Abstract
Purpose
CNS tumors are the most common second primary neoplasm (SPN) observed after childhood cancer in Britain, but the relationship of risk to doses of previous radiotherapy and chemotherapy is uncertain.
Methods
The British Childhood Cancer Survivor Study is a national, population-based, cohort study of 17,980 individuals surviving at least 5 years after diagnosis of childhood cancer. Linkage to national, population-based cancer registries identified 247 SPNs of the CNS. Cohort and nested case-control studies were undertaken.
Results
There were 137 meningiomas, 73 gliomas, and 37 other CNS neoplasms included in the analysis. The risk of meningioma increased strongly, linearly, and independently with each of dose of radiation to meningeal tissue and dose of intrathecal methotrexate. Those whose meningeal tissue received 0.01 to 9.99, 10.00 to 19.99, 20.00 to 29.99, 30.00 to 39.99 and ≥ 40 Gy had risks that were two-fold, eight-fold, 52-fold, 568-fold, and 479-fold, respectively, the risks experienced by those whose meningeal tissue was unexposed. The risk of meningioma among individuals receiving 1 to 39,40 to 69, and at least 70 mg/m2 of intrathecal methotrexate was 15-fold, 11-fold, and 36-fold, respectively, the risk experienced by those unexposed. The standardized incidence ratio for gliomas was 10.8 (95% CI, 8.5 to 13.6). The risk of glioma/primitive neuroectodermal tumors increased linearly with dose of radiation, and those who had CNS tissue exposed to at least 40 Gy experienced a risk four-fold that experienced by those who had CNS tissue unexposed.
Conclusion
The largest-ever study, to our knowledge, of CNS tumors in survivors of childhood cancer indicates that the risk of meningioma increases rapidly with increased dose of radiation to meningeal tissue and with increased dose of intrathecal methotrexate.
INTRODUCTION
A serious consequence of treatment for childhood cancer is the development of second primary neoplasms (SPNs).1 SPNs of the CNS are the most frequent types of SPNs observed in Britain.2 An increased risk of such neoplasms after treatment with cranial irradiation has been reported.3–6
It is of particular interest to evaluate risks of brain tumor among individuals exposed during childhood, because the risk appears to be higher for that population than for those individuals exposed during adulthood.7 Survival after CNS SPNs is generally poor, particularly after second primary gliomas, although survival is somewhat better after second primary meningiomas.8
CNS SPNs are mainly meningiomas and gliomas. Meningiomas may develop as a result of cranial irradiation,3,5 including irradiation from atomic bombs.9 The effect of cranial irradiation on the risk of subsequent gliomas has been investigated, but the dose response appears weaker than for meningiomas.3,4 Possible effects of chemotherapy on the risk of CNS SPNs are poorly understood.4,10
Two published studies have investigated the dose-response relationship between radiation and risk of CNS SPNs in survivors of childhood cancer.3,4 The larger study, carried out as part of the Childhood Cancer Survivor Study (CCSS),had less than half the number of CNS SPNs available to this study.3
We carried out the largest-ever population-based cohort and nested case-control study, to our knowledge, to investigate the risk of CNS SPNs in survivors of childhood cancer and to relate this risk to treatment and genetic susceptibility factors.
METHODS
Tumor localization and radiation dosimetry case-control study methods information is described in the Appendix (online only).
British Childhood Cancer Survivor Study
The British Childhood Cancer Survivor Study (BCCSS)11 is a population-based cohort study of late-treatment toxicities in 17,980 individuals in Britain diagnosed with cancer when they were less than 15 years of age, between 1940 and 1991, who survived at least 5 years from diagnosis. Survivors at least 16 years of age and contactable through their general practitioners were sent a questionnaire (N = 13,211). Ethical approval was obtained from a multicenter research ethics committee and from every local research ethics committee in Britain (n = 212).
The BCCSS cohort was linked to the National Health Service Central Registers (NHSCR). Such linkage of the entire population-based cohort with the national population-based cancer and death registration systems provides a means of informing the BCCSS when a survivor develops an SPN or dies.
Ascertainment, Definition, Confirmation, and Classification of CNS SPNs
Ascertainment of SPNs was first obtained from population-based record linkage through NHSCR. However, for survivors who had completed a questionnaire, SPN diagnosis also was crossed-checked with information provided by the survivor in a questionnaire. This resulted in the identification of a small number of additional meningiomas that were reported on the questionnaires but that were not ascertained by NHSCR. All cases, irrespective of the sources of ascertainment, were included in the case-control study. SPN status was confirmed principally from histopathology reports and occasionally from radiologic reports through writing to clinicians and pathologists. For survivors of a first primary CNS glioma who developed a potential CNS SPN that was also a glioma, all relevant diagnostic reports were considered by an international expert in pediatric neuropathology (D.E.) to determine whether they represented two separate primary neoplasms. A number of potential second primary gliomas were excluded in this way.
SPNs were coded to the International Classification of Diseases of Oncology (ICD-O-3) codes.12 Cases were BCCSS cohort members who developed an SPN of any histology or behavior (ICD-O-3 fifth digit code 0-3) with a primary site in the CNS, meninges, or intracranial endocrine glands (ICD-O-3: C70.0 to 72.9, C75.1 to 75.3).
SPNs were grouped into five categories: meningioma (ICD-O-3 codes 9530 to 9539); glioma and other neuroepithelial neoplasms (ICD-O-3 codes 9380 to 9523, excluding codes 9470 to 9473); primitive neuroectodermal neoplasms (ICD-O-3 codes 9470 to 9473); schwannomas (ICD-O-3 codes 9560 to 9561); and other/unclassified CNS neoplasms.
Numbers Included in Cohort and Case-Control Study
All confirmed benign and malignant CNS SPNs occurring after 5-year survival and before December 31, 2002, were included in the cohort and nested case-control study (N = 247). Of the 247 SPNs in the cohort study, 243 were matched and included in the case-control study. The remaining four were never matched to a control because of lost or destroyed records for the case (n = 2) or registration after completion of data collection (n = 2).
