Abstract
Pregnancy is a complex state where changes in maternal physiology have evolved to favor the development and growth of the placenta and the fetus. These adaptations may affect preexisting disease or result in pregnancy-specific disorders. Similarly, variations in physiology may alter the pharmacokinetics or pharmacodynamics that determines drug dosing and effect. It follows that detailed pharmacologic information is required to adjust therapeutic treatment strategies during pregnancy. Understanding both pregnancy physiology and the gestation-specific pharmacology of different agents is necessary to achieve effective treatment and limit maternal and fetal risk. Unfortunately, most drug studies have excluded pregnant women based on often-mistaken concerns regarding fetal risk. Furthermore, over two-thirds of women receive prescription drugs while pregnant, with treatment and dosing strategies based on data from healthy male volunteers and non-pregnant women, and with little adjustment for the complex physiology of pregnancy and its unique disease states. This review will describe basic concepts in pharmacokinetics and their clinical relevance and highlight the variations in pregnancy that may impact the pharmacokinetic properties of medications.
Keywords: Pregnancy, Pharmacology, Pharmacokinetics, Drug transport
Introduction
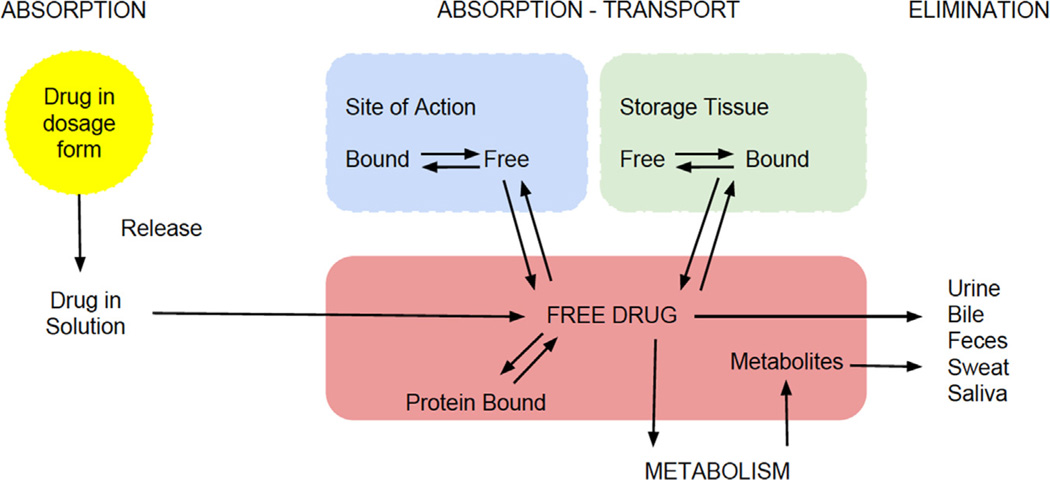
Various medications are used during pregnancy despite a lack of data in this unique setting.1,2 Treatment and dosing strategies are based on standard adult doses despite the fact that dosing, safety, and efficacy were determined in healthy, and mostly male, individuals.3 In some instances, treatment may be withheld from pregnant women due to concerns about maternal or fetal safety. Recent studies in clinical therapeutics in pregnancy suggest a myriad of changes that affect the pharmacologic properties of drugs. A fundamental concept in pharmacology is that a drug must reach the target tissues at sufficient concentration to exert its therapeutic effects without causing significant adverse events. Pharmacokinetics (PK) describes the time course of drug concentration in the body. It involves the evaluation of drug absorption, distribution, metabolism, elimination, and transport (Fig.). Various computational models are commonly used to estimate drug PK parameters, but they are beyond the scope of this article. Still, understanding drug-specific PK properties and gestation-specific variations allows for improved treatment and dosing strategies, which can improve treatment efficacy and limit maternal and fetal risks. As such, this review will focus more on the clinical relevance and application of PK parameters and less on the mathematical methods for parameter estimation.
Fig.
The pharmacokinetic process.
Drug absorption
Drug absorption is the movement of drug from the site of administration into the systemic circulation. Drug absorption is commonly characterized as bioavailability, the fraction or percentage of active drug medication that reaches the systemic circulation intact by any route.4 Drugs that are administered intravascularly are 100% bioavailable since they are delivered directly into the bloodstream. However, most drugs are administered extravascularly and are expected to act systemically. For this reason, absorption and bioavailability are a prerequisite for pharmacologic action of a drug. Delays or drug loss during absorption may contribute to variation in drug response and side effects and may lead to treatment failure. Intramuscular and subcutaneous administration may lead to a delay in time to reach maximal concentration but has less effect on bioavailability. Increased local blood flow and vasodilation are thought to facilitate drug absorption following intramuscular or subcutaneous drug delivery, although specific drug data are lacking. The greatest variability in drug absorption is seen when a medication is administered orally. For orally administered medications, the bioavailability is affected by the amount absorbed across the intestinal epithelium, as well as first-pass metabolism as the drug crosses the intestine and the liver on its way to the systemic circulation. Stomach pH, food, gut transit time, gut metabolism, uptake, and efflux transport processes may impact oral drug bioavailability.
