Introduction
Solid organ transplant patients are susceptible to rare and unusual dermatoses as a result of their chronic immunosuppression. Trichodysplasia spinulosa (TS) is one such rare clinical entity observed predominantly in immunosuppressed patients with a history of either solid organ transplantation on immunosuppressive therapy or hematologic malignancies treated with chemotherapy. Herein we present 2 cases of TS that were successfully treated with compounded topical cidofovir.
Case 1
A 36-year-old African-American man with a kidney transplant related to end-stage renal disease from uncontrolled hypertension presented with a 6-month history of a skin-colored follicular eruption beginning on the central part of the face and progressing to involve his entire body. The eruption occurred 8 months after transplantation, and his immunosuppressant regimen included mycophenolate mofetil, tacrolimus, and prednisone. Prior treatments included topical antifungals, metronidazole cream, imiquimod 5% cream, and cidofovir 1% cream as well as oral valganciclovir for 1 month, all of which were ineffective.
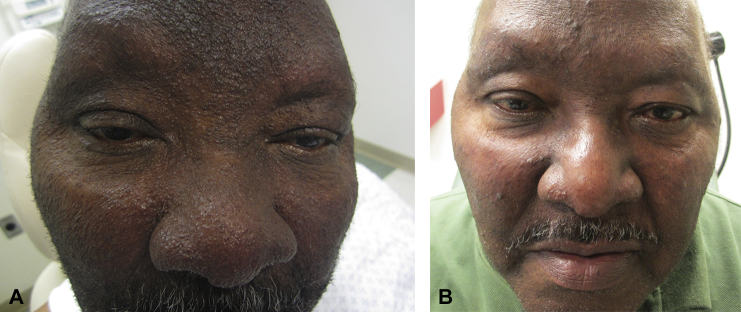
Physical examination found skin-colored to lightly erythematous follicular papules and follicular spinelike projections (Fig 1, A). These spines were distributed densely in hair-bearing skin on the head and neck and more sparsely throughout the trunk and extremities. His nose was disfigured with markedly thickened and pebbled skin. There was madarosis of the eyebrows and a leonine facies appearance.
Fig 1.
A, Presentation of multiple follicular spines, madarosis, and leonine facies. B, Smoother skin in affected areas after topical cidofovir 3% cream twice daily for 3 months.
Histopathology found increased cellularity of the nucleated eosinophilic inner root sheath cells with trichohyalin granules. There were also perinuclear eosinophilic globules consistent with TS. Polymerase chain reaction for the NCCR, LT1, and VP1 regions of the TS polyomavirus (TSPyV) confirmed the presence of the virus in a biopsy specimen. Complete sequencing of this isolate found that the strain was 99.98% identical to the WK164 strain.1
We began treatment with compounded topical cidofovir 3% cream and weekly monitoring of renal function and blood counts. He tolerated using the medicine once daily to his head and neck and then subsequent use to his extremities. He began showing improvement at 1 month, and within 3 months his skin was much smoother with resolution of the follicular spines and regrowth of his eyebrows (Fig 1, B).
Case 2
A 62-year-old Haitian man with a kidney transplant secondary to end-stage renal disease for hypertension and type II diabetes had a quickly evolving pruritic facial eruption, which began 6 months after transplantation. His immunosuppressant regimen included mycophenolate mofetil, tacrolimus, and prednisone. Prior ineffective treatments included topical cidofovir 1% cream.
On physical examination, there were diffuse small flesh-colored and hyperpigmented papules with central spiny projections on bilateral external ears and frontal/crown of scalp and face, with fewer such lesions on the neck; there was madarosis and thickened skin of the medial bilateral cheeks and nose (Fig 2, A).
Fig 2.
A, Diffuse small hyperpigmented papules with spiny projections. B, Resolution of lesions with smoother skin after 2 months of twice-daily topical cidofovir 3% cream.
Histopathology found mild follicular dilatation and keratotic plugging within the infundibulum. There was dystrophy of the inner root sheath along with pink irregular trichohyalin granules and apoptotic cells (Fig 3).
Fig 3.