Matching Criteria for Case-Control Study
A single control was randomly selected from the entire underlying population-based BCCSS cohort and was matched to each case on the following criteria: age at original cancer diagnosis in 3-year age bands (0 to 2, 3 to 5, 6 to 8, 9 to 11, and 12 to 14 years); sex (male or female); interval from first primary neoplasm (FPN) diagnosis (free of CNS SPN) for control that was at least as long as the interval between FPN and SPN in the case; and cases of bilateral or known family history (ie, heritable) retinoblastoma were matched to controls with the same original FPN diagnosis.
We did not match on other genetic conditions, but adjustment was made in the analysis. However, our measure of genetic susceptibility was crude and was related to associated genetic conditions without family history or biologic material (Appendix).
Quantifying Exposure to Chemotherapy: Case-Control Study
We subdivided treatment into cycles or courses; for each drug and each cycle, we recorded the dates of start and end of administration, the total dose (as milligrams per meter squared), and the route of administration. We summed across cycles the total cumulative dose received (as milligrams per meter squared) for each drug.
Because of the relatively small number of cases and the heterogeneity of multiple-agent therapy, we considered drugs in terms of exposure groups, as follows: alkylating agents; anthracyclines; intrathecal antimetabolites (without exception, methotrexate); nonintrathecal antimetabolites; epipodophyllotoxins; and vinca alkaloids. We used two methods of combining exposures to drugs within each exposure group.
Scores method. Tucker et al13 proposed a scores method of combining exposures to drugs; for example, a cumulative alkylating agent score was obtained by assigning patients a score of 0, 1, 2, or 3 for each alkylating agent used, depending on whether they received none or the lower, middle, or upper third of the distribution of total doses per meter squared for that agent, respectively. The cumulative alkylating agent score was the sum of the scores for each alkylating agent. A similar process was undertaken for each of the other exposure groups.
Equivalent milligrams per meter squared method. We assumed all drugs within a particular exposure group are equally carcinogenic for a specified amount of drug given per meter squared. For this approach, the total cumulative exposure per patient was obtained by summing cumulative exposures to each individual drug within the exposure group; drugs for which individuals were unexposed received the value of 0 mg/m2.
After analysis that used both methods, it became clear the results were similar; therefore, we only present results relating to the equivalent milligrams per meter squared analysis.
Statistical Analysis
Individuals entered risk at 5-year survival and exit date was December 31, 2002, if the survivor was still alive and had not developed an SPN. Otherwise, the exit date was the date of SPN, date of death, or date of loss to follow-up, whichever occurred first, provided it was not later than December 31, 2002. We report results that were statistically significant at the 5% level, by using two-tailed tests. All confidence intervals are 95%.
Cumulative risk: cohort study. We estimated cumulative incidence of SPN by treating death as a competing risk in terms of time from 5-year survival.14 Log-rank tests were undertaken to determine statistical significance of the effect of treatment on cumulative risk.
Standardized incidence ratio and absolute excess risk: cohort study. Standardized incidence ratio (SIR) and absolute excess risk were calculated with Stata 9.0 (STATA, College Station, TX)15 for gliomas. SIR is the ratio of observed (O) to expected (E) numbers of neoplasms. E was estimated from rates in the general population of England and Wales.
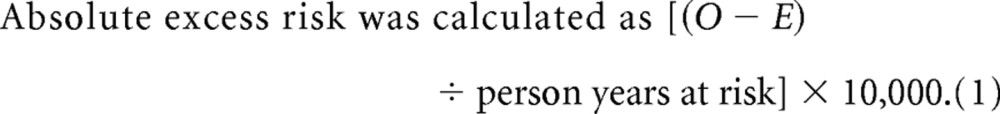 |
.
Conditional logistic regression: case-control study. To investigate variation in the risk of meningioma or glioma/primitive neuroectodermal tumor (PNET) in relation to levels of cumulative exposure to radiation or cytotoxic drug dose (D), the following linear model was fitted:
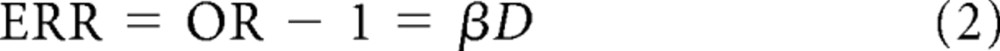 |
in which excess relative risk (ERR), which is the odds ratio (OR) minus 1, is expressed as a linear function of dose. Consequently, β is a measure of the ERR per Gy or ERR per milligram per meter squared (Appendix).
RESULTS
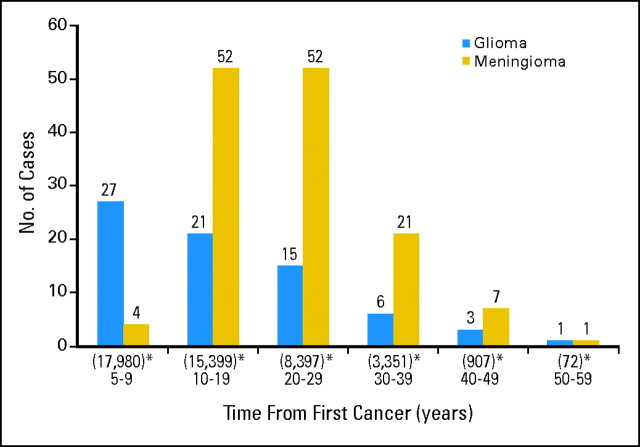
Total person-years of follow-up was 310,816 years from 5-year survival; the mean follow-up was 17.3 years per survivor. We identified 247 SPNs (137 meningiomas, 73 gliomas, 16 schwannomas, nine PNETs and 12 other SPNs; Appendix Table A1, online only). The interval between FPN and SPN ranged from 5 to 52 years (Appendix Fig A1, online only). The mean interval was 20.5 years overall but varied by SPN type, as follows: meningiomas, 23.1 years; schwannomas, 20.0 years; gliomas, 17.4 years; and PNET, 9.2 years.
Cohort Analysis
SIR for gliomas overall was 10.8 (95% CI, 8.5 to 13.6; Table 1). It was highest after leukemia (SIR, 16.7; 95% CI, 10.1 to 26.1) and CNS FPN (SIR, 18.5; 95% CI, 12.7 to 26.2). The SIR was higher after radiotherapy treatment (14.3; 95% CI, 10.9 to 18.7) compared with after treatment without radiotherapy (SIR, 6.1; 95% CI, 3.1 to 11.0), P = .008. The SIR was 15.3 (95% CI, 10.3 to 21.9) after chemotherapy treatment compared with 10.2 (95% CI, 7.1 to 14.1) after treatment without chemotherapy (P = .096). The SIR increased among those treated more recently (P < .001). The SIR decreased with increasing follow-up (P = .001).