Nausea and vomiting in early pregnancy may decrease the amount of drug available for absorption following oral administration. Therefore, oral medications should be administered when nausea is minimal. Gastric acid production is also decreased during pregnancy, whereas mucus secretion is increased, leading to an increase in gastric pH.5,6 These changes can increase ionization of weak acids (e.g., aspirin) and reduce their absorption, and weak bases (e.g., caffeine) will diffuse more readily since they will be primarily unionized. In addition, the slower intestinal motility and decreased gastric acid secretion in pregnancy could alter drug absorption and oral bioavailability. However, no confirmatory evidence validates these assumptions. In fact, studies on β-lactam antibiotics used for asymptomatic bacteruria found no difference in the bioavailability of the drugs (given orally and intravenously) between late pregnancy and postpartum.7,8 Meanwhile, increased cardiac output and intestinal blood flow may allow for increased drug absorption overall. Taken together, these data suggest that gastrointestinal changes during pregnancy have an overall minimal effect on the bioavailability and therapeutic effect of most oral drugs, especially with repeated dosing. Little information is available on changes in drug absorption for other routes of administration during pregnancy.
Drug distribution
Distribution describes the reversible transfer of a drug between different locations following its entry into the systemic circulation. The volume of distribution (Vd) is used to indicate how extensively a systemic dose of medication is ultimately dispersed throughout the body. It is a theoretical volume that an administered drug would occupy if it were uniformly distributed at a concentration observed in plasma. The Vd is important to determine the loading dose of a drug needed to achieve a certain therapeutic concentration. Drugs that predominantly remain within the vascular system will have a Vd estimate close to plasma volume, whereas drugs that are not bound to any proteins in the body will have a Vd estimate close to total body water. Drugs that are highly bound to tissues, with a small proportion remaining in the intravascular space, will have a very high Vd. By comparison, drugs that are highly bound to plasma proteins and/or have a large molecular weight will tend to concentrate intravascularly and will have a small Vd. A drug’s volume of distribution is useful in estimating the dose required to achieve a given plasma concentration. Drug distribution is influenced by various factors including tissue perfusion, tissue binding, lipid solubility, and plasma protein binding. Variations in Vd mainly affect the plasma concentration of the drug, which can directly impact a drug’s therapeutic and adverse effects.
Cardiovascular changes during pregnancy include an increase in cardiac output starting in early pregnancy, plateauing by 16 weeks of gestation ~7 L/min and remaining elevated until delivery.9 A parallel increase is also noted for stroke volume starting at 20 weeks of gestation and a gradual increase occurs with maternal heart rate reaching 90 beats per min at rest in the third trimester.10 Pregnancy is also marked by ~42% increase in plasma volume, reaching over 3.5 L at 38 weeks of gestation, with parallel increases in total body water and in all body fluid compartments.9 Expanded extracellular volume and total body water will increase volume of distribution for hydrophilic drugs, leading to lower plasma concentrations. In addition, maternal body fat expands by approximately 4 kg, increasing the volume of distribution for lipophilic drugs. However, little information is available to assess contribution of adipose tissue to altered drug disposition during pregnancy.
On the other hand, plasma protein binding of drugs decreases during pregnancy due to reduced concentrations of both albumin and alpha 1-acid glycoprotein.11–13 In normal pregnancy, albumin concentrations decrease on average by 1% at 8 weeks, 10% at 20 weeks, and 13% at 32 weeks.14 Certain pathophysiologic conditions can lead to even lower albumin levels. Decreased protein binding leads to higher concentrations of free drug (for drugs that have limited clearance) and favors more distribution to tissues. These changes can be clinically significant for certain drugs. For example, for phenytoin and tacrolimus, efficacy and toxicity are expected to be related to unbound drug concentration in plasma. During pregnancy, both the drugs exhibit an increased unbound fraction due to lower albumin concentrations and increased clearance.15,16 A dose titration strategy based on maintaining total blood/plasma concentration in the therapeutic range can lead to increased free drug concentrations and increase the likelihood of drug-related toxicity. In pregnancy, a more thorough approach would be to monitor free drug concentrations and adjust drug dosing to maintain the unbound concentration within its therapeutic range.
Gestation-specific changes also include an increase in uterine perfusion and the addition of the feto-placental compartment. Blood flow to the uterus increases 10-fold from 50 to 500 mL/min at term. In general, small-molecular-weight and lipophilic drugs readily cross the placenta. The fetus and the amniotic fluid can act as additional compartments, leading to increased drug accumulation and an apparent increase in volume of distribution of certain drugs.
Drug metabolism
Drug metabolism involves chemical modification of a drug through specialized enzymatic systems. For some medications, administered as inactive pro-drugs, metabolism is necessary to convert the drug into an active compound. For most drugs, metabolism leads to loss of drug activity. The liver accounts for the metabolism of a vast majority of drugs. Other organs including the intestine and the placenta can also contribute to the clearance of certain drugs. Metabolic enzyme activity is highly variable, affected by ethnicity, gender, age, and enzyme polymorphisms. Certain enzymes are involved in the metabolism of numerous drugs and create a potential for co-administered medications to impact drug clearance.
Clearance is a major pharmacokinetic parameter of a drug; it determines drug exposure as measured by the area under the plasma concentration vs time curve17 and a body’s overall ability to eliminate a drug. Systemic clearance of a drug is the sum of all the clearances by various organs. Clearance is the volume of blood/plasma that is completely cleared off the drug in a unit of time. The clearance of a drug in the liver is determined by hepatic blood flow and the extraction ratio of the drug in the liver. The extraction ratio (ER) refers to the proportion of a drug taken up from the hepatic arterial circulation into hepatocytes, making it available for subsequent metabolism. For high ER drugs (e.g., morphine and propranolol), overall hepatic elimination is limited only by hepatic perfusion (blood flow). In contrast, hepatic clearance of low ER drugs (e.g., diazepam, fluoxetine, or caffeine) is limited by intrinsic metabolic capacity of hepatic cells and the unbound fraction of the drug in plasma, and it would be changed little by changes in hepatic perfusion.
