pairs used to express peptides.
Inhibition of Nonclassical Signaling Using Peptides and Peptidomimetics
The S1 (PPPHPHARIK) and S1s peptides (HPKPARIPHPK) were synthesized by the University of Pittsburgh Genomics and Proteomics Core Laboratories with an acetylated N-terminus and a fluorescein isothiocyanate tag placed at the C terminus. The synthesis of the D1 and D2 peptidomimetics was described previously [37]. Cultured cells were incubated with S1 or S1s peptides (5 μM) or D1 or D2 peptidomimetics (100 nM) for 4 h prior to stimulation with T (100 nM, 15 min) or vehicle for assays of protein phosphorylation or concurrently with T (100 mM) or vehicle for 24 h prior to assays of luciferase activity.
Transfections and Luciferase Assays
The 15P-1 Sertoli cells were transfected using Fugene (Roche, Indianapolis, IN) according to the manufacturer's instructions with PSALuc + the empty pCMV5 vector, pCD315mARΔexon3, or pSG5HA-AR. The concentration of expression vector plasmid was made equal for all transfections with the addition of pCMV5 as needed. In other studies, PSALuc was cotransfected with pCMV5, pSG5HA-AR, pSG5HA-AR + pDC315H-K-AR122, or pSG5HA-AR + pCMV5ARtfm. Twenty-four hours after transfection the cells were treated for 24 h with T or ethanol as a control and in some cases S1 and S1s peptides or D1 or D2 peptidomimetics. The cells were washed once with PBS (137 mM NaCl, 2,7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) and then lysed using Reporter Lysis Buffer (Promega, Madison, WI). Relative light units of luciferase activity were detected using Luciferase Reagent (Perkin Elmer, Waltham, MA) on Wallac Victor2 1420 Multilabel Counter (Perkin Elmer). Activity was then normalized to the protein concentration of the samples as detected using Protein Assay Reagent (BioRad, Richmond, CA).
Infection of Cultured Cells with Adenovirus and Western Immunoblotting
Adenovirus constructs (5 × 1010 particles/ml) were added to the media of cultured cells. After 3 days, the cells were stimulated with T (100 mM) or vehicle for 15 min. To prepare whole cell extracts for direct analysis by Western immunoblot, cells were washed once with PBS and then lysed on the plates using boiling Laemmli sample buffer to minimize phosphatase activity. Cell lysates were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and incubated with primary antibodies, followed by horseradish peroxidase-conjugated second antibody. The antigen-antibody complex was visualized with Millipore Immobilon Western Chemiluminescent HRP substrate (Millipore Corporation, Billerica, MA). Protein concentrations were not performed prior to SDS-PAGE, but equal loading of samples was confirmed with control antisera including those against nonphosphorylated proteins.
Cetrorelix Treatments of Rats
Cetrorelix acetate (C-5249; Sigma Chemical Company, St. Louis, MO) (0.5 mg/ml resuspended in dH2O and 5.25% glucose) or 5.25% glucose only was injected (100 μl i.p.) into adult rats (>70 days old). After 7 h, injections of T enanthate (TE; 200 mg/ml in ethanol, 50 μl via im) and T propionate (TP; 5 mg/ml in ethanol, 1 ml i.p.) or ethanol alone were performed. The rats were euthanized after the 7-h Cetrorelix treatment or 60 min after subsequent T injections. Three rats were assayed for each treatment condition. Each testis was dissected into thirds for histological and immunocytochemistry analysis for fixation in 4% paraformaldehyde, whole-cell-extract isolation, and intratesticular T determination.
Determination of Intratesticular T Concentrations
Frozen testis tissue from Cetrorelix-treated rats (100 mg) was transferred to a 14-ml round-bottom tube containing 0.1% gel PBS and a known amount of tritiated T (2000 cpm) and homogenized. Homogenized tissue was transferred to a clean borosilicate glass tube. Diethyl ether was added (5 ml) and contents were vortexed for 3 min. The tube was placed in liquid nitrogen to freeze aqueous contents and ether was decanted into a clean borosilicate glass tube. Extraction was repeated using 5 ml diethyl ether with vortexing for 3 min. The extracted material was combined, frozen in liquid nitrogen, and evaporated in a 40°C water bath. T was reconstituted in 1 ml of 0.1% gel PBS by vortexing for 3 min at room temperature. T concentrations were measured using a solid-phase radioimmunoassay kit (Total T [TKTT5], Coat-A-Count; Diagnostic Products Corporation, Los Angeles, CA) [38]. The mean sensitivity of the assay was 0.020 ng/ml. The intra-assay and interassay coefficients of variation were less than 7.8% and 8.5%, respectively.
Immunocytochemistry and Immunofluorescence
Immunostaining of testis tissue was performed on paraffin-embedded sections (5 μm) from paraformaldehyde- (4%, overnight) or Bouin-fixed adult rat testis as previously described [39]. Testis sections were deparaffinized in xylene and rehydrated. The sections were subjected to antigen retrieval in citrate buffer (10 mm citrate, pH 6.0, containing 0.1% Tween-20) at 95°C for 30 min and then at room temperature for 30 min. The sections were washed two times for 5 min in PBS and blocked for 1 h in goat, donkey, or horse serum at room temperature. The testis sections were then incubated 12–16 h with nonimmune serum or antiserum. For immunofluorescence studies, Cy3- and Alexa488-conjugated secondary antisera were added, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI), and immunostaining was detected using a Nikon Provis II fluorescence microscope or confocal microscope. A charge-coupled video camera system was used to capture images. Quantitation of immunoreactivity within a region of interest was determined using integrated optical density measurements with Bioquant Nova Prime software (v 6.90.10; Bioquant Image Analysis Corp., Nashville, TN). Five replicate images were processed for each treatment group. All files were digitally processed with Photoshop (Adobe Systems, Inc., San Jose, CA).
Isolation of Testis Explants and Culture
Agarose platforms were produced by pipetting melted 1.5% agarose in water into cell-cloning wells followed by the aspiration of some agarose to allow the formation of a deep meniscus or “volcano crater.” The cloning well was removed and agarose platforms were placed into a 12-well plate and soaked in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine for at least 2 h. Testes were isolated from rats, decapsulated, and cut into fragments 2–4 mm in diameter. In some cases the tissue fragments were incubated for 2–5 h with peptides or adenovirus constructs in 12-well plates with 300 μl media with rocking to alternate tissue exposure to media and the atmosphere. Prior to placing the tissue fragments onto the agarose platforms, fresh DMEM media + T (100 nM) + follicle-stimulating hormone (FSH; 100 ng/ml) was added to the 12-well plate containing the agarose platforms until the agarose was three-fourths submerged. In some cases T was omitted from the media. Testis fragments were then carefully placed upon the agarose using a scupula to avoid tissue damage and cultured 2–7 days at 33°C. The tissue explants were “spritzed” daily by the addition of 50 μl of media + T + FSH with or without peptides or adenovirus to the top of the explant.
RNA Isolation, RT-PCR, and Quantitative Real-Time PCR
RNA was obtained from Sertoli cells using the method of Chomczynski and Sacchi [40]. Complementary DNAs were synthesized from Sertoli cell RNA by reverse transcription as previously described [41]. For quantitative PCR (qPCR), oligonucleotide primers (Table 3) were designed using Primer Express v. 1.5 (ABI Prism; Applied Biosystems, Foster City, CA). Primer sequences were compared by a BLAST search to ensure there was no homology with other rat genes and were independently validated [42]. The qPCR amplifications were performed in the ABI Prism 7900HT Sequence Detection System v 2.3 (Applied Biosystems) as previously described [41]. Ppia (peptidylprolyl isomerase A, commonly known as cyclophilin) or Gapdh was used as an endogenous control. The means (±SEM) of three to five individual experiments were determined for each treatment group for each gene of interest.
TABLE 3.
Oligonucleotides utilized for qPCR.
Injection of Adenovirus into Seminiferous Tubules
Wild-type mice were injected via the efferent ducts with 15 μl of adenovirus (1 × 1010 particles/ml) using an Eppendorf Transferman NK2 micromanipulator and Femtojet microinjector (150–200 psi; Eppendorf North America, Hauppage, NY) [43, 44]. Using trypan blue tracking dye, it was observed that at least 70% of the visible seminiferous tubule volume was normally filled with the adenovirus samples. Three (14-day-old mice) or four (adult mice) days after injection the mice were euthanized and each testis cut in half, with one half frozen for biochemical analysis and one half fixed in 4% paraformaldehyde for morphological and immunohistochemical analysis. Testes from two 14-day-old and three adult mice were assayed.
BTB Integrity
To assay BTB integrity, three mice each that had been injected earlier with Adβ-gal, AdS1, or AdS1s were anesthetized with Avertin (Sigma Chemical Company) and the testes were surgically exposed. A small hole was placed in the tunica with a forceps through which 30 μl of a 10 mg/ml solution of EZ-Link Sulfo-NHS-LC-Biotin (#21335; Thermo Scientific, Rockford, IL) was injected into the testis interstitium. After 30 min, the mice were euthanized and testes were isolated and fixed in 4% paraformaldehyde. The biotin tracer in testis cross sections was detected using a Vectastain Elite ABC kit (Vector Labs, Burlingame, CA) such that the cross sections were incubated with ABC reagent for 30 min, washed, and then incubated with ImmPACT diaminobenzidine peroxidase substrate (Vector Labs) followed by staining with hematoxylin. The presence of the biotin tracer was detected by microscopy. BTB integrity was also assessed by immunofluorescence assays of testis sections using antisera against N-cadherin.
