Introduction
Field cancerization in organ transplant recipients (OTRs) is a frequent occurrence, setting the stage for multiple squamous cell carcinomas (SCCs). Immunosuppression directly promotes keratinocyte cancer formation and reduces tumor immunity. Photo damage is amplified in OTRs via several mechanisms including potentiation of the tumor-promoting effects of calcineurin inhibitors and anti-infective medications such as voriconazole. The challenge with field cancerization in OTRs is to stay ahead of repetitive epithelial tumor formation. We present a strategy to treat field cancerization using ingenol mebutate and discuss other nonregistered modalities and treatment options.
Case report
We report the case of a 76-year-old male recipient of a ABO-incompatible living donor kidney in 2008 for underlying autosomal dominant polycystic kidney disease. He showed significant field cancerization of the skin with large areas of sun damage and extensive areas of actinic keratosis. These areas had multiple epithelial tumors that developed over time. His immunosuppressive medication had been adjusted to avoid azathioprine, a known ultraviolet (UV) A photosensitizer and DNA damaging agent, and to account for his increased incidence of skin cancer. He currently was maintained on tacrolimus, 1.5 mg orally daily, and mycophenolate mofetil, 500 mg orally daily.
Field cancerization of this patient's skin has resulted in 5 SCCs, 2 basal cell carcinomas (BCCs), multiple SCCs in situ (Bowen's disease) and actinic keratoses (AKs) over the last 13 years. The invasive SCCs and BCCs were surgically excised. Acitretin titrated up to 25 mg daily was started in 2007 to reduce his rate of SCC formation. With his immunosuppressive medication at low levels and acitretin chemoprevention at full dosage, we focused on repeat topical treatments for his field cancerization. Field treatment on the scalp and face consisted of various topical modalities including cryotherapy, imiquimod, and photodynamic therapy (PDT) with varying success. 5-Flurouracil was not used, as the patient refused daily application of the cream and long treatment courses. Because of reduced compliance of our patient in the past and because of his other chronic illness, we decided for physician-directed field treatment to ensure application and efficacy. Ingenol mebutate was considered and used when other therapies began to fail.
When used, cryotherapy was performed every 3 months on suspicious lesions and led to clinical remission and regression of treated spots but had no impact on the larger area of field cancerization. Side effects included oozing, crusting, inflammation, and depigmentation of the treated areas. Imiquimod was used intermittently between 2001 and 2011, both before and after transplantation, 3 times per week for 4 weeks inducing erythema, inflammation, and erosions. Clinically, field cancerization improved in the treated areas, whereas actinic keratosis tended to relapse within several months after the last application. Because of increasing numbers of intraepithelial lesions and reduced efficacy of self-medication with imiquimod, photodynamic therapy was started in November 2011. Ten cycles of PDT were performed on 5 anatomic areas of the patient's face and scalp. Despite clinical improvement, the patient was unable to tolerate the pain from PDT illumination, and he refused further PDT therapy in January 2013.