Hepatic drug metabolism includes phase I (oxidation, reduction, or hydrolysis) reactions that introduce more polar or reactive moieties into drug molecules, followed in many cases by phase II (conjugation) reactions to glucuronic acid, sulfate, or other moieties that favor excretion into urine or bile. Oxidative phase I reactions are predominantly carried out by the cytochrome P450 (CYP) family of enzymes that differ in their substrate specificity. The activities of CYP3A4 (50–100%), CYP2A6 (54%), CYP2D6 (50%), and CYP2C9 (20%) are all increased during pregnancy (Table 1).18–22 Changes in CYP3A4 activity lead to increased metabolism of drugs such as glyburide, nifedipine, and indinavir. By contrast, some CYP isoforms demonstrate decreased activity during pregnancy. CYP1A2 and CYP2C19 appear to undergo a gradual decrease in activity with advancing gestation,17,23,24 though with uncertain effects on drug therapy. The activity of phase II enzymes, including uridine 5’-diphosphate glucuronosyltransferases (UGTs), is also altered during pregnancy, with a 200% increase in UGT1A4 activity during the first and second trimesters and a 300% increase during the third trimester.25 This change leads to lower concentrations of UGT1A4 substrates such as lamotrigine, leading directly to poorer seizure control with advancing gestation in the absence of appropriate dose titration.26 The effects of pregnancy on enzyme activity can also vary with maternal genotype. A recent study on the PK of nifedipine, used for tocolysis, noted differences in drug clearance due to genetic variability in a specific allele of the CYP3A5 coding gene.27 Similarly, methadone metabolism varied with the specific genotype of CYP2B6.28 Enzyme activity varies with ethnicity, gender, age, and certain disease states that are unrelated to pregnancy. Commonly, enzymes have numerous substrates, and different drugs may undergo metabolism by several enzymes. This overlap may lead to changes in metabolic activity when certain medications are co-administered. In primary cultures of human hepatocytes, 17-hydroxyprogesterone caproate (17-OHPC) (a drug used to prevent preterm delivery) modestly increased the activity of CYP2C19. It follows that dosage of CYP2C19 substrates, for example, tricyclic antidepressants, proton pump inhibitors, and propranolol, may have to be increased in patients on 17-OHPC.29
Table 1.
Pregnancy-induced physiologic changes during near term.
| System (reference) | Parameter | Non-pregnant | Pregnant |
|---|---|---|---|
| Cardiovascular64,71,72 | Cardiac output [L/min] | 4.0 | 6.0 |
| Heart rate [beats per min] | 70 | 90 | |
| Stroke volume [mL] | 65 | 85 | |
| Plasma volume [L] | 2.6 | 3.5 | |
| Respiratory73,74 | Total lung capacity [mL] | 4225 | 4080 |
| Residual volume [mL] | 965 | 770 | |
| Tidal volume [mL] | 485 | 680 | |
| Liver75 | Portal vein blood flow [L/min] | 1.25 | 1.92 |
| Hepatic artery blood flow [L/min] | 0.57 | 1.06a | |
| Renal76 | Glomerular filtration rate [mL/min] | 97 | 144 |
| Serum creatinine [mg/dL] | 0.7 | 0.5 |
Not statistically significant.
Changes in drug metabolism can have implications for drug dosages in pregnancy. For drugs with a narrow therapeutic window, an increased clearance during pregnancy can lead to sub-therapeutic concentrations and worsening disease control. Conversely, to avoid increased toxicity, drug doses may need to be adjusted in the postpartum period, when pregnancy-related metabolic enzyme activity changes resolve.
Drug elimination
Renal drug excretion depends on GFR, tubular secretion, and reabsorption. GFR is 50% higher by the first trimester and continues to increase until the last week of pregnancy.30 If a drug is solely excreted by glomerular filtration, its renal clearance is expected to parallel changes in GFR during pregnancy. For example, cefazolin and clindamycin exhibit increased renal elimination during pregnancy.7,31 Despite a uniform increase in GFR during pregnancy, differences in renal tubular transport (secretion or reabsorption) can result in differing effects on renally cleared drugs.32,33 Specifically, the clearance of lithium is doubled during the third trimester compared to preconception.34 By comparison, the clearance of digoxin, which is 80% renally-cleared, is merely 20–30% higher during the third trimester compared to postpartum.35,36 Furthermore, the clearance of atenolol is only 12% higher across pregnancy.18,37 Such variations in drug clearances limit generalization about the effect of pregnancy on renally eliminated drugs and point to important but less-known gestational changes in renal tubular transporters.