Quantification of Vacuole Areas
The relative total cross-section areas of seminiferous tubules and vacuoles within the tubules were obtained from images of testis tissue sections of mice treated with Adβ-gal (n = 3) or AdS1 (n = 3). Using ImageJ software [45], the basement membrane of each tubule cross section was outlined and the area within the tubule cross section was determined. The areas of vacuoles within seminiferous tubules were measured similarly. A vacuole was defined as a well-circumscribed, mostly circular space either completely devoid of spermatocytes and other germ cells or containing apoptotic cell remnants signified by loss of normal nuclei structure or opacity. Seminiferous tubule lumens were not included in vacuole measurements but were included in total cross-sectional area. The sum of vacuole area for each cross section was divided by the total cross-sectional area of each seminiferous tubule and multiplied by 100 to derive percentage vacuole area per seminiferous tubule cross section. The percentage vacuole area per seminiferous tubule cross section was averaged for at least 10 circular seminiferous tubule cross sections per experimental animal. Differences in mean percentage vacuole area were analyzed for statistical significance using a one-tailed, equal-variance t-test (P < 0.05).
Statistical Analysis
Immunoreactive signals from Western blot films were scanned with an Epson 1600 Expressions scanner using Epson Scan software and quantified with ImageJ. For Western blots, the mean ± SEM relative signal intensities were determined for at least three independent experiments. Unless otherwise indicated, results were analyzed by ANOVA with Newman-Keuls protected least significant difference at a 5% significance level utilizing GraphPad Prism 4.3 software (GraphPad Software, San Diego, CA).
RESULTS
Deletion of AR Exon 3 Blocks Classical and Nonclassical Signaling
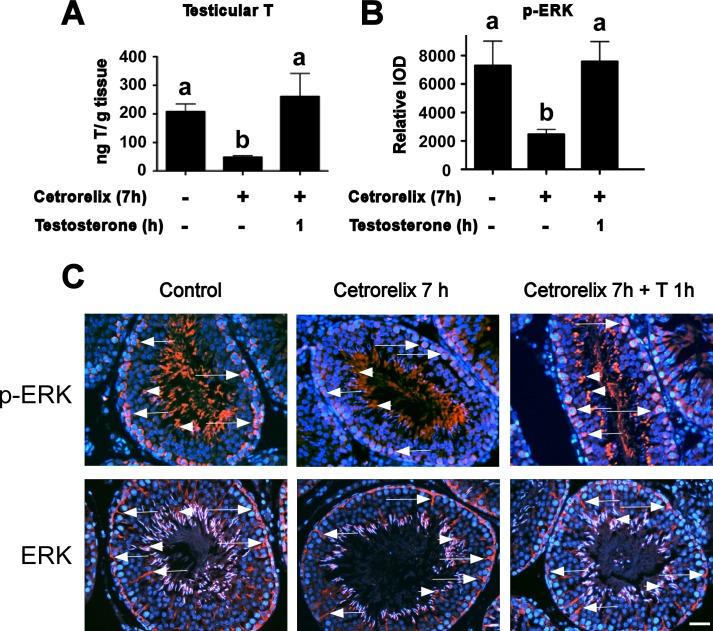
Although both classical and nonclassical T signaling have been described in cultured Sertoli cells (Fig. 1A), the contributions that each pathway makes to maintain spermatogenesis in vivo have not been investigated. It has been proposed that only the classical pathway is required for spermatogenesis because germ cell development was interrupted during meiosis in transgenic mice in which 39 amino acids encoding exon 3 of AR containing a portion of the DNA binding domain was removed [46]. However, the effects of this AR mutation on nonclassical T signaling were not assessed. We recreated the exon 3-deleted mouse AR cDNA and constructed a plasmid (pDC315mARΔexon3) that expressed this mutant protein. The 15P-1 Sertoli cell line was transfected with pDC315mARΔexon3 and the PSALuc plasmid in which luciferase expression is driven by the T-responsive prostate-specific antigen (PSA) promoter. Cotransfection with an empty expression vector did not alter luciferase activity after stimulation with T (100 nM). These data combined with Western blot analysis of AR levels indicate that 15P-1 cells have low AR activity (Fig. 1B). T-mediated transcription was induced 12-fold by a plasmid expressing wild-type AR (ARwt). In contrast, T stimulation after cotransfection of a plasmid expressing ARΔexon3 did not increase luciferase activity, confirming that the ability of the ARΔexon3 mutant to activate transcription in response to T is dramatically decreased [46, 47].
FIG. 1.
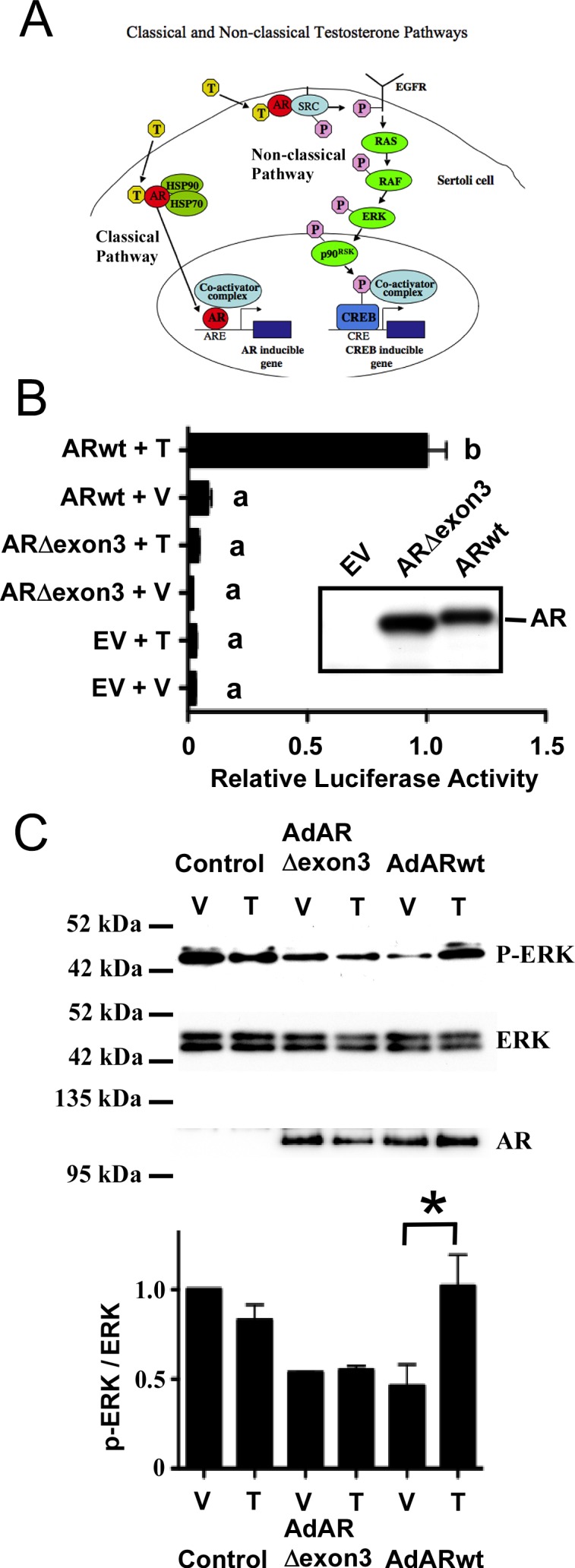
Deletion of exon 3 inhibits phosphorylation of ERK by the nonclassical T signaling pathway. A) The classical and nonclassical T signaling pathways. In the classical pathway (left) T diffuses through the plasma membrane and binds AR. A conformational change in AR allows the receptor to be released from heat shock proteins (HSP). AR then translocates to the nucleus, where it binds to AREs, recruits coactivator proteins, and regulates gene expression. In the nonclassical pathway (right), T stimulation transiently increases AR localization to the plasma membrane and results in AR interacting with and activating SRC tyrosine kinase. Activation of SRC can alter many physiological processes, including the phosphorylation and activation of the EGF receptor that in turn activates the MAP kinase cascade (RAF, MEK, and ERK). Further signaling through p90RSK kinase causes phosphorylation of the CREB transcription factor, resulting in increased transcription of CREB-regulated genes. B) The 15P-1 Sertoli cells were transfected with the PSALuc reporter plasmid and an empty expression vector (EV) or plasmids expressing ARΔexon3 or ARwt. The cells were stimulated with vehicle (V) or 100 nM T (T) for 24 h. The relative luciferase activity is reported relative to PSALuc + ARwt after 24 h stimulation with T (=1.0). Error bars show SEM for three experiments. Values with different lowercase letters differ significantly (P < 0.05). Inset: Western blot of whole cell extracts from 15P-1 cells transfected with empty vector (EV) or plasmids expressing ARΔexon3 or ARwt probed with antisera against AR. C) 15P-1 Sertoli cells were infected with adenovirus constructs expressing AdGFP (Control), exon 3-deleted AR (AdARΔexon3), or ARwt. The p-ERK, ERK, and AR levels were determined by Western blot after stimulation (10 min) with vehicle (V) or 100 nM T (T). Quantitation of the Western blot experiments is shown in the graph below. Relative p-ERK and ERK signal intensities were determined and the p-ERK/ERK ratio was determined for each sample. The p-ERK/ERK values obtained were normalized to the vehicle treated control condition (=1). The mean p-ERK/ERK values and SEM are shown for three experiments. A paired one-tailed t-test showed statistically significant differences (P < 0.05) between the control and T-treated samples only after transduction of the virus expressing ARwt (asterisk).
