Introduction
Solid organ transplant recipients (SOTR) suffer an overall 2-fold increased risk of any malignancy compared with the general population. Multiple strategies exist to address this risk in these patients. Revision of immunosuppression is the cornerstone to reducing the risk of skin cancer development. Here we present a patient with a remarkable clinical response to immunosuppression revision.
Case report
A 61-year-old man with a history of a kidney transplantation in 2004 secondary to polycystic kidney disease and a kidney–liver transplant in 2008 for decompensated cryptogenic cirrhosis maintained on tacrolimus, prednisone, and mycophenolate presented to our office with an undifferentiated tumor of the left parotid with distant metastases to the skin. The patient's medical history included multiple cutaneous squamous cell and basal cell carcinomas and a family history of polycystic kidney disease in his father and sister.
Five months prior, he presented to his outside dermatologist with several nodules on the left side of the face. A parotid fine-needle aspiration found atypical cells suspicious for a neoplasm without obvious lineage differentiation. Left superficial parotidectomy found a high-grade malignant tumor with areas of necrosis. The margins were positive, and periparotid lymph nodes were involved. Immunostains were strongly positive for vimentin and focally positive for S100, with absence of staining for a broad panel of epithelial, melanocytic, and hematopoietic markers (Fig 1, A and B). The final pathologic diagnosis was pleomorphic undifferentiated epithelioid cell neoplasm.
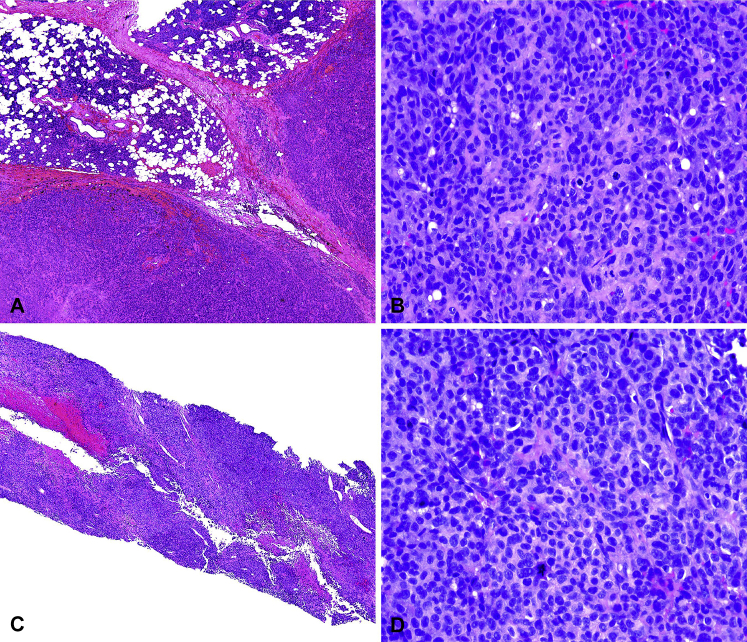
Fig 1.
A, Low-power image of tumor nodules (lower portion of Fig 1, A) in parotid gland (upper portion of figure), partially surrounded by a fibrous capsule. B, Higher magnification shows sheets of pleomorphic undifferentiated epithelioid cells with numerous mitoses. Per report of an outside facility that originally prepared the slides and read the pathology, a panel of immunostains were performed including AE/AE3, EMA, CK5/6, CK7, p40, CK18, 34BE12, p63, calponin, SMA, MITF, MART-1 and HMB-45, GFAP, LCA, CD3, CD20, CD138, CD34, ERG, TTF-1, desmin were negative. Tumor was strongly vimentin positive and focally positive for S100. C, Lower power image of needle biopsy from the chest wall shows sheets of pleomorphic cells, focal necrosis, and hemorrhage. D, High-power view of undifferentiated pleomorphic epithelioid cells and bizarre mitotic figures, histologically identical to the tumor in the parotid gland. (Hematoxylin-eosin stain; original magnifications: A and C, ×4; B and D, ×40.)