Drug transport
Drug transporters are widely expressed in several organs (Table 2). For example, intestinal luminal transporters can affect drug absorption from the gastrointestinal tract—those in hepatic sinusoids determine drug uptake into hepatocytes where they may undergo biotransformation, transporters in biliary canaliculi govern secretion into bile, and transporters on both the apical and the basolateral surfaces of renal epithelial cells govern tubular secretion and reabsorption. Together, their distribution, substrate specificity, and activities are important determinants governing drug absorption, excretion, and, in many cases, the extent of drug entry into target organs. Knowledge of drug transporter expression and function is necessary for a complete understanding of drug absorption, distribution, elimination, and effect. In addition, fetal development is dependent upon the transport of nutrients by the placenta toward the fetal side and that of products of fetal metabolism for elimination by the mother.38 The placenta produces and secretes hormones that affect the maternal physiology and endocrine state.35,39 The transport role is mediated by the syncytiotrophoblasts, the functional cell of the placenta. These cells have a polarized plasma membrane consisting of a brush border at the maternal side and a border membrane on the fetal side. Compounds transported between the mother and the fetus are carried by the maternal circulation within the uterine vasculature directly through the intervillous spaces and then the syncytiotrophoblasts. Thereafter, blood flows from the fetal side of the placental villi through the fetal capillary endothelium to reach the fetal circulation. Most xenobiotics cross the placental barrier by simple diffusion. Protein binding, degree of ionization, lipid solubility, and molecular weight can affect placental transport. In fact, small, lipid-soluble, ionized, and poorly protein-bound molecules cross the placenta easily. For other substrates, the placenta facilitates maternal to fetal transport through the polarized expression of various transporters.40 Transporters enable transport of specific endogenous substrates (such as cytokines, nucleoside analogs, and steroid hormones); however, exogenous compounds with similar structures may also interact with them (Table 3).
Table 2.
Pregnancy-induced enzyme-specific changes.
| Enzyme (references) | Pregnancy-induced change | Potential substrates in obstetrics |
|---|---|---|
| CYP3A419,20,77,78 | Increased | Glyburide, nifedipine, and indinavir |
| CYP2D677,79 | Increased | Metoprolol, dextromethorphan, paroxetine, duloxetine, fluoxetine, and citalopram |
| CYP2C918,80 | Increased | Glyburide, NSAIDs, phenytoin, and fluoxetine |
| CYP2C1918,80 | Decreased | Glyburide, citalopram, diazepam, omeprazole, pantoprazole, and propranolol |
| CYP1A217,23,77,81 | Decreased | Theophylline, clozapine, olanzapine, ondansetron, and cyclobenzaprine |
| UGT1A482–84 | Increased | Lamotrigine |
| UGT1A1/925 | Increased | Acetaminophen |
| NAT217,24,85 | Decreased | Caffeine |
Table 3.
Major drug transporters, their location, and common substrates.
| Transporter | Tissues/cells | Selected substrates | Selected inhibitors |
|---|---|---|---|
| P-gp | Intestinal enterocytes, kidney proximal tubule, hepatocytes, brain endothelial cells, and placenta | Glyburide, digoxin, loperamide, ritonavir, and St. John's Wort | Verapamil and cyclosporine |
| BCRP | Intestinal enterocytes, hepatocytes, kidney proximal tubule, brain endothelial cells, placenta, and mammary glands | Glyburide, statins, porphyrins, and methotrexate | Oestrone and 17 β-estradiol |
| MRP2 | Hepatocytes, kidney proximal tubule, and enterocytes (luminal) | Glutathione and glucuronide conjugates and methotrexate | Cyclosporine and efavirenz |
| MRP3 | Hepatocytes, kidney proximal tubule, and enterocytes (basolateral) | Glyburide, estradiol 17 β-glucuronide, methotrexate, and glucuronate conjugates | Delavirdine and efavirenz |
| MRP4 | Kidney proximal tubule, choroid plexus, hepatocytes, and platelets | Furosemide, adefovir, tenofovir, and methotrexate | Celecoxib and diclofenac |
| MDR3 | Hepatocytes | Digoxin | Verapamil and cyclosporine |
| OAT1 | Kidney proximal tubule and placenta | Acyclovir, zidovudine, lamivudine, adefovir, and cidofovir | Probenecid and novobiocin |
| OAT3 | Kidney proximal tubule, choroid plexus, and blood–brain barrier | NSAIDs, cefaclor, ceftizoxime, and furosemide | Probenecid and novobiocin |
| OCT1 | Hepatocytes and endothelial cells | Metformin, N-methylpyridinium, pindolol, procainamide, ranitidine, and amantadine | Quinine, quinidine, and disopyramide |
| OCT2 | Kidney proximal tubules and peripheral neurons | Metformin and N-methylpyridinium | Cimetidine, cetirizine, and quinidine |
| OATP2B1 | Hepatocytes and endothelial cells | Glyburide, statins, and fexofenadine | Rifampicin and cyclosporine |
| MATE1 | Kidney proximal tubule, liver, and skeletal muscle | Metformin and N-methylpyridinium | Cimetidine, quinidine, and procainamide |
| MATE2-K | Kidney proximal tubule | Metformin and N-methylpyridinium | Cimetidine, quinidine, and pramipexole |
| PEPT1 | Intestinal enterocytes and kidney proximal tubule | Cephalexin, cefadroxil, valacyclovir, enalapril, and captopril | Glycyl-proline |