The 15P-1 cells were infected with adenovirus constructs expressing either ARwt (AdARwt) or ARΔexon3 (AdARΔexon3). The two AR isoforms were expressed at similar levels. Although basal phosphorylated ERK (p-ERK) levels were elevated after transduction of a control virus for reasons that are not yet understood, stimulation with T increased ERK phosphorylation (a marker of nonclassical pathway activation) in the presence of ARwt but not ARΔexon3 or control virus (Fig. 1C). Collectively, these results indicate that the failure of the AR lacking exon 3 to support spermatogenesis could be due to the lack of either classical or nonclassical signaling activity.
Nonclassical T Signaling Increases ERK Phosphorylation in Sertoli Cells In Vivo
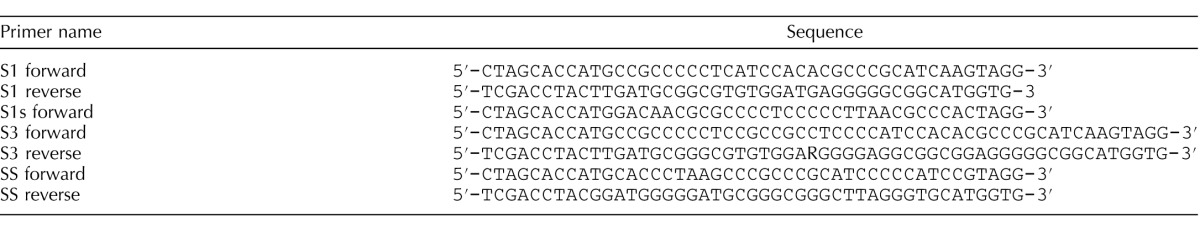
To assay nonclassical signaling in vivo, we used a rat model in which T levels could be rapidly manipulated. Adult rats were injected i.p. with the GnRH antagonist Cetrorelix (50 μg/100 μl) to transiently decrease gonadotropin (luteinizing hormone [LH] and FSH) secretion and T production. After 7 h, testicular T levels fell to 23% of control levels, in agreement with previous studies (Fig. 2A) [48–50]. Subsequent injections of TP (5 mg i.p.) plus TE (10 mg i.m.) caused intratesticular T levels to increase to 125% of control levels after 1 h.
FIG. 2.
T stimulates rapid ERK phosphorylation in Sertoli cells in vivo. A) T levels in control testes and after 7 h of Cetrorelix treatment or 7 h Cetrorelix followed by 1 h T are shown. B) The p-ERK immunostaining was quantified as relative integrated optical density (IOD) in stage VII tubules after treatment with vehicle (Control), 7 h after Cetrorelix treatment, and Cetrorelix (7 h) + T for 1 h. For A and B, error bars show SEM for three experiments. ANOVA statistical analysis with Newman-Keuls PLSD was used. Values with different lowercase letters differ significantly (P < 0.05). C) Representative immunofluorescence microscopy images of p-ERK or ERK (red) staining in stage VII tubules in control and after Cetrorelix (7 h) or Cetrorelix + TP and TE (1 h) treatments. Arrows indicate p-ERK staining in the BTB region and arrowheads denote p-ERK staining associated with elongated spermatids. Additional staining of ERK is localized to the Sertoli cell cytoplasm. Nuclei are stained blue with DAPI. Bar = 100 μm.
In vivo, AR expression in Sertoli cells is low and difficult to detect except during stages VII–VIII of the spermatogenic cycle [19, 24], and spermatogenesis is most sensitive to decreased T levels during these stages [1, 8, 10, 14, 25]. Therefore, we focused on assaying the phosphorylation of ERK kinase, a key component in the nonclassical T signaling pathway in cross sections of stage VII seminiferous tubules. In the testes of rats treated with vehicle control, p-ERK immunostaining was present in the Sertoli cell cytoplasm associated with the BTB region and Sertoli cell-elongated spermatid adhesion sites. The p-ERK immunostaining decreased to 34% of control levels 7 h after Cetrorelix injection but was restored to 103% of control levels 1 h after injection of TP and TE (Fig. 2, B and C). Immunostaining for total ERK did not change significantly after the changes in T levels. These data indicate that nonclassical signaling occurs in vivo because ERK phosphorylation is rapidly elevated at cell adhesion sites in Sertoli cells in response to increased T.
H-K-AR122 Is a Dominant Negative Inhibitor of Classical T Signaling
To identify the effects of blocking the classical pathway, we employed a previously characterized dominant negative AR mutant, HDAC1-KRAB-AR122 (renamed H-K-AR122) [51]. The H-K-AR122 construct has histone deacetylase 1 and Kruppel-associated box transcriptional repressor domains fused to an AR deletion mutant lacking the first half of its transactivating N-terminal domain [51]. Transient transfection studies of 15P-1 Sertoli cells using the PSALuc reporter plasmid again showed that AR-driven promoter activity was low in the presence of vehicle or T (Fig. 3A). However, cotransfection of a plasmid expressing ARwt increased T-mediated PSA promoter activity over 4-fold. In a control cotransfection, a plasmid encoding a mutant AR (Arg734Gln) that cannot bind T and was originally identified in rats having a tfm phenotype (ARtfm) [32] did not interfere with ARwt induction of T-mediated transcription. However, cotransfection with a plasmid expressing H-K-AR122 dramatically decreased T-mediated activation of transcription by ARwt. These results were in agreement with previous studies that showed transcription mediated by a constitutively active AR was inhibited 10-fold by H-K-AR122 [51]. To determine whether H-K-AR122 affected nonclassical T signaling, primary Sertoli cell cultures from rats were infected with an adenovirus construct expressing H-K-AR122 (AdH-K-AR122). Expression of H-K-AR122 did not inhibit T-mediated increases in ERK phosphorylation (Fig. 3B). Together, these data indicate that H-K-AR122 is a selective inhibitor of the classical pathway that does not affect nonclassical signaling.
FIG. 3.
H-K-AR122 and S1 are pathway-selective inhibitors of classical or nonclassical T signaling, respectively. A) 15P-1 Sertoli cells transfected with the PSALuc reporter plasmid and plasmids expressing ARwt, ARwt + ARtfm, or ARwt + H-K-AR122 (AR122) were stimulated with vehicle (V) or T for 24 h. The relative luciferase levels were determined relative to empty vector + vehicle (EV + V, =1.0) Error bars show SEM for four experiments. B) Primary rat Sertoli cell cultures were infected with adenovirus constructs expressing β-galactosidase (β-gal), H-K-AR122, or the S1 or control S1s peptide. The cells were stimulated for 15 min with 100 nM T (T) 24 h after adenovirus infection. Western analyses of whole cell extracts are shown for p-ERK and total ERK (T-ERK). Quantitation of p-ERK/ERK values normalized to Ad H-K-AR122 + T (left) or AdS1s+ T (right) (=1) for three experiments is shown below the Western blots. C) Primary Sertoli cell cultures were incubated for 2 h with S1 or S1s peptides (5 μM) or D1 or D2 peptidomimetics (100 nM) prior to a 15-min stimulation with T (100 nM). Western immunoblots for p-ERK and ERK are shown. Quantitations of the p-ERK/ERK values from four experiments were normalized to S1s + T (=1). For B and C, paired one-tailed t-tests revealed statistically significant differences (P < 0.05) where indicated with an asterisk (*). D) The 15P-1 Sertoli cells transfected with the PSALuc reporter plasmid and a plasmid expressing ARwt incubated with S1 or S1s (5 μM) or D1 or D2 (100 nM) during a 24-h stimulation with vehicle (V) or 100 nM T (n = 3). Values with different lowercase letters were found to differ significantly by ANOVA (P < 0.05).
The S1 Peptide and D1 Peptidomimetic Are Selective Inhibitors of the Nonclassical Pathway
The S1 peptide (aa 377–386 in hAR) corresponding to the proline-rich domain in AR blocks the nonclassical pathway by inhibiting AR-mediated activation of SRC kinase [52, 53]. Infection of cultured primary Sertoli cells with an adenovirus expressing S1 (AdS1) blocked T-mediated phosphorylation of ERK but the AdS1s adenovirus expressing a scrambled version of the S1 peptide (S1s) had no effect on ERK phosphorylation (Fig. 3B).