Table 1.
SIR for Glioma, P Value for Test of Heterogeneity and Trend in SIRs, and AERs
| Category | No. of Survivors in Category | No. Observed | No. Expected | SIR | 95% CI |
P |
AER | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Heterogeneity | Linear Trend | ||||||||
| Overall | 17,980 | 73 | 6.76 | 10.8 | 8.5 to 13.6 | 2.1 | 1.6 to 2.7 | ||
| Sex | .09 | ||||||||
| Male | 9,886 | 37 | 4.09 | 9.0 | 6.4 to 12.5 | 1.9 | 1.2 to 2.7 | ||
| Female | 8,094 | 36 | 2.68 | 13.4 | 9.4 to 18.6 | 2.3 | 1.5 to 3.2 | ||
| Age at original diagnosis, years | .31 | .19 | |||||||
| 0-4 | 8,248 | 34 | 2.82 | 12.0 | 8.3 to 16.8 | 2.1 | 1.3 to 2.9 | ||
| 5-9 | 4,812 | 21 | 1.70 | 12.3 | 7.6 to 18.9 | 2.4 | 1.3 to 3.5 | ||
| 10-14 | 4,920 | 18 | 2.24 | 8.0 | 4.8 to 12.7 | 1.9 | 0.9 to 2.9 | ||
| Year of diagnosis of childhood cancer | < .0016 | < .001 | |||||||
| 1940-1959 | 1,118 | 5 | 1.29 | 3.9 | 1.3 to 9.1 | 1.0 | 0.0 to 2.2 | ||
| 1960-1969 | 2,628 | 12 | 1.79 | 6.7 | 3.5 to 11.8 | 1.5 | 0.5 to 2.4 | ||
| 1970-1979 | 5,379 | 32 | 2.00 | 16.0 | 11.0 to 22.7 | 2.8 | 1.8 to 3.9 | ||
| 1980-1991 | 8,855 | 24 | 1.70 | 14.1 | 9.1 to 20.9 | 2.3 | 1.3 to 3.2 | ||
| Childhood cancer diagnosis | < .001 | ||||||||
| Leukemia | 4,851 | 19 | 1.13 | 16.7 | 10.1 to 26.1 | 2.8 | 1.4 to 4.1 | ||
| CNS tumor | 4,111 | 32 | 1.73 | 18.5 | 12.7 to 26.2 | 4.0 | 2.6 to 5.6 | ||
| Genetic retinoblastoma | 549 | 2 | 0.29 | 6.9 | 0.8 to 25.1 | 1.3 | |||
| Nongenetic retinoblastoma | 651 | 1 | 0.35 | 2.9 | 0.1 to 16.1 | 0.4 | |||
| Lymphoma | 2,206 | 7 | 0.97 | 7.2 | 2.9 to 14.9 | 1.6 | 0.2 to 2.9 | ||
| Other types of childhood cancer | 5,612 | 12 | 2.30 | 5.2 | 2.7 to 9.1 | 0.9 | 0.3 to 1.6 | ||
| Period of follow-up, years | .0032 | .0015 | |||||||
| 0-4 | 17,980 | 27 | 1.31 | 20.6 | 13.6 to 30.1 | 3.0 | 1.8 to 4.2 | ||
| 5-9 | 16,450 | 9 | 1.20 | 7.5 | 3.4 to 14.3 | 1.0 | 0.3 to 1.8 | ||
| 10-14 | 13,129 | 11 | 1.00 | 11.0 | 5.5 to 19.7 | 1.8 | 0.6 to 3.0 | ||
| 15-19 | 9,362 | 11 | 0.88 | 12.5 | 6.2 to 22.3 | 2.6 | 0.9 to 4.2 | ||
| 20-29 | 6,424 | 10 | 1.38 | 7.2 | 3.5 to 13.3 | 2.1 | 0.6 to 3.6 | ||
| ≥ 30 | 2,370 | 5 | 0.99 | 5.0 | 1.6 to 11.7 | 2.6 | 0.0 to 5.5 | ||
| Treatment for original childhood cancer | |||||||||
| Radiotherapy | 9,223 | 57 | 4.00 | 14.3 | 10.9 to 18.7 | .008 | 3.0 | 2.1 to 3.8 | |
| No radiotherapy | 3,835 | 11 | 1.79 | 6.1 | 3.1 to 11.0 | 1.2 | 0.3 to 2.0 | ||
| Chemotherapy | 6,633 | 30 | 1.96 | 15.3 | 10.3 to 21.9 | .096 | 2.6 | 1.6 to 3.6 | |
| No chemotherapy | 6,038 | 36 | 3.54 | 10.2 | 7.1 to 14.1 | 2.3 | 1.5 to 3.2 | ||
Abbreviations: SIR, standardized incidence ratio; AER, absolute excess risk.