| PEPT2 | Kidney proximal tubule, choroid plexus, and lung | Cephalexin, valacyclovir, enalapril, and captopril | Zofenopril and fosinopril |
A number of placental drug transporters have been identified, including the family of multi-drug resistance protein (MRPs). Phosphoglycoprotein (P-gp) and breast cancer resistance protein (BCRP) are the most studied so far and will be discussed in greater detail. P-gp is expressed on the apical microvillous surface, whereas BCRP is mostly identified on the basolateral membrane and fetal blood vessels.41–44 Their polarized distribution may reflect a difference in their role. Transporters on the apical membrane are thought to allow selective substrates to be transmitted to the fetus and hence may protect the fetus by extruding harmful xenobiotics. Both the transporters have a wide array of substrates. Those of P-gp include endogenous compounds such as cortisol, aldosterone, and bilirubin as well as various drugs such as antibiotics, antiretrovirals, and steroids.45,46 Substrates of BCRP include antibiotics, antiretrovirals, calcium channel blockers, estrogen, and porphyrins.45,47,48 These transporters have a number of overlapping substrates for which they have differing affinities.49,50
A limited number of studies have examined the gestational changes of placental drug transporters and decrease near term.51,52 Investigations of BCRP changes have yielded inconsistent results with reported increase, decrease, or unchanged expression with advancing gestation.53,54 These differences may be related to the variation in tissues used in each study. Furthermore, evidence on the regulation of placental drug transporters expression is scarce. Both estrogen and progesterone appear to increase expression of P-gp and BCRP in trophoblast cell lines.46,55,56 In vivo studies describe an increase in maternal and fetal glucocorticoids with advancing gestation in parallel with a decrease in P-gp. Surprisingly, studies investigating a possible direct link demonstrate that prolonged exposure to dexamethasone increased P-gp and decreased BCRP expression in mice.57,58 By comparison, treatment of trophoblast cells with inflammatory cytokines or simulated infection in pregnant rats resulted in decreased P-gp and BCRP expression.59,60 Also, P-gp and BCRP expression is lower in preterm placentas and placentas from women with preeclampsia compared to term placentas from uncomplicated pregnancies, suggesting a role for hypoxia in mediating these transporters.57
Selective serotonin reuptake inhibitors (SSRIs) including fluoxetine, sertraline, and paroxetine inhibit P-gp in vitro.61 Along with a decrease in P-gp expression late in gestation, an inhibition of its function may result in fetal and maternal consequences. The most recent guidelines for treatment of depression during pregnancy recommend using the lowest effective dose of SSRIs.62 Maternal SSRI use in the first and the third trimesters has been linked to congenital anomalies and neonatal complications, respectively.63 A clear link between inhibition of P-gp, neonatal pulmonary hypertension or tachypnea, and prenatal exposure to SSRIs remains to be determined. Anti-seizure drugs appear to exhibit an inhibitory effect on carnitine placental transport.64,65 Carnitine deficiency has been linked to apnea, cardiac arrest, and cardiac hypertrophy.66 Carnitine is mainly actively transported through two transporters.66–68 One of these transporters—carnitine/organic cation transporter (OCTN2)—is located on the apical membrane of the syncytiotrophoblasts and is inhibited by some anti-seizure drugs such as valproic acid and phenytoin.69,70
Pathophysiologic states may also alter transporter expression. P-gp and BCRP expression was lower in placentas from women with preeclampsia compared to term placentas from uncomplicated pregnancies.57 It is unknown whether transporter expression and activity are altered further in other conditions affecting pregnancy.
Conclusion
Pregnancy involves various changes in maternal physiology and disease. It logically follows that drug disposition and effects are altered in pregnancy. Historically, concerns about fetal safety have limited pharmacotherapy during pregnancy and have hampered drug studies during pregnancy. Although these concerns have validity, pregnant women require medications for medical disorders, and pregnancy does not eliminate the need for therapy. Recent studies on pharmacology in pregnancy highlight the complexity of drug distribution and response in light of the dynamic process of gestation. To extrapolate drug dosage and expected responses from non-pregnant populations is inappropriate and may cause harm for pregnant women. Rather, a structured approach to study the pharmacokinetic and pharmacodynamic properties of drugs used in pregnancy should be followed. In the absence of data specific to a drug, close monitoring of the patient is the most logical step to optimize drug therapy in pregnant subjects.
Acknowledgments
Maisa Feghali is supported by the National Institutes of Heath through Grant Number KL2 TR000146. Steve Caritis and Raman Venkataramanan are supported in part by the Obstetric-Fetal Pharmacology Research Centers, Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 5 U10 HDO47905.