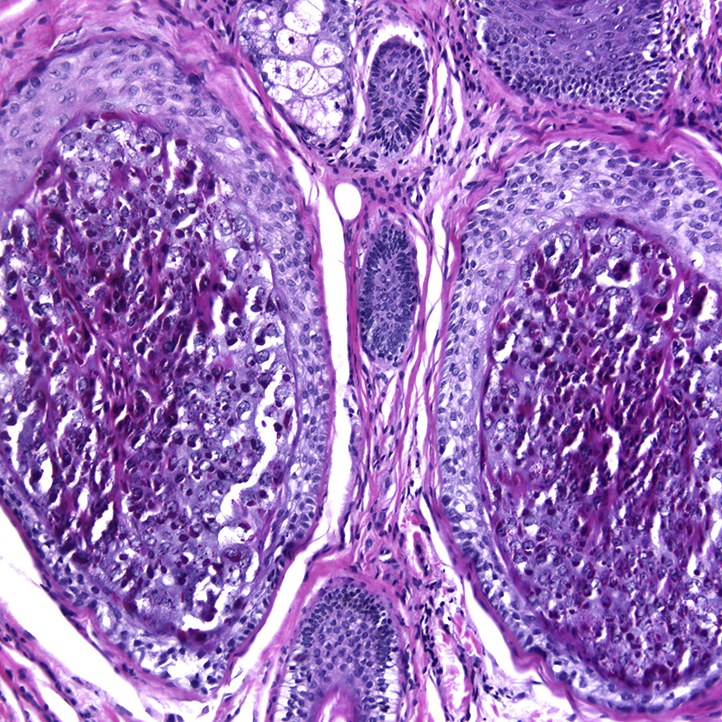
Histopathology of TS with increased cellularity of nucleated eosinophilic inner root sheath cells with trichohyalin granules.
Treatment with topical cidofovir 3% cream applied twice daily to the affected areas resulted in improvement of his eruption within 2 months (Fig 2, B).
Discussion
TS is a rare clinical entity observed predominantly in immunosuppressed patients with a history of either solid organ transplantation on immunosuppressive therapy or hematologic malignancies treated with chemotherapy. TS often presents with numerous flesh-colored to erythematous papules featuring a central keratotic follicular spine. The distribution favors the glabella, mid-face, chin, and ears and less so the trunk and extremities. There are varying degrees of alopecia in areas of high follicular density, such as on the face, although the scalp is less affected.2 Lesions in TS are frequently asymptomatic but may be associated with mild pruritus. Nasal disfigurement akin to that seen in sarcoidosis and rosacea may develop from thickening of the skin resulting in leonine facies.3
TS was previously identified under various monikers including viral-associated trichodysplasia, cyclosporine-induced folliculodystrophy, pilomatrix dysplasia, and trichodysplasia of immunosuppression.4 Since the original description by Izakovic et al,5 the histologic findings of TS are highly distinctive and unique. Light microscopy shows pronounced distention of anagen follicles with abnormal maturation, and inner root sheath cells contain abundant trichohyalin granules. In 1999, Haycox et al,6 used electron microscopy to show the presence of intranuclear viral particles of the Polyomaviridae family. By sequencing the viral genome, Van der Meijden et al,7 recently determined TS to be caused by a new human polyomavirus and named it trichodysplasia spinulosa-associated polyomavirus (TSPyV).