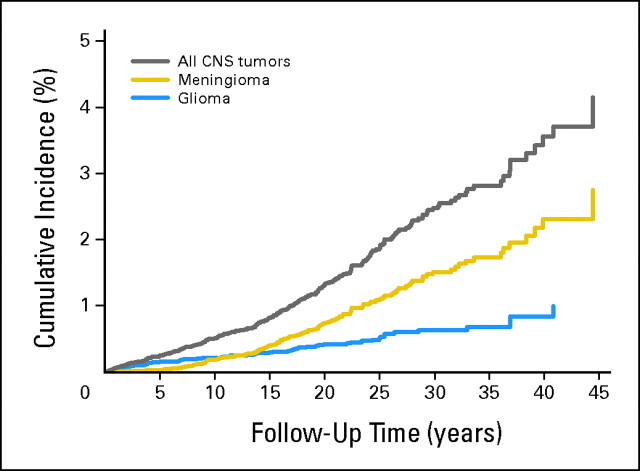
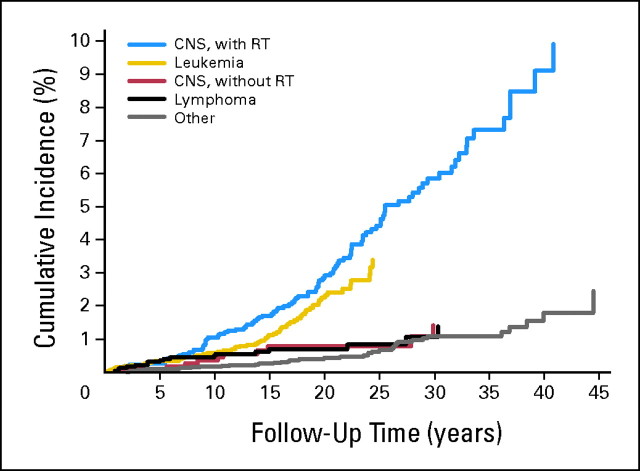
Cumulative incidence was 3.6% (95% CI, 2.9% to 4.3%) by 40 years of follow-up for all SPNs, comprising 2.3% (95% CI, 1.8% to 3.0%) for meningiomas and 0.8% (95% CI, 0.6% to 1.2%) for gliomas. Corresponding cumulative incidences were highest after CNS FPN 6.5% (95% CI, 5.0% to 8.2%) and leukemia FPN 3.4% (95% CI, 2.5% to 4.5%). Corresponding cumulative incidences after irradiated CNS FPN were 9.1% (95% CI, 7.9% to 11.7%), 6.3% (95% CI, 4.5% to 8.5%) for meningiomas, and 2.4% (95% CI, 1.3% to 4.1%) for gliomas, compared with 1.4% (95% CI, 0.6% to 2.8%) after nonirradiated CNS FPN (log-rank P < .001; Appendix Figs A2 and A3, online only).
Case-Control Study
Table 2 provides a summary of evidence of the influence of cumulative exposure to radiation and cytotoxic drugs on the risk of meningioma and glioma/PNET. The ERR of meningioma increased linearly both with increased cumulative exposure to radiation in Gy (P < .001) and with increased cumulative exposure to intrathecal methotrexate in milligrams per meter squared (P = .015 adjusted for radiation exposure). In terms of model 1, specified in the Appendix, the estimates for β1 and β2 were 5.1 (95% CI, 0.7 to 107.7) per Gy and 2.2 (95% CI, 0.1 to 64.4) per mg/m2, respectively. For no other group of cytotoxics investigated was there statistically significant variation in ERR of meningioma with increased cumulative exposure measured in milligrams per meter squared. The ERR of glioma/PNET increased linearly with increased cumulative exposure to radiation in Gy (P < .001). In terms of model 1, the estimate for β1 was .079 (95% CI, 0.021 to .229) per Gy. For none of the groups of cytotoxics investigated was there statistically significant variation in the ERR of glioma/PNET, with increased cumulative exposure measured in mg/m2.
Table 2.
Influence of Increased Cumulative Exposure to Radiation and Chemotherapeutic Drugs on the ERR of Meningioma and Glioma/PNET
| Exposures Included in the Model | Meningioma |
Glioma/PNET |
||||
|---|---|---|---|---|---|---|
| Deviance | df | LRT P | Deviance | df | LRT P | |
| Null model | 127.54 | 92 | 81.79 | 59 | ||
| Radiation, Gy* | 50.83 | 91 | < .001† | 69.58 | 58 | < .001† |
| Radiation* + alkylating agents mg/m2* | 49.47 | 90 | .245‡ | 69.55 | 57 | .864‡ |
| Radiation* + anthracyclines* | 50.58 | 90 | .621‡ | 68.89 | 57 | .407‡ |
| Radiation* + intrathecal methotrexate* | 44.87 | 90 | .015‡ | 68.81 | 57 | .379‡ |
| Radiation* + nonintrathecal antimetabolites* | 49.22 | 90 | .205‡ | 69.24 | 57 | .561‡ |
| Radiation* + epipodophyllotoxins* | 49.69 | 90 | .287‡ | 68.96 | 57 | .429‡ |
| Radiation* + vinca alkaloids* | 48.12 | 90 | .100‡ | 69.55 | 57 | .854‡ |
NOTE. Radiation dosed in Gy; chemotherapeutic drugs dosed in milligrams per meter squared.
Abbreviations: ERR, excess relative risk; PNET, primitive neuroectodermal tumor; df, degrees of freedom; LRT, likelihood ratio test.
Fitted as a linear trend in continuous dose (model 1).
LRT P value relative to null model.
LRT P value relative to model with radiation fitted as a linear trend in continuous dose.
Appendix Table A2 (online only) summarizes an investigation of evidence for nonlinearity in dose responses, interaction between exposures and potential effects of age at exposure, and time since exposure on the ERR of meningioma and glioma/PNET. The effect of cumulative exposure to radiation on the ERR of each of meningioma and glioma/PNET is well explained by a linear relationship. There was evidence of nonlinearity in the effect of cumulative exposure to intrathecal methotrexate on the ERR of meningioma (P = .004 for a cubic relationship), but such a complex dose-response relationship would need independent confirmation before being regarded as robust. There was no evidence of an interaction between cumulative exposure to radiation and intrathecal methotrexate on the ERR of meningioma. Neither age at exposure nor time since exposure revealed evidence of an effect on the ERR of meningioma. As indicated in Appendix Table A2, there is statistically significant decline in the ERR of glioma/PNET with increasing age at exposure (P = .033) but not with time since exposure. Appendix Table A2 also shows that, for meningioma, there is no statistically significant variation of ERR with genetic susceptibility. However, for PNET/glioma, there is statistically significant variation (P = .016), and risks appear markedly elevated in the susceptible group: the excess relative risk per Gy is higher by a factor of 5.69 × 105 (95% CI, 2.30 to >106; Appendix).