Postoperative positron emission tomography (PET) scan suggested persistent periparotid and cervical lymph node activity, splenomegaly, and distant metastases to the skin with increased uptake in the left parietal scalp and left anterior chest wall. Core biopsy from the left chest lesion and left scalp found a high-grade undifferentiated epithelioid cell neoplasm identical to the parotid tumor, confirming distant cutaneous metastatic disease (Fig 1, C and D). These metastases were completely excised, and he underwent a modified neck dissection, which found involvement of multiple cervical lymph nodes.
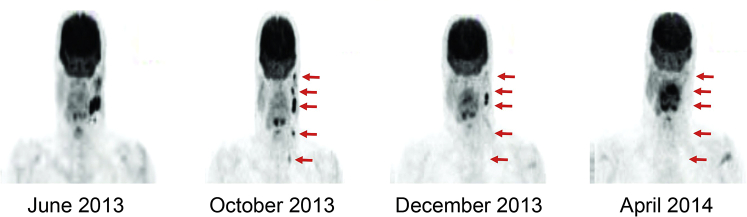
At the time of presentation to our transplant dermatology clinic, the patient had not undergone treatment. In coordination with the transplant team, recommendations were made to switch from mycophenolate to the mammalian target of rapamycin (mTOR) inhibitor sirolimus while continuing on tacrolimus and prednisone, 5 mg daily. PET scan at this time found progression of disease with increase in size of the left parotid bed avid nodule from 0.9 × 0.6 cm to 1.3 × 0.8 cm and involvement of new left cervical and left parathyroid lymph nodes (Fig 2). Given the aggressive features of this tumor, the calcineurin inhibitor, tacrolimus, was tapered off. Without any other intervention or change in immunosuppression, repeat PET scan 1 month after discontinuing tacrolimus and 3 months after starting sirolimus found significant improvement with resolution of most periparotid and cervical nodes. Repeat PET scan 4 months off calcineurin inhibitor and 6 months on sirolimus found no evidence of disease (Fig 2). The patient's graft functions remained stable, and his only side effects included nonhealing ulcers of the shin and mild proteinuria.
Fig 2.
PET scan consolidations show progression of high-grade undifferentiated tumor of the left parotid with metastasis to scalp and left subcutaneous breast between June and October 2013. No evidence of disease on PET scan 6 months after starting mTOR inhibitor sirolimus and 4 months after discontinuing calcineurin inhibitor tacrolimus.
Discussion
Here we describe a SOTR with an aggressive undifferentiated epithelioid tumor in the parotid gland with skin metastases who had no evidence of disease after converting to an mTOR inhibitor and discontinuing his calcineurin inhibitor and mycophenolate without any other systemic therapy. To date, the benefits of revising immunosuppression regimens have been best studied in the treatment of SOTRs with cutaneous squamous cell carcinoma (SCC); however, there is supporting evidence that decreasing immunosuppression and changing to an mTOR inhibitor may be important in the prevention and treatment of other tumors, including those that are high grade and metastatic as in this case.
SOTRs suffer an overall 2-fold increased risk of any malignancy when compared with the general population. The most common malignancy is SCC with an approximately 100-fold increased relative risk. However, there is at least a 5-fold increased relative risk of basal cell carcinoma, Kaposi's sarcoma, Non-Hodgkin's lymphoma, liver cancer, anal and vulvar cancer, and SCC of the lip compared with normal population. Other poorly differentiated tumors, such as undifferentiated pleomorphic sarcoma, have been reported to be more common and show a higher risk of metastases and mortality in SOTRs.1
Revision of immunosuppression regimen in SOTRs to manage SCC is recommended in patients with SCC at high risk of metastasis, patients with life-threatening cancer, or those with rapid development of SCC (more than 5–10 per year). Early minimization of immunosuppression or stopping immunosuppressive therapy altogether will significantly reduce the development of new SCC at 5 years in SOTRs and has been shown to improve outcomes for patients with aggressive SCC.2 In a case series examining cutaneous undifferentiated pleomorphic sarcoma in SOTRs at several institutions, decreasing immunosuppression was also noted to improve outcomes.1