Additional studies were performed using peptidomimetics, which are small organic molecules that do not possess a peptide backbone but have the capacity to interact with the binding pockets of target proteins because of the arrangement of functional groups into a specific three-dimensional pattern [37]. The D1 peptidomimetic was designed to mimic the FXXLF motif in the amino terminal region of AR. In contrast, the control D2 peptidomimetic mimics the LXXLL motif and disrupts the interaction of AR with NR box-containing proteins [37]. T-mediated phosphorylation of ERK in primary cultures of Sertoli cells was inhibited by a 4-h pretreatment with 100 nM D1 relative to D2. The D2 peptidomimetic did not alter the phosphorylation of ERK after stimulation with T as was shown previously (Fig. 3C) [37]. The relative inhibition of T-mediated ERK phosphorylation by D1 was similar to that resulting from preincubation of Sertoli cells with S1 peptide (1 μM).
To determine whether the peptides or peptidomimetics altered classical T signaling, 15P-1 Sertoli cells were cotransfected with the PSALuc reporter plasmid and the AR expression vector. Prior to stimulation with vehicle or T (100 mM) for 24 h, S1 or S1s peptides (5 μM) or D1 or D2 peptidomimetics (100 nM) were added to the cultures. None of the peptides or peptidomimetics altered the T-mediated increase in transcription from the PSA promoter (Fig. 3D). The lack of effect upon T-mediated transcription by the peptidomimetics is in agreement with a previous study that showed D1 has no effect on androgen-mediated transcription and that expression of AR rescues D2-mediated suppression of androgen-induced transactivation [37]. These studies indicate that the S1 peptide and the D1 peptidomimetic are selective inhibitors of the nonclassical pathway.
Pathway-Selective Activators and Inhibitors Alter T-Mediated Gene Expression in Testis Explants
As a first test to determine whether classical and nonclassical T signaling is required for spermatogenesis, we employed a testis explant model. In agreement with previous studies [54, 55], we found that the morphology of the explants cultured on agarose platforms can be maintained for at least 7 days (Fig. 4). Based on this result, we performed all additional studies within 6 days of initiating the explant cultures.
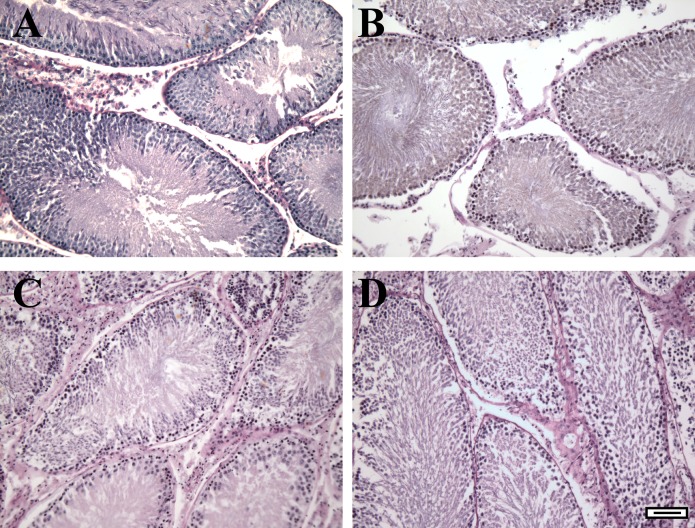
FIG. 4.
Testis explants are maintained in culture for at least 7 days. Testis explants (3 mm2) were fixed immediately after isolation (A) or cultured on agar slabs in serum-free media lacking T for 2, 4, or 7 days (B–D) followed by fixation and embedding in paraffin. Explant cross sections were stained with periodic acid-Schiff-hematoxylin. Bar = 100 μm.
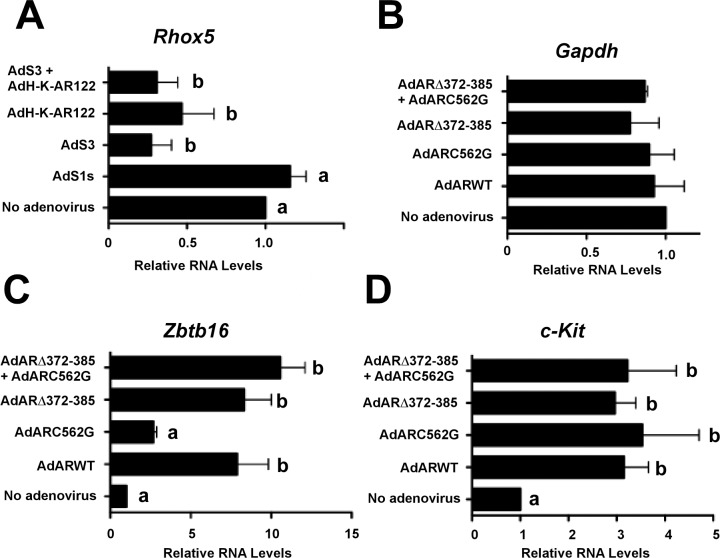
Studies of pathway-specific regulation of T-mediated transcription were performed by transducing testis explants on the first day of culture with the adenovirus constructs AdS1s, AdS3 (expresses the S3 peptide that is functionally identical to S1 but contains an additional four prolines at the amino terminus [52, 53]), AdH-K-AR122, or AdS3 + AdH-K-AR122. RNA was extracted from the explants 6 days after adenovirus transduction and RT-qPCR assays were performed to determine the expression levels of Rhox5, a homeobox gene expressed only in Sertoli cells that is highly inducible by T via the classical signaling pathway [56–58]. Previously, studies of factors regulating Rhox5 were challenging because Rhox5 expression is rapidly extinguished after the culture of Sertoli cells alone [57]. However, Rhox5 mRNA expression was detected in the testis explants, indicating that they can be used to assay the expression of Sertoli cell-specific genes regulated by T (Fig. 5A). Transduction with the AdS3 adenovirus decreased Rhox5 expression by 73%. The AdH-K-AR122 adenovirus lowered Rhox5 mRNA levels by 53%, whereas the combination of adenovirus constructs expressing both pathway inhibitors (AdH-K-AR122 + AdS3) decreased Rhox5 expression by 69%. Efficient uptake of adenovirus by Sertoli cells has been shown by our group and others [59–62]; however, we cannot rule out the possibility that the adenoviruses could also infect peritubular cells and inhibit AR-mediated functions that are required to support Rhox5 expression in Sertoli cells [63]. Nevertheless, these data indicate that Rhox5 mRNA levels, previously shown to be regulated by the classical pathway [64], are also regulated via the nonclassical signaling pathway.
FIG. 5.
Inhibitors of classical and nonclassical T signaling disrupt spermatogenesis in testis explants. A) Testis explants from 35-day-old wild-type rats were cultured with no adenovirus or adenovirus constructs expressing S1s, S3, H-K-AR122, or S3 + H-K-AR122. After 6 days, RNA was extracted from the explants and reverse transcription (RT) assays performed followed by qPCR quantitation of relative Rhox5 RNA levels. Data shown are representative of assays from three isolations of explants. B–D) Testis explants from 35-day-old tfm rats were infected with no adenovirus or adenovirus constructs expressing ARwt (WT), nonclassical pathway activator (ARC562G), classical pathway activator (ARΔ372-385), or both activators (ARC562G + ARΔ372-385). The relative RNA levels and SEM for Gapdh (B, n = 3), Zbtb16 (C, n = 3), and c-Kit (D, n = 5) were assayed using RNA extracted from the explants 6 days after adenovirus infection and made relative to no adenovirus (=1). ANOVA statistical analysis with Newman-Keuls PLSD was used. Values with different lowercase letters differ significantly (P < 0.05).
Activation of Either the Classical or Nonclassical Pathway in Sertoli Cells Can Alter Gene Expression in Germ Cells
Rhox5 regulates the expression of genes that modulate Sertoli cell metabolism and the production of factors needed by germ cells [65, 66]. The findings that inhibitors of T signaling can regulate Rhox5 gene expression in explants raised the possibility that pathway-specific activators could be used in explants to alter Sertoli cell functions that support germ cell development. To test this hypothesis, testis explants were cultured from AR-defective tfm rats, in which spermatogenesis is halted during meiosis. The explants were transduced with our previously characterized AdARΔ372-385 or AdARC562G adenoviruses expressing AR mutants that selectively activate either the classical or nonclassical pathway, respectively [35].
The qPCR analysis performed 6 days after adenovirus transduction showed that expression of the housekeeping gene Gapdh in tfm explants was not altered in response to adenovirus constructs expressing activators of the classical or nonclassical pathways or ARwt (Fig. 5B). In contrast, transduction of the tfm testis explants with an adenovirus expressing ARwt resulted in a 7.8-fold increase in expression of the Zbtb16 gene (also known as Plzf) encoding a transcription factor that is expressed exclusively in spermatogonial stem cells (SSCs) and undifferentiated spermatogonia [67, 68] (Fig. 5C). The adenovirus activating the nonclassical pathway increased Zbtb16 expression by 2.7-fold, but this difference from the no-adenovirus control was found not to be statistically significant. In contrast, activation of only the classical pathway increased mean levels of Zbtb16 mRNA in spermatogonia by 8.3-fold. Activation of both the classical and nonclassical pathways also increased Zbtb16 expression (10.6-fold). Analysis of c-Kit mRNA, which is a marker for differentiated spermatogonia [69–72], showed that c-Kit expression was stimulated to a similar extent after expression of ARwt, the classical pathway or the nonclassical pathway activator alone, or the combination of both activators (3.1-, 3.0-, 3.5-, and 3.2-fold, respectively) (Fig. 5D). These data indicate that of the spermatogonia-specific mRNAs, expression of Zbtb16 is more effectively induced after activation of the classical versus the nonclassical T signaling pathway in Sertoli cells. In contrast, c-Kit expression is elevated after activating either the classical or the nonclassical pathway.