Tables 3 and 4 summarize variation in relative risks (RRs) of meningioma and glioma/PNET across increasing levels of cumulative exposure to radiation from radiotherapy. These tables provide RRs associated with each exposed level compared with the baseline level comprising those unexposed. For meningioma and glioma/PNET, the RR associated with an exposure of at least 40 Gy was 479 times and four times, respectively, that experienced in unexposed tissue.
Table 3.
Variation in RR of Meningioma Across Different Levels of Radiation Exposure Resulting From Radiotherapy
| Level of Exposure, Gy | Cases |
Controls |
RRs |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted* |
Adjusted for Intrathecal Methotrexate Exposure*† |
|||||||||
| No. | Mean Dose (Gy) | No. | Mean Dose (Gy) | RR | 95% CI | P | RR | 95% CI | P | |
| Incomplete information | 25 | 25 | ||||||||
| 0 | 3 | 39 | 1 | 1 | ||||||
| 0.01-9.99 | 6 | 2.66 | 33 | 0.88 | 1.1 | 0.1 to 7.5 | .962 | 1.8 | < 0.8 to 39.3 | .632 |
| 10.00-19.99 | 12 | 16.58 | 10 | 15.17 | 35.1 | 2.9 to 419.1 | .005 | 8.4 | 6.4 to < 10.7 | .078 |
| 20.00-29.99 | 39 | 24.76 | 15 | 23.29 | 57.8 | 6.1 to 544.5 | < .001 | 51.6 | 5.5 to < 69.5 | < .001 |
| 30.00-39.99 | 21 | 34.73 | 8 | 34.66 | 69.6 | 6.6 to 736.4 | < .001 | 567.9 | 29.3 to < 773.6 | < .001 |
| ≥ 40.00 | 28 | 46.97 | 4 | 43.38 | 94.2 | 8.7 to 1,014.0 | < .001 | 479.1 | 25.0 to < 657.2 | < .001 |
| Total | 134 | 134 | ||||||||
Abbreviation: RR, relative risk.
Likelihood ratio test for evidence of heterogeneity in RR across different levels of exposure to radiation: P < .001 for unadjusted analysis and for adjusted analysis.
Adjusted for intrathecal methotrexate exposure fitted as a categoric exposure variable.
Table 4.
Variation in RR of Glioma/PNET Across Different Levels of Radiation Exposure Resulting From Radiotherapy
| Level of Exposure, Gy | Cases |
Controls |
Unadjusted Analysis |
||||
|---|---|---|---|---|---|---|---|
| No. | Mean Dose (Gy) | No. | Mean Dose (Gy) | RR | 95% CI | P | |
| Incomplete information | 13 | 13 | |||||
| 0 | 13 | 17 | 1 | ||||
| 0.01-9.99 | 11 | 0.78 | 27 | 0.79 | 0.5 | 0.2 to 1.5 | .230 |
| 10.00-19.99 | 4 | 18.45 | 9 | 17.48 | 0.5 | 0.1 to 2.3 | .406 |
| 20.00-29.99 | 18 | 23.57 | 8 | 23.84 | 2.6 | 0.9 to 8.0 | .084 |
| 30.00-39.99 | 6 | 35.17 | 3 | 34.00 | 3.4 | 0.5 to 23.0 | .215 |
| ≥ 40.00 | 16 | 45.04 | 4 | 46.43 | 4.4 | 1.2 to 16.4 | .028 |
| Total* | 81 | 81 | |||||
Abbreviations: RR, relative risk; PNET, primitive neuroectodermal tumor.
Likelihood ratio test for evidence of heterogeneity in RRs across different levels of exposure to radiation: P for unadjusted analysis = .0013.
Table 5 summarizes the variation in RR of meningioma across different levels of cumulative exposure to intrathecal methotrexate, analogous to Tables 3 and 4. After adjustment for radiotherapy exposure, the RR of meningioma associated with a cumulative exposure of at least 70 mg/m2 of intrathecal methotrexate was 36 times that experienced in those unexposed to intrathecal methotrexate.
Table 5.
Variation in RR of Meningioma Across Different Levels of Intrathecal Methotrexate Exposure
| Level of Exposure, mg/m2 | Cases |
Controls |
RRs |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted for Radiation Exposure* |
|||||||||
| No. | Mean Dose (mg/m2) | No. | Mean Dose (mg/m2) | RR | 95% CI | P | RR | 95% CI | P | |
| Incomplete information | 22 | 22 | ||||||||
| 0 | 79 | 97 | 1 | 1 | ||||||
| 1-39 | 12 | 31 | 4 | 34 | 4.1 | 1.2 to 14.1 | .024 | 15.4 | 2.2 to 179.6 | .004 |
| 40-69 | 5 | 49 | 7 | 52 | 1.2 | 0.3 to 4.7 | .817 | 10.8 | 1.3 to 143.0 | .027 |
| ≥ 70 | 16 | 542 | 4 | 82 | 6.4 | 1.7 to 23.5 | .006 | 35.6 | 4.8 to 599.4 | < .001 |
| Total† | 134 | 134 | ||||||||
NOTE. Four and two of the 33 exposed patients and 15 exposed controls, respectively, received leucovorin after intrathecal methotrexate.
Abbreviation: RR, relative risk.
Adjusted for radiation fitted as a linear trend in continuous cumulative radiation exposure (model 2).
Likelihood ratio test for evidence of heterogeneity in RRs across different levels of exposure to intrathecal methotrexate: P for unadjusted analysis = .002; P for adjusted analysis = .001.
DISCUSSION
The risk of meningioma after radiation was strongly and linearly related to dose. The risk of glioma also increased (linearly) with increasing radiation dose. There was modification of risk of glioma by age at childhood cancer diagnosis and genetic susceptibility. Increased exposure to intrathecal methotrexate significantly increases risk of meningioma, which is a novel finding. The risk among those exposed to at least 70 mg/m2 of intrathecal methotrexate was 36-fold that among those unexposed.