REFERENCES
- 1.Chambers CD, Polifka JE, Friedman JM. Drug safety in pregnant women and their babies: ignorance not bliss. Clin Pharmacol Ther. 2008;83(1):181–183. doi: 10.1038/sj.clpt.6100448. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51.e51–51.e58. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas SH, Yates LM. Prescribing without evidence—pregnancy. Br J Clin Pharmacol. 2012;74(4):691–697. doi: 10.1111/j.1365-2125.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LZ B. Pharmacokinetics: absorption, distribution, and elimination. In: BG K, editor. Basic and Clinical Pharmacology. Appleton & Lange: East Norwalk: Conn; 1992. [Google Scholar]
- 5.Vasicka A, Lin TJ, Bright RH. Peptic ulcer and pregnancy, review of hormonal relationships and a report of one case of massive gastrointestinal hemorrhage. Obstet Gynecol Surv. 1957;12(1):1–13. doi: 10.1097/00006254-195702000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Waldum HL, Straume BK, Lundgren R. Serum group I pepsinogens during pregnancy. Scand J Gastroenterol. 1980;15(1):61–63. doi: 10.3109/00365528009181433. [DOI] [PubMed] [Google Scholar]
- 7.Philipson A. Pharmacokinetics of ampicillin during pregnancy. J Infect Dis. 1977;136(3):370–376. doi: 10.1093/infdis/136.3.370. [DOI] [PubMed] [Google Scholar]
- 8.Philipson A, Stiernstedt G, Ehrnebo M. Comparison of the pharmacokinetics of cephradine and cefazolin in pregnant and non-pregnant women. Clin Pharmacokinet. 1987;12(2):136–144. doi: 10.2165/00003088-198712020-00004. [DOI] [PubMed] [Google Scholar]
- 9.Qasqas SA, McPherson C, Frishman WH, Elkayam U. Cardiovascular pharmacotherapeutic considerations during pregnancy and lactation. Cardiol Rev. 2004;12(4):201–221. doi: 10.1097/01.crd.0000102420.62200.e1. [DOI] [PubMed] [Google Scholar]
- 10.Pirani BB, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. J Obstet Gynaecol Br Commonw. 1973;80(10):884–887. doi: 10.1111/j.1471-0528.1973.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheung CK, Lao T, Swaminathan R. Urinary excretion of some proteins and enzymes during normal pregnancy. Clin Chem. 1989;35(9):1978–1980. [PubMed] [Google Scholar]
- 12.Erman A, Neri A, Sharoni R, et al. Enhanced urinary albumin excretion after 35 weeks of gestation and during labour in normal pregnancy. Scand J Clin Lab Invest. 1992;52(5):409–413. doi: 10.3109/00365519209088376. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi M, Ueda Y, Hoshimoto K, et al. Changes in urinary excretion of six biochemical parameters in normotensive pregnancy and preeclampsia. Am J Kidney Dis. 2002;39(2):392–400. doi: 10.1053/ajkd.2002.30561. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MM, Scott JM, McPartlin JM, Fernandez-Ballart JD. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am J Clin Nutr. 2002;76(3):614–619. doi: 10.1093/ajcn/76.3.614. [DOI] [PubMed] [Google Scholar]
- 15.Hebert MF, Zheng S, Hays K, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95(7):908–915. doi: 10.1097/TP.0b013e318278d367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perucca E, Crema A. Plasma protein binding of drugs in pregnancy. Clin Pharmacokinet. 1982;7(4):336–352. doi: 10.2165/00003088-198207040-00004. [DOI] [PubMed] [Google Scholar]
- 17.Brazier JL, Ritter J, Berland M, Khenfer D, Faucon G. Pharmacokinetics of caffeine during and after pregnancy. Dev Pharmacol Ther. 1983;6(5):315–322. doi: 10.1159/000457332. [DOI] [PubMed] [Google Scholar]
- 18.Hebert MF, Easterling TR, Kirby B, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84(2):248–253. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
- 19.Hirt D, Treluyer JM, Jullien V, et al. Pregnancy-related effects on nelfinavir-M8 pharmacokinetics: a population study with 133 women. Antimicrob Agents Chemother. 2006;50(6):2079–2086. doi: 10.1128/AAC.01596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villani P, Floridia M, Pirillo MF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br J Clin Pharmacol. 2006;62(3):309–315. doi: 10.1111/j.1365-2125.2006.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tracy TS, Venkataramanan R, Glover DD, Caritis SN National Institute for Child Health and Human Development Network of Maternal–Fetal-Medicine Unit. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Carter BL, Driscoll CE, Smith GD. Theophylline clearance during pregnancy. Obstet Gynecol. 1986;68(4):555–559. [PubMed] [Google Scholar]
- 23.Tsutsumi K, Kotegawa T, Matsuki S, et al. The effect of pregnancy on cytochrome P4501A2, xanthine oxidase, and N-acetyltransferase activities in humans. Clin Pharmacol Ther. 2001;70(2):121–125. doi: 10.1067/mcp.2001.116495. [DOI] [PubMed] [Google Scholar]
- 24.Grosso LM, Bracken MB. Caffeine metabolism, genetics, and perinatal outcomes: a review of exposure assessment considerations during pregnancy. Ann Epidemiol. 2005;15(6):460–466. doi: 10.1016/j.annepidem.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Pennell PB, Newport DJ, Stowe ZN, Helmers SL, Montgomery JQ, Henry TR. The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology. 2004;62(2):292–295. doi: 10.1212/01.wnl.0000103286.47129.f8. [DOI] [PubMed] [Google Scholar]
- 26.de Haan GJ, Edelbroek P, Segers J, et al. Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology. 2004;63(3):571–573. doi: 10.1212/01.wnl.0000133213.10244.fd. [DOI] [PubMed] [Google Scholar]