Inhibition of the Classical or Nonclassical Pathway Disrupts Spermatogenesis In Vivo
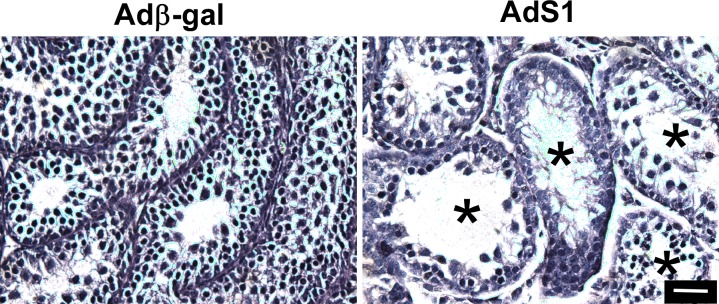
Our previous studies and those from other groups have shown that adenovirus constructs injected via the efferent ducts into the lumen of seminiferous tubules of adult mouse and rat testes are taken up only by Sertoli cells [59–62]. This cell-specific uptake occurs because only Sertoli cells have direct access to the nonreplicating adenovirus in the tubule lumen and Sertoli cells express receptors used by adenoviruses to enter the cell [44, 59–61, 73]. As a first test of the hypothesis that nonclassical signaling is required to maintain spermatogenesis, we delivered adenovirus vectors into the seminiferous tubules of 14-day-old mice. During this developmental period, pachytene spermatocytes first appear during the initial wave of spermatogenesis [74]. We found that 3 days after injection of AdS1, spermatogenesis was disrupted, with spermatocytes being lost in at least 50% of the tubule cross sections (Fig. 6A). This result was consistent with approximately 50% of tubule volume being filled with adenovirus + dye marker solution after injection (data not shown). No disruption of spermatogenesis was observed with the control Adβ-gal adenovirus and no immune response was detected after infection with either adenovirus. These results indicate that spermatogenesis is blocked during the meiosis stage of germ cell development after inhibition of the nonclassical pathway in vivo.
FIG. 6.
Inhibition of nonclassical T signaling blocks meiosis during the first wave of spermatogenesis. Hematoxylin staining of 18-day-old mouse testis 3 days after injection of control Adβ-gal or nonclassical pathway inhibitor AdS1. Asterisks (*) indicate tubules with disrupted spermatogenesis. The data shown are representative of two experiments. Bar = 100 μm.
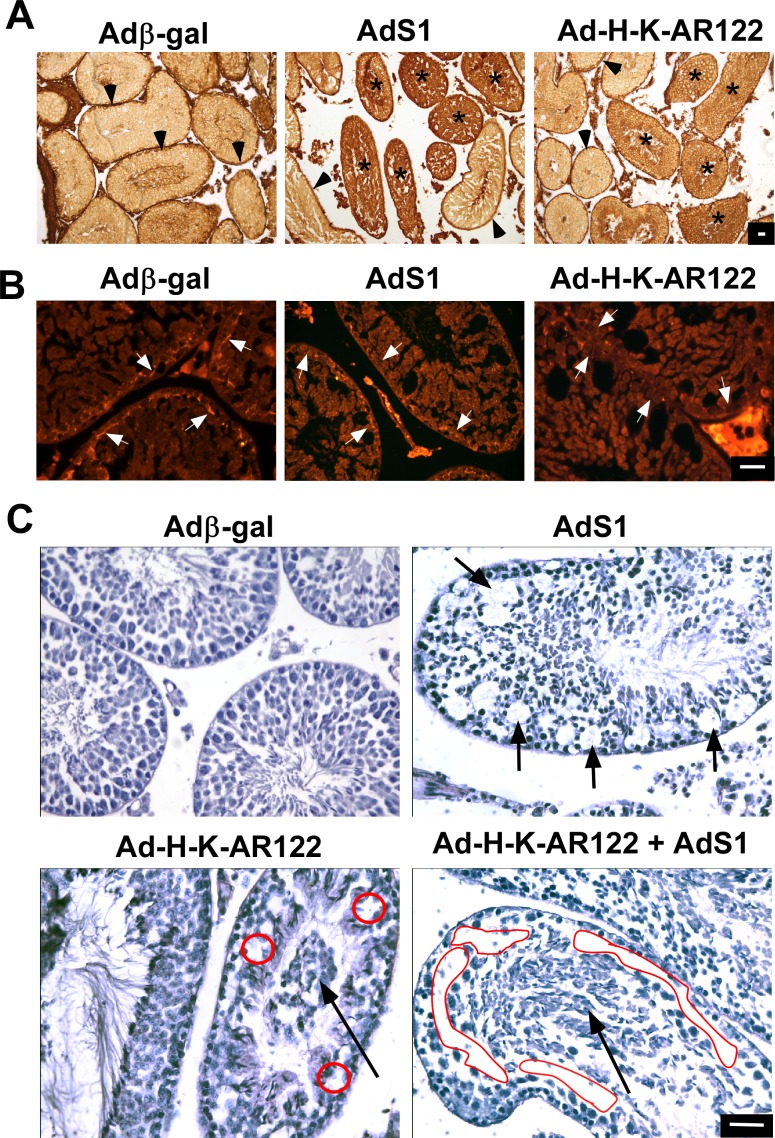
To assess the integrity of the BTB after inhibiting the nonclassical or classical pathways, additional adenovirus injection studies were performed using adult mice. Six days after adenovirus injection, a biotin tracer (EZ-Link Sulfo-NHS-LC-Biotin) that cannot transit through an intact BTB was injected into the interstitium of adenovirus-transduced testes 30 min before euthanasia as previously described [10]. These studies revealed that in testes injected with the control Adβ-gal adenovirus, the BTB was intact, as the biotin was excluded from all but the basement membrane portions of seminiferous tubules (Fig. 7A). In contrast, the biotin tracer was found throughout the interior regions of numerous tubules in testes injected with adenovirus constructs expressing the S1 nonclassical inhibitor or the H-K-AR122 classical pathway inhibitor. A major component of the BTB whose expression in Sertoli cells is supported by T signaling is the adherens protein N-cadherin [11]. Immunofluorescence studies revealed that N-cadherin expression at the BTB was decreased after injection of inhibitors of either the nonclassical or classical pathway (Fig. 7B). These findings indicate that the integrity of the BTB is disrupted after blocking either nonclassical or classical T signaling.
FIG. 7.
Inhibition of classical or nonclassical T signaling disrupts spermatogenesis in adult mice. A) Adult mouse testes were injected with adenovirus constructs expressing β-galactosidase (Adβ-gal, control), the S1 nonclassical pathway inhibitor (AdS1) or the classical pathway inhibitor (AdH-K-AR122). Prior to euthanasia, a biotin tracer was injected into the testes. Colorimetric staining for biotin (brown) showed that the biotin tracer lined the perimeter of testes injected with Adβ-gal (arrowheads) but biotin penetrated the BTB in testes injected with AdS1 or AdH-K-AR122 (asterisks). B) Immunofluorescence analysis of testes sections from A using antisera against N-cadherin shows that N-cadherin is present at the BTB after injection of control adenovirus (Adβ-gal) but expression at the BTB is reduced or absent after injection of AdS1 or AdH-K-AR122. Arrows indicate N-cadherin immunoreactivity that is present or reduced at the BTB. C) Hematoxylin staining of adult mouse testis 4 days after injection of Adβ-gal, AdS1, Ad-H-K-AR122, or AdS1 + Ad-H-K-AR122. Short arrows depict vacuoles lacking germ cells. Long arrows depict prematurely detached germ cells in the lumen. Red circles and ovals indicate regions lacking germ cells. For A, B, and C, the data shown are representative of three experiments. Bars = 100 μm.
Injection of Adβ-gal into the seminiferous tubules of adult mouse testes (70 days old) did not affect spermatogenesis or the morphology of the testis 4 days later (Fig. 7C). In contrast, injection of AdS1 increased the area of vacuoles within the seminiferous tubules where pachytene spermatocytes and round spermatids were absent. Specifically, the mean relative area of vacuoles within seminiferous tubules after injection of AdS1 (9.3% ± 0.5%) was 4.4-fold greater than after injection of Adβ-gal (2.2% ± 0.6%) (P < 0.05). Injection of AdH-K-AR122 caused the mislocalization of immature germ cells away from the basement membrane and toward the tubule lumen, suggesting that the germ cells had detached from Sertoli cells. Combined expression of the classical and nonclassical pathway inhibitors also resulted in the appearance of vacuoles, the loss of pachytene spermatocytes, plus a more generalized detachment of remaining spermatocytes and postmeiotic germ cells away from the basement membrane and into the tubule lumen. These results indicate that both T signaling pathways are required to maintain spermatogenesis.