The adjusted RR of meningioma among those irradiated with doses of at least 40 Gy was 479-fold that among those unexposed. The most comparable figure from the CCSS is 33-fold, which relates to those exposed to at least 45 Gy.3 ERR of meningioma in this study was 5.1 (95% CI, 0.7 to 107.7) per Gy. In the CCSS study,3 an ERR of 1.06 (95% CI, 0.21 to 8.15) per Gy for meningioma was reported. A study of atomic bomb survivors9 reported an ERR of 0.64 (95% CI, −0.01 to 1.8) per Sievert. A Franco/British study of survivors of childhood cancer reported an ERR for benign/unspecified brain tumors of greater than 1,000 (95% CI, 0.25 to > 1,000) per Gy.4 From studies of children treated for tinea capitis with cranial irradiation,16 the ERR for benign meningiomas was 4.63 (95% CI, 2.43 to 9.12) per Gy, which is similar to our estimate.16 We found no evidence of a significant association with known genetic susceptibility and risk of meningiomas, although there does appear to be evidence of such an association in other studies.17 Recent data concerning cranially irradiated survivors of childhood acute lymphoblastic leukemia in Israel indicate that those undergoing regular scanning with magnetic resonance imaging or computed tomography had a cumulative risk of 15% of developing a meningioma within 20 years of irradiation.5 Fifteen of 16 observed meningiomas were asymptomatic, and most were smaller than 4 cm in diameter.5 These investigators commented that the chances of success of complete resection are higher for smaller lesions and that symptomatic patients usually have larger tumors that require riskier surgery. We found that 6% of survivors of irradiated CNS tumors developed a meningioma after 40 years. Practices in the United Kingdom are ad hoc, and there is no agreed-upon, standardized screeningfor CNS SPNs. Computed tomography or magnetic resonance imaging for symptoms is routine in survivors attending long-term follow-up clinics.
The overall SIR for glioma was 10.8 (95% CI, 8.5 to 13.6) and was highest after leukemia and CNS childhood neoplasms, which in this cohort were commonly treated with high doses of cranial irradiation. This compares with an SIR of 8.7 (95% CI, 6.2 to 11.6) in the CCSS study.3 The RR of glioma/PNET was 4.4 (95% CI, 1.2 to 16.4) among those exposed to radiation doses of at least 40 Gy compared with those unexposed. The most comparable figure from the CCSS study was 17.5 (95% CI, 2.9 to 107.5), which related to those exposed to at least 45 Gy.3 We found an ERR for glioma/PNET of 0.079 (95% CI, 0.021 to 0.229) per Gy, which compares with 0.33 (95% CI, 0.07 to 1.71) reported by the CCSS,3 0.6 (95% CI, −0.2 to 2.0) from a study of the atomic bomb survivors,9 and 1.98 (95% CI, 0.73 to 4.69) per Gy from the tinea capitis cohort.16 We found modification of ERR with age at exposure (P = .033) and genetic susceptibility (P = .016). The CCSS study found no statistically significant association between ERR and age at exposure for either gliomas or meningiomas, and they did not include genetic susceptibility as a factor in their analysis.3 Preston et al9 reported a weak association (P = .06) with age at exposure to atomic radiation and risk of CNS tumors (excluding schwannomas), in which ERR was higher in those exposed when younger than the age of 20 years (and this was also the case when meningiomas were examined separately). The study of children with tinea capitis also reports an association with age at exposure for malignant brain tumors (but not for meningiomas).16
We estimate ERR associated with intrathecal methotrexate was 2.2 (95% CI, 0.1 to 64.4) per mg/m2. The CCSS did not investigate detailed dose response in relation to chemotherapy.3 The Franco/British study looked solely at the effect of alkylating agents on risk of developing CNS SPNs and found a slightly increased risk relating to one subgroup.4 The only previous paper reporting an association between intrathecal methotrexate and increased risk of brain tumors concerned an excess of gliomas after childhood acute lymphoblastic leukemia.10 Two other studies have examined the role of intrathecal chemotherapy on risk of gliomas18 and brain tumors19 after childhood acute lymphoblastic leukemia but failed to find an association. There were insufficient survivors in our cohort treated with intrathecal methotrexate without cranial irradiation to assess their risk of meningioma. It is important that such populations are investigated, because intrathecal methotrexate is still widely used to treat leukemia and lymphoma.20
There are important advantages associated with this study, including that it has a large-scale, population-based design and has careful estimation of treatment exposures. The principal limitations relate to the interpretation of the finding that the risk of meningioma increased with increased cumulative exposure to intrathecal methotrexate. There are two reasons to be cautious about interpreting this association as causal. First, as reported in the previous paragraph, there were insufficient survivors exposed to intrathecal methotrexate without cranial irradiation to assess their risk of meningioma separately, and residual confounding is a possibility. Second, if this relationship were causal, then one might anticipate that systematic methotrexate (to which all except two of those who received nonintrathecal antimetabolites were exposed) might reveal evidence of a relationship, but it did not.
In conclusion, the largest-ever study, to our knowledge, of CNS tumors in survivors of childhood cancer indicates that the risk of meningioma increases strongly, linearly, and independently with dose of radiation to meningeal tissue and with dose of intrathecal methotrexate. The risk of glioma/PNET increased linearly with dose of radiation.
Acknowledgment
We thank the Steering Group of the British Childhood Cancer Survivor Study (BCCSS) that comprises Douglas Easton (chair), PhD, Michael Hawkins (secretary), DPhil, Helen Jenkinson, MD, Meriel Jenney, MD, Emma Lancashire, PhD, Kathryn Pritchard-Jones, MD, Michael Stevens, MD, Charles Stiller, MSc, Elaine Sugden, MD, Andrew Toogood, MD, and Hamish Wallace, MD; the officers, centers, and individual members of the Children's Cancer and Leukemia Group, the Childhood Cancer Research Group, and the Regional Pediatric Cancer Registries; the collaboration of the Office for National Statistics, the General Register Office for Scotland, the National Health Service Central Registers, the regional cancer registries, health authorities, and area health boards that provided general practitioner names and addresses; the general practitioners nationwide who facilitated direct contact with survivors; all of the survivors who completed a 40-page questionnaire; our two funders: Cancer Research UK and the Kay Kendall Leukemia Fund; and all BCCSS staff, who have given many years of dedicated work to bring the BCCSS to fruition, particularly Julie Kelly who contributed importantly to this study.
Appendix
Genetic risk: case-control study.