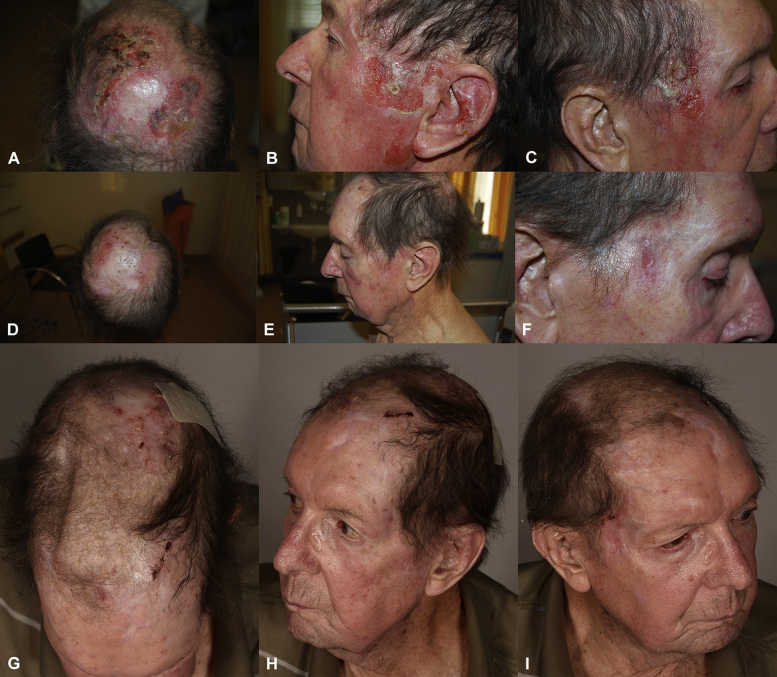
Field cancerization persisted, and in June 2013 multiple biopsies of the scalp again confirmed AKs that required treatment. Radiotherapy, while commonly used in our department for immunocompetent patients to cure large areas such as balding scalp or face from field cancerization, was not an option, as our experience shows reduced efficacy and rapid recurrence of epithelial invasive skin cancers. PDT would have been a suitable option, but the patient refused further attempts of PDT because of pain. Without registered modalities available in this case, we searched for nonregistered alternative treatments to improve his field cancerization. Based on previous experience with a limited number of patients, we chose to apply ingenol mebutate (IM), 150 μg once, as a physician-directed treatment on a large area of field cancerization, mimicking the application of PDT. A single, physician-directed application in March 2014 extended the treatment area beyond the registered protocol of 75 cm2 to a larger area including the occiput, vertex, temporal fossa triangularis bilaterally, and the left ear. A pronounced reaction with erythema, erosions, and hemorrhagic, yellowish crusting developed on the next day (Fig 1, A-C). Erythema and erosions were present at follow-up 1 month later (Fig 1, D-F). At the last follow-up in September 2014, slightly erythematous macules with few interspersed crusts were present (Fig 1, G-I). We observed a full clinical remission of AK with good cosmetic outcome. The patient never reported any fever, chills, fatigue or malaise, during or after ingenol mebutate treatment. C-reactive protein never exceeded a level of 3.6 mg/L after IM cycles. Glomerular filtration rate remained at 30 mL/min or greater after all IM treatment cycles. No other signs of graft rejection were noted.
Fig 1.
Reaction to ingenol mebutate. Field cancerization of the skin was addressed with three cycles of ingenol mebutate (IM). Here, the third cycle of IM is depicted in an area larger than the registered 25 cm2. The first day after applying 150 μg IM gel showed a pronounced local reaction with erythema, erosions, and hemorrhagic, yellowish crusting of occiput and vertex (A), with erythema and erosions of the left temporal fossa triangularis and the left ear (B), as well of the right temporal fossa (C). Five weeks after stopping the third cycle of 150 μg IM gel the erythema and erosions had improved significantly on occiput and vertex (D), with slight erythema with no erosions of the left temporal fossa triangularis and the left ear (E) and atrophied macula with slight erythema and a central superficial erosion of the right temporal fossa (F). Twenty five weeks after stopping the third cycle of 150 μg IM gel, a whitish macule remained on the vertex with sporadic crusts (G), with complete clinical remission of AK of left temporal fossa triangularis and the left ear (H), and atrophied macula of right temporal fossa triangularis (I) without erythema in all treated areas.