DISCUSSION
Nonclassical actions of steroid hormones, including the activation of kinases and signaling cascades, have been studied in numerous cell culture systems, but the characterization of nonclassical actions in vivo is limited [75, 76] and nonclassical actions of androgen in vivo have not been reported previously. A recent study argued that nonclassical signaling did not contribute to maintaining spermatogenesis [46]. This study employed a naturally occurring AR mutation lacking exon 3 that binds T but causes complete androgen insensitivity (AIS) [47, 77]. Because exon 3 is required for DNA-binding activity and the ability to induce T-mediated transcription, the finding that spermatogenesis was blocked in transgenic mice expressing only this AR mutant in Sertoli cells was interpreted as evidence that only the classical pathway was required to maintain fertility [46]. However, our finding that the removal of the 39 amino acids encoded by exon 3 also extinguished nonclassical activity indicates that the associated AIS phenotype and disruption of spermatogenesis may result from the lack of classical, nonclassical, or both pathways. Further studies will be required to determine how the loss of exon 3 inhibits nonclassical signaling, but it is possible that an alteration in the conformation of AR occurs that does not allow AR to interact with Src kinase.
We found evidence for nonclassical signaling occurring in Sertoli cells in vivo by injecting rats with the GnRH antagonist Cetrorelix followed by injections of T to rapidly decrease and then increase intratesticular T levels. The corresponding loss and reestablishment of p-ERK immunoreactivity in Sertoli cells indicates that the nonclassical T signaling pathway is active in these cells. The rapid T-mediated increase in p-ERK levels in Sertoli cells is unlikely to result from classical T signaling, because accumulation of T in the testis after T restoration would not be instantaneous and initiating T-mediated gene expression requires at least 45 min, with protein production occurring even later [28]. Instead, the increase in P-ERK levels in Sertoli cells is characteristic of the nonclassical T signaling that is observed within minutes of T stimulation [31]. It is possible that estradiol could contribute to activation of the nonclassical pathway in Sertoli cells, because estradiol can be derived from T by aromatase enzymes that are present in testicular cells (reviewed in [78]) and estradiol has been reported to activate the MAP kinase pathway in Sertoli cells [79]. However, we showed previously that T and the nonaromatizable R1881 androgen activate the nonclassical pathway and that activation requires the AR [31]. Furthermore, T levels in the testis are far greater than that of estradiol, causing T to be the major stimulus for the nonclassical pathway in vivo. Based on these data, we interpret our results as the first evidence that nonclassical T signaling occurs in Sertoli cells in vivo.
The culturing of testis explants containing intact seminiferous tubules and accompanying interstitial cells allowed investigation of classical and nonclassical T signaling in a model testis environment that supports spermatogenesis. The organ culture method used permits the explants access to media as well as an oxygenated atmosphere. The long-term culture of explants using this strategy has been shown to permit the eventual production of sperm from neonatal testes [54]. Our ability to maintain the integrity of the cultured testis tissue for at least 7 days allowed for the use of adenovirus expression vectors in a testis-like environment and the time required for gene expression and intracellular signaling events in Sertoli cells to be translated into alterations in spermatogenesis processes.
Testis explants provide a unique opportunity to study expression of the Sertoli cell-specific Rhox5 mRNA that is dramatically up-regulated in the presence of T in vivo [56–58] but extinguished rapidly in cultures of isolated primary Sertoli cells under normal conditions [57]. The finding that the classical pathway inhibitor H-K-AR122 decreased Rhox5 mRNA levels was expected because the Rhox5 promoter has been shown to contain four AREs [80–82]. However, the inhibition of Rhox5 expression by a nonclassical inhibitor was unexpected and is the first example of T-mediated gene expression being regulated by both the classical and nonclassical pathways. It is possible that nonclassical signaling may activate transcription from a previously characterized second, distal Rhox5 promoter or alleviate repression associated with a promoter regulatory region that is adjacent to the ARE-containing region [64]. Nonclassical signaling also may contribute to the induction of Rhox5 via the activation of kinases that alter the activity of factors regulating the AR-mediated transcription of Rhox5 [83]. Additional studies will be required to determine the mechanism by which Rhox5 mRNA levels are regulated by the nonclassical pathway.
The functional importance of the classical and nonclassical pathways in Sertoli cells was shown further in gene expression assays using testis explants from rats lacking AR activity. Introduction of an adenovirus expressing an AR mutant that can only activate classical T signaling increased expression of the gene encoding ZBTB16, a transcription factor expressed only in the least mature germ cells, including SSCs and undifferentiated spermatogonia derived from SSCs [67, 68]. Because T and AR do not directly regulate developing germ cells [18–21], alterations in gene expression in germ cells due to the activation of the classical and nonclassical pathways must be indirect. It is possible that activation of the classical pathway allows Sertoli cells to provide a better niche for SSCs and undifferentiated spermatogonia or the production of a factor that induces expression of the Zbtb16 gene. Activation of the nonclassical pathway did not produce statistically significant differences in Zbtb16 expression. Thus, the nonclassical pathway may be less effective in supporting SSCs and SSC gene expression. The idea that activation of either T signaling pathway in Sertoli cells can alter gene expression in germ cells was supported by similar classical and nonclassical pathway-mediated increases in the expression of c-Kit, a gene expressed predominantly in differentiated spermatogonia. This T-mediated alteration of germ cell gene expression agrees with a previous finding that AR-regulated genes in Sertoli cells regulate RNA transcripts expressed in differentiated spermatogonia and are involved in the regulation of spermatogonial differentiation [84].
The classical and nonclassical activators produced by adenoviruses are not likely to act directly on germ cells because germ cells in vivo are not efficiently transduced with adenovirus [59–62]. Furthermore, gene-targeting studies in mice show that ablating any potential AR expression in germ cells does not result in functional defects [85, 86]. Therefore, the use of testis explants combined with adenovirus-mediated expression of regulatory factors may provide a strategy to identify testicular factors and mechanisms that regulate stem cell renewal and spermatogonia differentiation as well as later germ cell developmental processes.
Androgens and AR are required to maintain the integrity of the BTB [10, 11], and we found that inhibition of either T signaling pathway disrupts BTB integrity. The finding that the nonclassical pathway is required to maintain the BTB is consistent with earlier findings that SRC family kinases, a target of nonclassical T signaling, are associated with and required for maintaining the BTB [87] and that T is required for the transit of BTB proteins out of the membrane during the remodeling of the BTB [88]. In addition, ERK kinase activity is associated with maintenance of the BTB [89, 90]. The finding that classical T signaling is required to maintain the BTB is supported by previous studies showing that knockout of AR activity in Sertoli cells decreases the expression of genes encoding BTB-associated proteins including N-cadherin, claudin 11, occludin, gelsolin, JAM3, and claudin 3 [9–11]. However, it is not yet known whether expression of identified genes encoding BTB-associated proteins is enhanced by classical or nonclassical signaling or both. Additional studies also are required to determine whether inhibition of nonclassical signaling causes the mislocalization of BTB-associated proteins away from the plasma membrane.
In rodents, germ cells enter prophase of meiosis coincident with the formation of the BTB [91, 92] and disruption of the BTB inhibits the differentiation of spermatogonia into spermatocytes [93]. These required properties of the BTB may explain the lack of spermatocyte formation after injection of an adenovirus expressing an inhibitor of the nonclassical pathway into the testes of 14-day-old mice and the similar loss of spermatocytes in adult testes. However, other consequences of blocking nonclassical T signaling, including inhibiting the production of factors required by the germ cells or the misregulation of cell-cell attachment proteins, may also contribute to the loss of spermatocytes as well as affect other stages of the spermatogenesis process.
An adenovirus construct expressing the classical pathway inhibitor H-K-AR122 also disrupted spermatogenesis such that germ cells were prematurely released into the lumen of seminiferous tubules. One explanation for this result may be that expression of cell attachment proteins is inhibited because T stimulation has been shown to increase the expression of genes required for cell-cell attachment and some of these genes are regulated via the classical pathway [9–11]. The observed disruption of the BTB after inhibition of classical pathway signaling also may contribute to the loss of germ cells as the ablation of proteins required to maintain the BTB blocks spermatogenesis during meiosis and causes the loss of meiotic cells [62, 94, 95]. The more generalized disruption of spermatogenesis observed after inhibition of both the classical and nonclassical pathways in vivo was similar to the progressive loss of spermatocytes and absence of spermatids that is observed in Sertoli cell-specific AR knockout (SCARKO) mice [7, 8]. The increased disruption of spermatogenesis in the presence of inhibitors of both pathways as well as the differences in the patterns of disrupted spermatogenesis observed with either inhibitor alone indicates that the classical and nonclassical pathways support at least some distinct, complementary processes required to maintain spermatogenesis.