Twenty-six cases had a known associated genetic syndrome with an increased risk of brain or CNS second primary neoplasm (SPN). Twelve of 73 gliomas had associated neurofibromatosis (NF) type 1 (NF1), and five of 127 meningiomas had an associated genetic syndrome (one, NF1; two NF2; one, Gorlin's syndrome; one, tuberous sclerosis). Five schwannomas were associated with NF2, and three other SPNs (ie, a sarcoma, a malignant peripheral-nerve sheath tumor, and a malignant neoplasm of uncertain diagnosis) were associated with NF1. One other low-grade SPN (ie, hemangioblastoma) was associated with Von Hippel-Lindau syndrome. Two controls had NF1.
Tumor localization: case-control study.
Available records (including operative notes, pathology reports, radiology reports, and medical correspondence) were reviewed to determine as precisely as possible the location of the SPN. The SPN location was drawn on a three-dimensional grid map that included eight axial sections of the brain. Spinal tumors were defined by vertebral level. This method of tumor localization is described by Neglia et al.3
Radiation dosimetry: case-control study.
The original prescription sheets, planning diagrams, and treatment details were obtained from the treating hospitals. These were made anonymous, were electronically scanned, and were sent to the University of Texas M. D. Anderson Cancer Center in Houston, TX, for radiation dosimetry. Therapy information abstracted from the scanned records included dates, beam energy, field size, field location, and total dose. Therapy included in the analysis was given either prophylactically or as treatment of recognized disease.
For each patient, site of subsequent CNS SPN was characterized by calculation points within boundaries of tumor areas drawn on grid maps. Calculation points were evenly spaced within the SPN. The number of calculation points was dependent on SPN size and ranged from three to 125. Doses to the selected subset of points were averaged to estimate the dose to CNS SPN site for each case and for the matched control. Minimum and maximum doses for CNS SPN were also reported. Where subsequent SPNs could not be located specifically and when dose gradient across the brain was minimal, average dose to the whole brain was reported.
Doses to selected points were estimated by using one of two techniques: for points in the treatment beam, standard radiation dosimetry techniques were used (Br J Radiol 17:1-147, 1983 [suppl]); for points outside treatment beams, doses were estimated by using radiation measurements in water phantoms, applied to a three-dimensional mathematical phantom that simulates patients of various ages. Age of child at time of therapy was used to determine height and, subsequently, distance from treatment field to selected points. Complete description of dosimetry methods is given by Stovall et al (Stovall M, et al: Radiat Res 166:141-157, 2006).
Conditional logistic regression: case-control study.
To investigate variation in the risk of meningioma and glioma/primitive neuroectodermal tumor in relation to levels of cumulative exposure to radiation, from radiotherapy, and from specific groups of cytotoxic drugs, the following model was fitted:
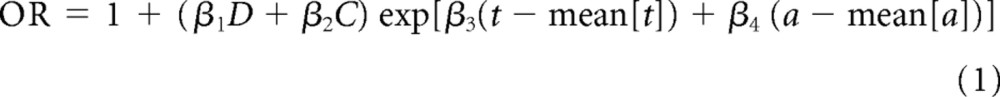 |
in which the odds ratio (OR) is for an individual t years after exposure at age a to a cumulative radiation dose of D Gy and a cumulative cytotoxic drug exposure of C mg/m2. Values β1 through β4 are regression coefficients, and the variables of time from exposure t and of age at exposure a were centered by subtracting the approximate mean values (mean[t] and mean [a]) in the case-control data relating to meningioma and glioma/primitive neuroectodermal tumor separately.
For analyses in which cumulative exposure to cytotoxic drugs was coded as a categoric variable, adjusted for cumulative radiation exposure, the following model was fitted:
in which f(C) is a piecewise constant (ie, dummy variable) function of the cytotoxic drug dose. In particular, this model was used to fit the data produced by using the scores methodology. These models and extensions with higher-order terms for radiation and cytotoxic drug exposure were fitted by using PECAN (Preston DL, et al: EPICURE 2.10. Seattle, WA, Hirosoft International, 1988 to 1998).
In models 1 and 2 above, the excess relative risk (ERR) is equal to the OR minus 1. Therefore, in particular, the model
 |
expresses the ERR as being linear in relation to cumulative radiation dose (D) in Gy, and therefore β1 is ERR per Gy.
Finally, to facilitate interpretation and comparison with previous work, we report the results of simply fitting exposure to radiation (Gy) and cytotoxic drugs (mg/m2) as categoric factors with several discrete levels. Such models provide the relative risks associated with each exposed level compared with the baseline level comprising those unexposed.
Fig A1.
Time to occurrence of subsequent glioma or meningioma from original cancer diagnosis. (*) Number entering risk intervals.
Fig A2.
Cumulative incidence of all second CNS tumors, meningiomas, and gliomas by period of follow-up from 5-year survival.
Fig A3.
Cumulative incidence of all second CNS tumors after specific types of childhood cancer and treatment. RT, radiotherapy.
Table A1.