Discussion
Actinic keratoses are proliferations of atypical keratinocytes in the epidermis caused by chronic UV radiation exposure. The presence of 2 or more AKs, SCC, or BCC on photo-damaged skin constitutes the diagnosis of field cancerization and increases the risk for subsequent invasive nonmelanoma skin cancer (NMSC).1 Although AKs are frequent in the general population, OTRs are at higher risk for AKs because of their exposure to immunosuppressive medications. These patients have SCC 65 to 250 times more frequently than those in the general population.2 The aim in treating AKs is to prevent the progression to invasive SCC. Preventive measures involve avoidance of unnecessary exposure to UV light and regular use of sunscreen to prevent or delay the development of NMSC in OTR.2 Treatment approaches to AKs can be divided into lesion directed (LD) or field directed (FD). LD therapies are preferred in patients with few isolated lesions and without elevated risk for invasive NMSC development. These LD therapies include local destruction, curettage, cryotherapy, or excision. FD therapies target clinically visible lesions and preclinical lesions with keratinocyte changes in the skin around the visible lesions. Topical treatments comprise 5-fluorouracil, diclofenac, imiquimod, topical retinoids, or ingenol mebutate. Other FD treatment modalities include PDT, radiotherapy, laser, chemical peels, dermabrasion, or skin grafting.3
Most topical therapies for AKs typically induce inflammation but must be applied for long periods of weeks to months. Topical IM has been registered in the United States and the European Union since 2012. IM is found to have equal efficacy against AK after only 3 days of application. Furthermore, IM appears to have a dual mode of action: (1) rapid necrosis within hours of application and (2) specific neutrophil-mediated, antibody-dependent cellular cytotoxicity within days.4 IM gel is available in 2 formulations: 150 μg/g used once daily for 3 consecutive days to the face or scalp or 500 μg/g used once daily on the trunk or extremities for 2 consecutive days. Application of IM results in complete cure rates for the field in the range of 34.1% on trunk and extremities and 42.2% on the face and scalp comparing to vehicle in the normal population. Local skin reactions are the most frequent adverse events with IM.5 Recent postmarketing reports of severe allergic reactions and herpes zoster after IM have led to a label change for IM by the US Food and Drug Administration. Such inflammatory reactions, however, can be welcome in the poorly responsive population of OTR with large areas of field cancerization. We believe that IM may show a similar benefit with tolerable safety in the population of OTR at high risk for AK and SCC development. Previous experience with topical imiquimod, an immunomodulator, has proven that these substances are safe in OTR without any indication of damage to the grafted organ.6 Recently, a US Food and Drug Administration–approved clinical review (Study number PEP005) of the topical treatment for AKs with IM 0.05% showed that a 100-cm2 treatment area is both safe and well tolerated with no increase in treatment-related adverse events compared with the treatment areas of 25, 50, or 75 cm2.7 A prospective trial will address the use of IM and its potential in OTR starting in 2015. Until then, IM seems a good second-line choice. Once data on the safety in OTR are available, IM may well be found suitable for first-line use in field cancerization.
The treatment of field cancerization is challenging, in particular in the heavily affected population of OTR. Our case illustrates that IM may be used on an area larger than registered with an effect resembling field cancerization treatment by PDT without eliciting systemic side effects. Although a multitude of topical treatment modalities are available for use in this setting, IM can be a useful addition to the armamentarium available in OTR, in particular as a physician-directed single application in a large field.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Hu B., Castillo E., Harewood L. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell. 2012;149:1207–1220. doi: 10.1016/j.cell.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Euvrard S., Kanitakis J., Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 3.Ferrandiz L., Ruiz-de-Casas A., Trakatelli M. Assessing physicians' preferences on skin cancer treatment in Europe. Br J Dermatol. 2012;167(Suppl 2):29–35. doi: 10.1111/j.1365-2133.2012.11084.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosen R.H., Gupta A.K., Tyring S.K. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. JAmAcad Dermatol. 2012;66:486–493. doi: 10.1016/j.jaad.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 5.Martin G., Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. JAm Acad Dermatol. 2013;68:S39–S48. doi: 10.1016/j.jaad.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich C., Bichel J., Euvrard S. Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br J Dermatol. 2007;157:25–31. doi: 10.1111/j.1365-2133.2007.08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku J. NDA 202833 PICATO (ingenol mebutate gel, PEP005 Gel) 2011