In the future, specific proteins and signaling pathways regulated by classical and nonclassical T signaling may be identified with the combined use of testis explants, adenovirus injection into testes, and transgenic mice in which endogenous AR is replaced by pathway-selective AR mutants. The finding that the S1 peptide or the D1 peptidomimetic blocks nonclassical signaling in Sertoli cells raises the possibility that delivery of these inhibitors specifically to the testis could act as a contraceptive to block sperm production in a reversible manner without interfering with processes that require classical T signaling in the testis or other tissues. The D1 peptidomimetic would have particular advantages as a male contraceptive because it is a lipophilic small organic compound that is not subject to protease degradation. It is also possible that pathway-selective activators or inhibitors could be delivered to other tissues, resulting in the activation or inhibition of specific T-mediated processes without altering functions regulated by the other pathway.
In summary, we have found that nonclassical T signaling occurs in Sertoli cells in vivo. Inhibitors of classical and nonclassical signaling are able to regulate Sertoli cell-specific gene expression in testis explants. Expression of classical or nonclassical pathway-activating AR mutants in Sertoli cells alters the mRNA levels for genes expressed in spermatogonia germ cells in testis explants. The production of either classical or nonclassical pathway inhibitors in Sertoli cells in vivo results in the disruption of the BTB and spermatogenesis. Together, our findings indicate that the classical and nonclassical pathways both contribute to maintaining spermatogenesis and male fertility.
ACKNOWLEDGMENT
We thank Drs. Elizabeth Wilson, Jorma Palvino, Ferdinando Auricchio, and Guido Jenster for plasmid and adenovirus constructs. We thank Dr. Tony Zeleznik for assisting in the editing of the manuscript. J.M.A. and G.V.R. have a U.S. patent application No. 13/683 932, entitled “Oligo-Benzamide Compounds and Their Use in Treating Cancers” by Jung-Mo Ahn et al. pending. J.M.A. has a U.S. patent 8 618 324 B2 issued, entitled “Composition and Method for Making Oligo-Benzamide Compounds.” These two patents cover the two peptidomimetics D1 and D2 referred to in this manuscript.
Footnotes
Supported by grants DOD W81XWH-12-1-0288 (G.V.R.), Welch Foundation AT-1595 (J.M.A.), NIH-HD055475 (K.E.O.), and NIH-HD43143 and PA Department of Health Research Formula Funds (W.H.W.). Presented in part at the 45th Annual Meeting of the Society for the Study of Reproduction, 12–15 August 2012, State College, Pennsylvania.
REFERENCES
- Sharpe RM. Regulation of spermatogenesis In Knobil E, Neil JD. (eds.), The Physiology of Reproduction . New York: Raven Press; 1994. 1363 1434 [Google Scholar]
- McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, De Kretser DM, Pratis K, Robertson DM. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Ruokonen A, Kontturi M, Koskela E, Vihko R. The simultaneous radioimmunoassay of seven steroids in human spermatic and peripheral venous blood. J Clin Endocrinol Metab. 1977;45:16–24. doi: 10.1210/jcem-45-1-16. [DOI] [PubMed] [Google Scholar]
- Comhaire FH, Vermeulen A. Testosterone concentration in the fluids of seminiferous tubules, the interstitium and the rete testis of the rat. J Endocrinol. 1976;70:229–235. doi: 10.1677/joe.0.0700229. [DOI] [PubMed] [Google Scholar]
- Turner TT, Jones CE, Howards SS, Ewing LL, Zegeye B, Gunsalus GL. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–1932. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology. 2003;144:509–517. doi: 10.1210/en.2002-220710. [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant'Agnese PA, deMesy-Bentley KL, Tzeng CR, Chang C. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems A, Batlouni SR, Esnal A, Swinnen JV, Saunders PT, Sharpe RM, Franca LR, De Gendt K, Verhoeven G. Selective ablation of the androgen receptor in mouse sertoli cells affects sertoli cell maturation, barrier formation and cytoskeletal development. PLoS One. 2010;5:e14168. doi: 10.1371/journal.pone.0014168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996;55:895–901. doi: 10.1095/biolreprod55.4.895. [DOI] [PubMed] [Google Scholar]
- Wong CH, Xia W, Lee NP, Mruk DD, Lee WM, Cheng CY. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology. 2005;146:1192–1204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Millar M, Maddocks S, Sharpe RM. Stage-dependent changes in spermatogenesis and Sertoli cells in relation to the onset of spermatogenic failure following withdrawal of testosterone. Anat Rec. 1993;235:547–559. doi: 10.1002/ar.1092350407. [DOI] [PubMed] [Google Scholar]
- Zhengwei Y, Wreford NG, Royce P, de Kretser DM, McLachlan RI. Stereological evaluation of human spermatogenesis after suppression by testosterone treatment: heterogeneous pattern of spermatogenic impairment. J Clin Endocrinol Metab. 1998;83:1284–1291. doi: 10.1210/jcem.83.4.4724. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, O'Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, Robertson DM. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546–556. doi: 10.1210/jcem.87.2.8231. [DOI] [PubMed] [Google Scholar]
- Matthiesson KL, Stanton PG, O'Donnell L, Meachem SJ, Amory JK, Berger R, Bremner WJ, McLachlan RI. Effects of testosterone and levonorgestrel combined with a 5alpha-reductase inhibitor or gonadotropin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men. J Clin Endocrinol Metab. 2005;90:5647–5655. doi: 10.1210/jc.2005-0639. [DOI] [PubMed] [Google Scholar]
- Grootegoed JA, Peters MJ, Mulder E, Rommerts FF, Van der Molen HJ. Absence of a nuclear androgen receptor in isolated germinal cells of rat testis. Mol Cell Endocrinol. 1977;9:159–167. doi: 10.1016/0303-7207(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Millar MR, Sharpe RM, Saunders PTK. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 1994;135:1227–1234. doi: 10.1210/endo.135.3.8070367. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Russell LD, Friel PJ, Griswold MD. Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation. Endocrinology. 2001;142:2405–2408. doi: 10.1210/endo.142.6.8317. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Glenister PH, Lamoreux ML. Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature. 1975;258:620–622. doi: 10.1038/258620a0. [DOI] [PubMed] [Google Scholar]
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- Griswold MD. Perspective on the function of Sertoli cells In Griswold MD. (ed.), Sertoli Cell Biology San Diego: Elsevier Science; 2005. 15 18 [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Walker WH. Molecular mechanisms of testosterone action in spermatogenesis. Steroids. 2009;74:602–607. doi: 10.1016/j.steroids.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Walker WH. Non-classical actions of testosterone and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1557–1569. doi: 10.1098/rstb.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradial receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;20:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Watkins SC, Walker WH. Testosterone activates MAP kinase via Src kinase and the EGF receptor in Sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265:8893–8900. [PubMed] [Google Scholar]
- Huang W, Shostak Y, Tarr P, Sawyers C, Carey M. Cooperative assembly of androgen receptor into a nucleoprotein complex that regulates the prostate-specific antigen enhancer. J Biol Chem. 1999;274:25756–25768. doi: 10.1074/jbc.274.36.25756. [DOI] [PubMed] [Google Scholar]
- Karvonen U, Kallio PJ, Janne OA, Palvimo JJ. Interaction of androgen receptors with androgen response element in intact cells. Roles of amino- and carboxyl-terminal regions and the ligand. J Biol Chem. 1997;272:15973–15979. doi: 10.1074/jbc.272.25.15973. [DOI] [PubMed] [Google Scholar]
- Shupe J, Cheng J, Puri P, Kostereva N, Walker WH. Regulation of Sertoli-germ cell adhesion and sperm release by FSH and nonclassical testosterone signaling. Mol Endocrinol. 2011;25:238–252. doi: 10.1210/me.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranathan P, Lee TK, Yang L, Centenera MM, Butler L, Tilley WD, Hsieh JT, Ahn JM, Raj GV. Peptidomimetic targeting of critical androgen receptor-coregulator interactions in prostate cancer. Nat Commun. 2013;4:1923. doi: 10.1038/ncomms2912. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Ramaswamy S, Plant TM. Gonadotropin-independent proliferation of the pale type A spermatogonia in the adult rhesus monkey (Macaca mulatta) Biol Reprod. 2005;73:222–229. doi: 10.1095/biolreprod.104.038968. [DOI] [PubMed] [Google Scholar]
- Puri P, Walker WH. The tyrosine phosphatase SHP2 regulates Sertoli cell junction complexes. Biol Reprod. 2013;88:59. doi: 10.1095/biolreprod.112.104414. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Wood MA, Mukherjee P, Toocheck CA, Walker WH. Upstream stimulatory factor induces Nr5a1 and Shbg gene expression during the onset of rat Sertoli cell differentiation. Biol Reprod. 2011;85:965–976. doi: 10.1095/biolreprod.111.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol. 2006 doi: 10.1002/0471142727.mb1508s73. (eds.) Unit 15.8. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogura A, Ikegawa M, Inoue K, Ogonuki N, Tashiro K, Toyokuni S, Honjo T, Shinohara T. Adenovirus-mediated gene delivery and in vitro microinsemination produce offspring from infertile male mice. Proc Natl Acad Sci U S A. 2002;99:1383–1388. doi: 10.1073/pnas.022646399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH. Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P, Robson M, Spaliviero J, McTavish KJ, Jimenez M, Zajac JD, Handelsman DJ, Allan CM. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology. 2009;150:4755–4765. doi: 10.1210/en.2009-0416. [DOI] [PubMed] [Google Scholar]