- 27.Haas DM, Quinney SK, Clay JM, et al. Nifedipine pharmacokinetics are influenced by CYP3A5 genotype when used as a preterm labor tocolytic. Am J Perinatol. 2013;30(4):275–281. doi: 10.1055/s-0032-1323590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogen DL, Perel JM, Helsel JC, et al. Pharmacologic evidence to support clinical decision making for peripartum methadone treatment. Psychopharmacology. 2013;225(2):441–451. doi: 10.1007/s00213-012-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Alshabi AM, Caritis S, Venkataramanan R. Impact of 17-alpha-hydroxyprogesterone caproate on cytochrome P450s in primary cultures of human hepatocytes. Am J Obstet Gynecol. 2014;211(4):412.e411–412.e416. doi: 10.1016/j.ajog.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 30.Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18(2):152–161. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- 31.Chamberlain A, White S, Bawdon R, Thomas S, Larsen B. Pharmacokinetics of ampicillin and sulbactam in pregnancy. Am J Obstet Gynecol. 1993;168(2):667–673. doi: 10.1016/0002-9378(93)90515-k. [DOI] [PubMed] [Google Scholar]
- 32.Allegaert K, van Mieghem T, Verbesselt R, et al. Cefazolin pharmacokinetics in maternal plasma and amniotic fluid during pregnancy. Am J Obstet Gynecol. 2009;200(2):170.e171–170.e177. doi: 10.1016/j.ajog.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 33.Muller AE, Mouton JW, Oostvogel PM, et al. Pharmacokinetics of clindamycin in pregnant women in the peripartum period. Antimicrob Agents Chemother. 2010;54(5):2175–2181. doi: 10.1128/AAC.01017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 35.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43(8):487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 36.Schou M, Amdisen A, Steenstrup OR. Lithium and pregnancy. II. Hazards to women given lithium during pregnancy and delivery. Br Med J. 1973;2(5859):137–138. doi: 10.1136/bmj.2.5859.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luxford AM, Kellaway GS. Pharmacokinetics of digoxin in pregnancy. Eur J Clin Pharmacol. 1983;25(1):117–121. doi: 10.1007/BF00544027. [DOI] [PubMed] [Google Scholar]
- 38.Hebert MF, Carr DB, Anderson GD, et al. Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol. 2005;45(1):25–33. doi: 10.1177/0091270004269704. [DOI] [PubMed] [Google Scholar]
- 39.St-Pierre MV, Serrano MA, Macias RI, et al. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1495–R1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- 40.Ushijima K, Kimura A, Inokuchi T, et al. Placental transport of bile acids: analysis of bile acids in maternal serum and urine, umbilical cord blood, and amniotic fluid. Kurume Med J. 2001;48(2):87–91. doi: 10.2739/kurumemedj.48.87. [DOI] [PubMed] [Google Scholar]
- 41.Enders AC, Blankenship TN. Comparative placental structure. Adv Drug Deliv Rev. 1999;38(1):3–15. doi: 10.1016/s0169-409x(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 42.Nagashige M, Ushigome F, Koyabu N, et al. Basal membrane localization of MRP1 in human placental trophoblast. Placenta. 2003;24(10):951–958. doi: 10.1016/s0143-4004(03)00170-x. [DOI] [PubMed] [Google Scholar]
- 43.Mylona P, Hoyland JA, Sibley CP. Sites of mRNA expression of the cystic fibrosis (CF) and multidrug resistance (MDR1) genes in the human placenta of early pregnancy: no evidence for complementary expression. Placenta. 1999;20(5–6):493–496. doi: 10.1053/plac.1999.0400. [DOI] [PubMed] [Google Scholar]
- 44.Meyer Zu Schwabedissen HE, Grube M, Heydrich B, et al. Expression, localization, and function of MRP5 (ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: effects of gestational age and cellular differentiation. Am J Pathol. 2005;166(1):39–48. doi: 10.1016/S0002-9440(10)62230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raggers RJ, van Helvoort A, Evers R, van Meer G. The human multidrug resistance protein MRP1 translocates sphingolipid analogs across the plasma membrane. J Cell Sci. 1999;112(Pt 3):415–422. doi: 10.1242/jcs.112.3.415. [DOI] [PubMed] [Google Scholar]
- 46.Yeboah D, Sun M, Kingdom J, et al. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can J Physiol Pharmacol. 2006;84(12):1251–1258. doi: 10.1139/y06-078. [DOI] [PubMed] [Google Scholar]
- 47.Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther. 2005;312(1):44–52. doi: 10.1124/jpet.104.073320. [DOI] [PubMed] [Google Scholar]
- 48.Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38(7–8):802–832. doi: 10.1080/00498250701867889. [DOI] [PubMed] [Google Scholar]
- 49.Robey RW, To KK, Polgar O, et al. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61(1):3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polgar O, Robey RW, Bates SE. ABCG2: structure, function and role in drug response. Expert Opin Drug Metab Toxicol. 2008;4(1):1–15. doi: 10.1517/17425255.4.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 52.Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95(26):15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2006;27(6–7):602–609. doi: 10.1016/j.placenta.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Gil S, Saura R, Forestier F, Farinotti R. P-glycoprotein expression of the human placenta during pregnancy. Placenta. 2005;26(2–3):268–270. doi: 10.1016/j.placenta.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 55.Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R963–R969. doi: 10.1152/ajpregu.00173.2005. [DOI] [PubMed] [Google Scholar]
- 56.Meyer zu Schwabedissen HE, Dreisbach A, Hammer E, et al. Direct mass spectrometric identification of ABCB1 (P-glycoprotein/MDR1) from the apical membrane fraction of human placenta using fourier transform ion cyclotron mass spectrometry. Pharmacogenet Genomics. 2006;16(6):385–389. doi: 10.1097/01.fpc.0000215064.83599.47. [DOI] [PubMed] [Google Scholar]
- 57.Evseenko DA, Paxton JW, Keelan JA. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos. 2007;35(4):595–601. doi: 10.1124/dmd.106.011478. [DOI] [PubMed] [Google Scholar]