Conversion to an mTOR inhibitor such as sirolimus may provide an additional antitumor effect in high-risk transplant patients.3 Mammalian target of rapamycin is part of the family of phosphatidylinositol-3 kinase (PI3K)-related kinases that is phosphorylated via PI3K/AKT signaling pathway and activates downstream of cellular growth signaling.4 In SOTRs, conversion to sirolimus-based immunosuppression decelerated the incidence of new SCC and induced regression of existing skin lesions.5 A trial involving 120 SOTRs and another with 830 patients receiving sirolimus or continued on calcineurin inhibitors found significantly longer duration of survival free of SCC after 2 years in the sirolimus group, and these patients experienced up to half the number of new SCCs compared with those on calcineurin inhibitors with no difference in graft function.6, 7 A significant decrease in all malignancies was noted in 5 randomized trials comparing sirolimus therapy with other modes of immunosuppression in SOTRs. There was a similar trend that did not reach statistical significance in a recent large, randomized multicenter study.6, 8
The effectiveness of mTOR inhibitor immunotherapy in preventing and treating SCC and other malignancies in transplant patient may not be surprising given mTOR activates cellular growth. Aberration of the mTOR/PI3K/AKT pathway is suspected in many malignancies including cutaneous SCC, which is found to express significant phospho-mTOR immunoreactivity.9, 10 Use of mTOR inhibitors show promising results in treatment of advanced renal cell carcinoma, breast carcinoma, mantle cell lymphoma, endometrial cancer, glioblastoma, neuroendocrine tumors, and soft tissue sarcomas.4 Side effects of mTOR inhibitors are not trivial and should be taken into consideration when initiating therapy in a patient. Common adverse events include peripheral edema, increased triglyceride and cholesterol levels, hypertension, constipation or diarrhea, nausea and abdominal pain, and anemia/thrombocytopenia.
This case provides a successful example of applying lessons learned from managing SCCs in SOTRs with the addition of sirolimus therapy and reduction of overall immunosuppression, coupled with surgical excision, to eradicate an undifferentiated metastatic malignancy.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.McCoppin H.H., Christiansen D., Stasko T. Clinical spectrum of atypical fibroxanthoma and undifferentiated pleomorphic sarcoma in solid organ transplant recipients: a collective experience. Dermatol Surg. 2012;38:230–239. doi: 10.1111/j.1524-4725.2011.02180.x. [DOI] [PubMed] [Google Scholar]
- 2.Moloney F.J., Elly P.O., Ay E.W., Conlon P., Murphy G.M. Maintenance versus reduction of immunosuppression in renal transplant recipients with aggressive squamous cell carcinoma. Dermatol Surg. 2004;30:674–678. doi: 10.1111/j.1524-4725.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc K.G., Jr., Hughes M.P., Sheehan D.J. The role of sirolimus in the prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Dermatol Surg. 2011;37:744–749. doi: 10.1111/j.1524-4725.2011.01973..x. [DOI] [PubMed] [Google Scholar]
- 4.Guertin D.A., Sabatini D.M. Defining the role of mTOR in cancer. Cancer cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Salgo R., Gossmann J., Schofer H. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant. 2010;10:1385–1393. doi: 10.1111/j.1600-6143.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 6.Alberu J., Pascoe M.D., Campistol J.M. Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation. 2011;92:303–310. doi: 10.1097/TP.0b013e3182247ae2. [DOI] [PubMed] [Google Scholar]
- 7.Euvrard S., Morelon E., Rostaing L. Sirolimus and secondary skin-cancer prevention in kidney transplantation. New Engl J Med. 2012;367:329–339. doi: 10.1056/NEJMoa1204166. [DOI] [PubMed] [Google Scholar]
- 8.Mathew T., Kreis H., Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446–449. doi: 10.1111/j.1399-0012.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 9.Karayannopoulou G., Euvrard S., Kanitakis J. Differential expression of p-mTOR in cutaneous basal and squamous cell carcinomas likely explains their different response to mTOR inhibitors in organ-transplant recipients. Anticancer Res. 2013;33:3711–3714. [PubMed] [Google Scholar]
- 10.Nielsen T., West R. Translating gene expression into clinical care: sarcomas as a paradigm. J Clin Oncol. 2010;28:1796–1805. doi: 10.1200/JCO.2009.26.1917. [DOI] [PubMed] [Google Scholar]