- Quigley CA, Evans BA, Simental JA, Marschke KB, Sar M, Lubahn DB, Davies P, Hughes IA, Wilson EM, French FS. Complete androgen insensitivity due to deletion of exon C of the androgen receptor gene highlights the functional importance of the second zinc finger of the androgen receptor in vivo. Mol Endocrinol. 1992;6:1103–1112. doi: 10.1210/mend.6.7.1508223. [DOI] [PubMed] [Google Scholar]
- Halmos G, Schally AV, Pinski J, Vadillo-Buenfil M, Groot K. Down-regulation of pituitary receptors for luteinizing hormone-releasing hormone (LH-RH) in rats by LH-RH antagonist Cetrorelix. Proc Natl Acad Sci U S A. 1996;93:2398–2402. doi: 10.1073/pnas.93.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek TK, Joshi AR, Sanyal A, Dighe RR. Insights into male germ cell apoptosis due to depletion of gonadotropins caused by GnRH antagonists. Apoptosis. 2007;12:1085–1100. doi: 10.1007/s10495-006-0039-3. [DOI] [PubMed] [Google Scholar]
- Hakola K, Pierroz DD, Aebi A, Vuagnat BA, Aubert ML, Huhtaniemi I. Dose and time relationships of intravenously injected rat recombinant luteinizing hormone and testicular testosterone secretion in the male rat. Biol Reprod. 1998;59:338–343. doi: 10.1095/biolreprod59.2.338. [DOI] [PubMed] [Google Scholar]
- Bramlett KS, Dits NF, Sui X, Jorge MC, Zhu X, Jenster G. Repression of androgen-regulated gene expression by dominant negative androgen receptors. Mol Cell Endocrinol. 2001;183:19–28. doi: 10.1016/s0303-7207(01)00636-0. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Varricchio L, De Falco A, Castoria G, Arra C, Yamaguchi H, Ciociola A, Lombardi M, Di Stasio R, Barbieri A, Baldi A, Barone MV, et al. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007;26:6619–6629. doi: 10.1038/sj.onc.1210487. [DOI] [PubMed] [Google Scholar]
- Auricchio F, Migliaccio A, Castoria G. Sex-steroid hormones and EGF signalling in breast and prostate cancer cells: targeting the association of Src with steroid receptors. Steroids. 2008;73:880–884. doi: 10.1016/j.steroids.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- Sato T, Yokonishi T, Komeya M, Katagiri K, Kubota Y, Matoba S, Ogonuki N, Ogura A, Yoshida S, Ogawa T. Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc Natl Acad Sci U S A. 2012;109:16934–16938. doi: 10.1073/pnas.1211845109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JS, Wilkinson MF. Pem: a testosterone- and LH-regulated homeobox gene expressed in mouse Sertoli cells and epididymus. Dev Biol. 1996;179:471–484. doi: 10.1006/dbio.1996.0276. [DOI] [PubMed] [Google Scholar]
- Sutton KA, Maiti S, Tribley WA, Lindsey JS, Meistrich ML, Bucana CD, Sanborn BM, Joseph DR, Griswold MD, Cornwall GA, Wilkinson MF. Androgen regulation of the Pem homeodomain gene in mice and rat Sertoli and epididymal cells. J Androl. 1998;19:21–30. [PubMed] [Google Scholar]
- Rao MK, Wayne CM, Meistrich ML, Wilkinson MF. Pem homeobox gene promoter sequences that direct transcription in a Sertoli cell-specific, stage-specific, and androgen-dependent manner in the testis in vivo. Mol Endocrinol. 2003;17:223–233. doi: 10.1210/me.2002-0232. [DOI] [PubMed] [Google Scholar]
- Scobey M, Bertera S, Somers J, Watkins S, Zeleznik A, Walker W. Delivery of a cyclic adenosine 3′, 5′-monophosphate response element-binding protein (CREB) mutant to seminiferous tubules results in impaired spermatogenesis. Endocrinology. 2001;142:948–954. doi: 10.1210/endo.142.2.7948. [DOI] [PubMed] [Google Scholar]
- Blanchard KT, Boekelheide K. Adenovirus-mediated gene transfer to rat testis in vivo. Biol Reprod. 1997;56:495–500. doi: 10.1095/biolreprod56.2.495. [DOI] [PubMed] [Google Scholar]
- Hooley RP, Paterson M, Brown P, Kerr K, Saunders PT. Intra-testicular injection of adenoviral constructs results in Sertoli cell-specific gene expression and disruption of the seminiferous epithelium. Reproduction. 2009;137:361–370. doi: 10.1530/REP-08-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehashi M, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Ogura A, Shinohara T. Adenovirus-mediated gene delivery into mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2007;104:2596–2601. doi: 10.1073/pnas.0609282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009;23:4218–4230. doi: 10.1096/fj.09-138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JA, II, Wilkinson MF. The Rhox genes. Reproduction. 2010;140:195–213. doi: 10.1530/REP-10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Dandekar D, O'Shaughnessy PJ, De Gendt K, Verhoeven G, Wilkinson MF. Androgen-induced Rhox homeobox genes modulate the expression of AR-regulated genes. Mol Endocrinol. 2010;24:60–75. doi: 10.1210/me.2009-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JA., II The role of Rhox homeobox factors in tumorigenesis. Front Biosci (Landmark Ed) 2013;18:474–492. doi: 10.2741/4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Ahn HW, Chu T, Bowden W, Gassei K, Orwig K, Rajkovic A. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev Biol. 2012;361:301–312. doi: 10.1016/j.ydbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios F, Filipponi D, Campolo F, Gori M, Bramucci F, Pellegrini M, Ottolenghi S, Rossi P, Jannini EA, Dolci S. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J Cell Sci. 2012;125:1455–1464. doi: 10.1242/jcs.092593. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–1392. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve A. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Han L, Kim HN, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS, Chambliss KL, Shaul PW, Roberson PK, Weinstein RS, Jilka RL, Almeida M, et al. Non-nuclear-initiated actions of the estrogen receptor protect cortical bone mass. Mol Endocrinol. 2013;27:649–656. doi: 10.1210/me.2012-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IA, Evans BA. Complete androgen insensitivity syndrome characterized by increased concentration of a normal androgen receptor in genital skin fibroblasts. J Clin Endocrinol Metab. 1986;63:309–315. doi: 10.1210/jcem-63-2-309. [DOI] [PubMed] [Google Scholar]
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas TF, Siu ER, Esteves CA, Monteiro HP, Oliveira CA, Porto CS, Lazari MF. 17beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol Reprod. 2008;78:101–114. doi: 10.1095/biolreprod.107.063909. [DOI] [PubMed] [Google Scholar]
- Barbulescu K, Geserick C, Schuttke I, Schleuning WD, Haendler B. New androgen response elements in the murine pem promoter mediate selective transactivation. Mol Endocrinol. 2001;15:1803–1816. doi: 10.1210/mend.15.10.0708. [DOI] [PubMed] [Google Scholar]
- Geserick C, Meyer HA, Barbulescu K, Haendler B. Differential modulation of androgen receptor action by deoxyribonucleic acid response elements. Mol Endocrinol. 2003;17:1738–1750. doi: 10.1210/me.2002-0379. [DOI] [PubMed] [Google Scholar]
- Faus H, Haendler B. Androgen receptor acetylation sites differentially regulate gene control. J Cell Biochem. 2008;104:511–524. doi: 10.1002/jcb.21640. [DOI] [PubMed] [Google Scholar]
- Sen A, De Castro I, Defranco DB, Deng FM, Melamed J, Kapur P, Raj GV, Rossi R, Hammes SR. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Invest. 2012;122:2469–2481. doi: 10.1172/JCI62044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang G, Small CL, Liu Z, Weng CC, Yang L, Griswold MD, Meistrich ML. Gene expression alterations by conditional knockout of androgen receptor in adult Sertoli cells of Utp14b jsd/jsd (jsd) mice. Biol Reprod. 2011;84:400–408. doi: 10.1095/biolreprod.110.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, Tzeng CR, Chang C. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci U S A. 2006;103:18975–18980. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Weng CC, Shao SH, Zhou W, de Gendt K, Braun RE, Verhoeven G, Meistrich ML. Androgen receptor in Sertoli cells is not required for testosterone-induced suppression of spermatogenesis, but contributes to Sertoli cell organization in Utp14b jsd mice. J Androl. 2009;30:338–348. doi: 10.2164/jandrol.108.006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Lee WM. Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–665. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, Lui WY, Lee WM, Cheng CY. Tumor necrosis factor {alpha} reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont Y, Perey B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat. 1957;100:241–267. doi: 10.1002/aja.1001000205. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Ohkawa M, Oku R, Maekawa M, Yuasa S. Neonatally administered diethylstilbestrol retards the development of the blood-testis barrier in the rat. J Androl. 2001;22:413–423. [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Silvestrini B. Cheng CY. rpS6 Regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology. 2012;153:5036–5048. doi: 10.1210/en.2012-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaud-Guittot S, Meugnier E, Pesenti S, Wu X, Vidal H, Gow A, Le Magueresse-Battistoni B. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol Reprod. 2009;82:202–213. doi: 10.1095/biolreprod.109.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]