- 58.Coles LD, Lee IJ, Voulalas PJ, Eddington ND. Estradiol and progesterone-mediated regulation of P-gp in P-gp overexpressing cells (NCI-ADR-RES) and placental cells (JAR) Mol Pharm. 2009;6(6):1816–1825. doi: 10.1021/mp900077q. [DOI] [PubMed] [Google Scholar]
- 59.Petropoulos S, Gibb W, Matthews SG. Effect of glucocorticoids on regulation of placental multidrug resistance phosphoglycoprotein (P-gp) in the mouse. Placenta. 2010;31(9):803–810. doi: 10.1016/j.placenta.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 60.Petropoulos S, Gibb W, Matthews SG. Glucocorticoid regulation of placental breast cancer resistance protein (Bcrp1) in the mouse. Reprod Sci. 2011;18(7):631–639. doi: 10.1177/1933719110395399. [DOI] [PubMed] [Google Scholar]
- 61.Mason CW, Buhimschi IA, Buhimschi CS, Dong Y, Weiner CP, Swaan PW. ATP-binding cassette transporter expression in human placenta as a function of pregnancy condition. Drug Metab Dispos. 2011;39(6):1000–1007. doi: 10.1124/dmd.111.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss J, Dormann SM, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, Haefeli WE. Inhibition of P-glycoprotein by newer antidepressants. J Pharmacol Exp Ther. 2003;305(1):197–204. doi: 10.1124/jpet.102.046532. [DOI] [PubMed] [Google Scholar]
- 63.Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. Br Med J. 2001;323(7307):257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010–1015. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 65.Tuccori M, Testi A, Antonioli L, et al. Safety concerns associated with the use of serotonin reuptake inhibitors and other serotonergic/noradrenergic antidepressants during pregnancy: a review. Clin Ther. 2009;31(Pt 1):1426–1453. doi: 10.1016/j.clinthera.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Wu SP, Shyu MK, Liou HH, Gau CS, Lin CJ. Interaction between anticonvulsants and human placental carnitine transporter. Epilepsia. 2004;45(3):204–210. doi: 10.1111/j.0013-9580.2004.29603.x. [DOI] [PubMed] [Google Scholar]
- 67.Siu KL, Lee WH. Maternal diclofenac sodium ingestion and severe neonatal pulmonary hypertension. J Paediatr Child Health. 2004;40(3):152–153. doi: 10.1111/j.1440-1754.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- 68.Pons R, De Vivo DC. Primary and secondary carnitine deficiency syndromes. J Child Neurol. 1995;10(suppl 2):S8–S24. [PubMed] [Google Scholar]
- 69.Tamai I, Ohashi R, Nezu J, et al. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem. 1998;273(32):20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 70.Nakanishi T, Hatanaka T, Huang W, et al. Na+- and Cl−-coupled active transport of carnitine by the amino acid transporter ATB(0,+) from mouse colon expressed in HRPE cells and Xenopus oocytes. J Physiol. 2001;532(Pt 2):297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. J Am Med Assoc. 2005;293(19):2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 72.Nordeng H, Spigset O. Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy: effects on the infant. Drug Saf. 2005;28(7):565–581. doi: 10.2165/00002018-200528070-00002. [DOI] [PubMed] [Google Scholar]
- 73.Yeomans ER, Gilstrap LC. Physiologic changes in pregnancy and their impact on critical care. Crit Care Med. 2005;33(suppl 10):S256–S258. doi: 10.1097/01.ccm.0000183540.69405.90. [DOI] [PubMed] [Google Scholar]
- 74.Clark SL, Cotton DB, Lee W, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161(6 Pt 1):1439–1442. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 75.Bonica JJ, McDonald JS. Principles and Practice of Obstetric Analgesia and Anesthesia. 2nd. Vol. 1995. Baltimore, MD: Williams & Wilkins; 1995. [Google Scholar]
- 76.Nakai A, Sekiya I, Oya A, Koshino T, Araki T. Assessment of the hepatic arterial and portal venous blood flows during pregnancy with Doppler ultrasonography. Arch Gynecol Obstet. 2002;266(1):25–29. doi: 10.1007/pl00007495. [DOI] [PubMed] [Google Scholar]
- 77.Hodge LS, Tracy TS. Alterations in drug disposition during pregnancy: implications for drug therapy. Expert Opin Drug Metab Toxicol. 2007;3(4):557–571. doi: 10.1517/17425225.3.4.557. [DOI] [PubMed] [Google Scholar]
- 78.Tamminga WJ, Wemer J, Oosterhuis B, et al. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol. 1999;55(3):177–184. doi: 10.1007/s002280050615. [DOI] [PubMed] [Google Scholar]
- 79.Claessens AJ, Risler LJ, Eyal S, Shen DD, Easterling TR, Hebert MF. CYP2D6 mediates 4-hydroxylation of clonidine in vitro: implication for pregnancy-induced changes in clonidine clearance. Drug Metab Dispos. 2010;38(9):1393–1396. doi: 10.1124/dmd.110.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McGready R, Stepniewska K, Seaton E, et al. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol. 2003;59(7):553–557. doi: 10.1007/s00228-003-0651-x. [DOI] [PubMed] [Google Scholar]
- 81.Aldridge A, Bailey J, Neims AH. The disposition of caffeine during and after pregnancy. Semin Perinatol. 1981;5(4):310–314. [PubMed] [Google Scholar]
- 82.Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding: report of the Quality Standards Subcommittee and therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1247–1255. doi: 10.1111/j.1528-1167.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 83.Harden CL, Meador KJ, Pennell PB, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): II. Teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1237–1246. doi: 10.1111/j.1528-1167.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- 84.Harden CL, Hopp J, Ting TY, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): I. Obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1229–1236. doi: 10.1111/j.1528-1167.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- 85.Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46(9):1414–1417. doi: 10.1111/j.1528-1167.2005.10105.x. [DOI] [PubMed] [Google Scholar]