The seroprevalence of TSPyV appears to be high in the healthy population; however, clinical manifestations have thus far only been observed in immunosuppressed individuals, among whom viral seroprevalence approaches 90%.8 Renal and renal-pancreas transplant patients account for 40% of cases of TS; cardiac transplant patients comprise another 20%. Other affected patients have medical histories significant for pre–B-cell or T-cell acute lymphoblastic leukemia, chronic lymphocytic leukemia, lung transplantation, or lupus erythematosus.9
It has been hypothesized that the pathophysiology of TS lesions may involve unabated proliferation of inner root sheath cells infected by TSPyV. Ki-67 staining of lesional cells is found to be strongly positive, indicating rapid proliferation of the inner root sheath cells.10
Clinical improvement of lesions may be noted with either decreasing or discontinuing immunosuppressive therapy, although the latter is not always a viable option for solid organ transplant recipients. Resolution has also been reported after treatment of the underlying hematologic disease. Treatment with either oral valganciclovir or topical cidofovir of 1% to 3% is found to be successful in case reports.11, 12 Additionally, improvement of lesions has been described in isolated cases with use of oral or topical retinoids (eg, tazarotene 0.5% gel).13
These 2 cases uniquely highlight the efficacy of topical cidofovir 3% cream as a potent therapy for treatment-refractory TS. Both patients did not respond to cidofovir 1% cream with several months of application, and the higher concentration was found to be efficacious. Because of its toxicity, cidofovir requires specialty pharmacy compounding in a class II laminar flow hood. Monitoring of serum blood urea nitrogen and creatinine is recommended weekly for the first month, then biweekly thereafter, particularly in kidney transplant recipients. A complete blood count should be monitored monthly while on therapy.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Siebrasse E.A., Bauer I., Holtz L.R. Human polyomaviruses in children undergoing transplantation, United States, 2008-2010. Emerg Infect Dis. 2012;18:1676–1679. doi: 10.3201/eid1810.120359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celeiro-Munoz C., Gonzalez-Vilas D., Sanchez-Aguilar D., Suarez-Penaranda J.M. Viral-associated trichodysplasia secondary to antineoplastic treatment in a patient with lymphoblastic leukemia. Am J Dermatopathol. 2014;36:e105–e107. doi: 10.1097/DAD.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 3.Sperling L.C., Tomaszewski M.M., Thomas D.A. Viral-associated trichodysplasia in patients who are immunocompromised. J Am Acad Dermatol. 2004;50:318–322. doi: 10.1016/s0190-9622(03)01490-7. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt A.J., Sachs D.L., Shia J., Delgado R., Busam K.J. Virus-associated trichodysplasia spinulosa. Am J Surg Pathol. 2005;29:241–246. doi: 10.1097/01.pas.0000149691.83086.dc. [DOI] [PubMed] [Google Scholar]
- 5.Izakovic J., Buchner S.A., Duggelin M., Guggenheim R., Itin P.H. Hair-like hyperkeratoses in patients with kidney transplants. A new cyclosporin side-effect. Hautarzt. 1995;46:841–846. doi: 10.1007/s001050050350. [DOI] [PubMed] [Google Scholar]
- 6.Haycox C.L., Kim S., Fleckman P. Trichodysplasia spinulosa–a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268–271. doi: 10.1038/sj.jidsp.5640227. [DOI] [PubMed] [Google Scholar]
- 7.van der Meijden E., Janssens R.W., Lauber C., Bouwes Bavinck J.N., Gorbalenya A.E., Feltkamp M.C. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:e1001024. doi: 10.1371/journal.ppat.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Meijden E., Kazem S., Burgers M.M. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355–1363. doi: 10.3201/eid1708.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews M.R., Wang R.C., Reddick R.L., Saldivar V.A., Browning J.C. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa-associated human polyomavirus. J Cutan Pathol. 2011;38:420–431. doi: 10.1111/j.1600-0560.2010.01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazem S., van der Meijden E., Wang R.C. Polyomavirus-Associated Trichodysplasia Spinulosa Involves Hyperproliferation, pRB Phosphorylation and Upregulation of p16 and p21. PLoS One. 2014;9:e108947. doi: 10.1371/journal.pone.0108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wanat K.A., Holler P.D., Dentchev T. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219–223. doi: 10.1001/archdermatol.2011.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benoit T., Bacelieri R., Morrell D.S., Metcalf J. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871–874. doi: 10.1001/archdermatol.2010.175. [DOI] [PubMed] [Google Scholar]
- 13.Schwieger-Briel A., Balma-Mena A., Ngan B., Dipchand A., Pope E. Trichodysplasia spinulosa–a rare complication in immunosuppressed patients. Pediatr Dermatol. 2010;27:509–513. doi: 10.1111/j.1525-1470.2010.01278.x. [DOI] [PubMed] [Google Scholar]