Characteristics of SPNs of the CNS by Subcategory Compared With Controls and BCCSS
| Characteristic | CNS SPN* |
Case Control Study |
All of BCCSS (N = 17,980) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glioma (n = 73) |
Meningioma (n = 137) |
PNET (n = 9) |
Schwannoma (n = 16) |
All (N = 247) |
Cases (n = 243) |
Controls (n = 243) |
||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||||||||||
| Male | 37 | 50.7 | 66 | 48.2 | 4 | 44.4 | 6 | 37.5 | 119 | 48.2 | 117 | 48.1 | 117 | 48.1 | 9,886 | 55.0 |
| Female | 36 | 49.3 | 71 | 51.8 | 5 | 55.6 | 10 | 62.5 | 128 | 51.8 | 126 | 51.9 | 126 | 51.9 | 8,094 | 45.0 |
| Age at diagnosis of FPN, years | ||||||||||||||||
| 0-4 | 34 | 46.6 | 63 | 46.0 | 4 | 44.4 | 6 | 37.5 | 112 | 45.3 | 110 | 45.3 | 108 | 44.4 | 8,248 | 45.9 |
| 5-9 | 21 | 28.8 | 46 | 33.6 | 2 | 22.2 | 4 | 25.0 | 78 | 31.6 | 77 | 31.7 | 76 | 31.3 | 4,812 | 26.8 |
| 10-14 | 18 | 24.7 | 28 | 20.4 | 3 | 33.3 | 6 | 37.5 | 57 | 23.1 | 56 | 23.0 | 59 | 24.3 | 4,920 | 27.4 |
| Childhood cancer diagnostic group | ||||||||||||||||
| Leukemia | 19 | 26.0 | 40 | 29.2 | 4 | 44.4 | 2 | 12.5 | 69 | 27.9 | 69 | 28.4 | 39 | 16.0 | 4,851 | 27.0 |
| CNS tumor | 32 | 43.8 | 74 | 54.0 | 1 | 11.1 | 6 | 37.5 | 117 | 47.4 | 113 | 46.5 | 66 | 27.2 | 4,111 | 22.9 |
| Retinoblastoma | 3 | 4.1 | 11 | 8.0 | 1 | 11.1 | 1 | 6.3 | 16 | 6.5 | 16 | 6.6 | 22 | 9.1 | 1,200 | 6.7 |
| Hodgkin's disease | 4 | 5.5 | 3 | 2.2 | 0 | 0.0 | 1 | 6.3 | 10 | 4.0 | 10 | 4.1 | 18 | 7.4 | 1,325 | 7.4 |
| Non-Hodgkin's lymphoma | 3 | 4.1 | 2 | 1.5 | 2 | 22.2 | 0 | 0.0 | 7 | 2.8 | 7 | 2.9 | 8 | 3.3 | 881 | 4.9 |
| Wilms tumor | 3 | 4.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 1.6 | 4 | 1.6 | 28 | 11.5 | 1,478 | 8.2 |
| Neuroblastoma | 2 | 2.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 1.2 | 3 | 1.2 | 10 | 4.1 | 766 | 4.3 |
| Soft tissue sarcoma | 2 | 2.7 | 4 | 2.9 | 0 | 0.0 | 3 | 18.8 | 9 | 3.6 | 9 | 3.7 | 22 | 9.1 | 1,200 | 6.7 |
| Bone tumor | 2 | 2.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.8 | 2 | 0.8 | 18 | 7.4 | 643 | 3.6 |
| Other | 3 | 4.1 | 3 | 2.2 | 1 | 1.1 | 3 | 18.8 | 10 | 4.0 | 10 | 4.1 | 12 | 4.9 | 1,525 | 8.5 |
| Year of childhood cancer diagnosis | ||||||||||||||||
| 1940-1959 | 5 | 6.8 | 16 | 11.7 | 0 | 0.0 | 1 | 6.3 | 23 | 9.3 | 22 | 9.1 | 29 | 11.9 | 1,118 | 6.2 |
| 1960-1969 | 12 | 16.4 | 35 | 25.5 | 0 | 0.0 | 4 | 25.0 | 55 | 22.3 | 54 | 22.2 | 78 | 32.1 | 2,628 | 14.6 |
| 1970-1979 | 32 | 43.8 | 67 | 48.9 | 2 | 22.2 | 5 | 31.3 | 108 | 43.7 | 107 | 44.0 | 85 | 35.0 | 5,379 | 29.9 |
| 1980-1991 | 24 | 32.9 | 19 | 13.9 | 7 | 77.8 | 6 | 37.5 | 61 | 24.7 | 60 | 24.7 | 51 | 21.0 | 8,855 | 49.3 |
Abbreviations: SPNs, second primary neoplasms; BCCSS, British Childhood Cancer Survivor Study; PNET, primitive neuroectodermal tumor; FPN, first primary neoplasm.
Percentages of all CNS SPNs are as follows: glioma, 29.4%; meningioma, 55.5%; PNET, 3.7%; Schwannoma, 6.5%.
Table A2.
Investigation for Potential Nonlinearity in Dose Response, Interaction Between Exposures, Effect of Age at Exposure, Time Elapsed Since Exposure,and Genetic Susceptibility
| Exposures Included in the Model by Tumor Type | Analysis |
||
|---|---|---|---|
| Deviance | df | LRT P | |
| Meningioma | |||
| Null model | 127.54 | 92 | |
| Radiation linear in continuous dose | 50.83 | 91 | < .001* |
| Plus quadratic in continuous dose | 50.82 | 90 | .954† |
| Plus cubic in continuous dose | 50.72 | 89 | .748† |
| Plus quartic in continuous dose | 50.47 | 88 | .618† |
| Radiation linear in continuous dose + intrathecal methotrexate linear in continuous dose | 44.87 | 90 | .015† |
| Plus quadratic in continuous dose of intrathecal methotrexate | 43.67 | 89 | .274‡ |
| Plus cubic in continuous dose of intrathecal methotrexate | 35.48 | 88 | .004‡ |
| Plus quartic in continuous dose of intrathecal methotrexate | 35.48 | 87 | .931‡ |
| Radiation linear in continuous dose + intrathecal methotrexate linear in continuous dose + modification by genetic susceptibility | 44.78 | 89 | .780‡ |
| Radiation linear in continuous dose + intrathecal methotrexate linear in continuous dose + interaction | 44.69 | 89 | .676‡ |
| Radiation linear in continuous dose + intrathecal methotrexate linear in continuous dose + modification by age at exposure | 44.57 | 89 | .583‡ |
| Radiation linear in continuous dose + intrathecal methotrexate linear in continuous dose + modification by time since exposure | 44.19 | 89 | .539‡ |
| Radiation linear in continuous dose + intrathecal methotrexate linear in continuous dose + first primary cancer group | 35.02 | 81 | .362‡ |
| Glioma/PNET | |||
| Null model | 81.79 | 59 | |
| Radiation linear in continuous dose | 69.58 | 58 | < .001* |
| Plus quadratic in continuous dose | 67.12 | 57 | .117† |
| Plus cubic in continuous dose | 65.45 | 56 | .197† |
| Plus quartic in continuous dose | 64.64 | 55 | .367† |
| Radiation linear in continuous dose + modification by genetic susceptibility | 63.77 | 57 | .016† |
| Radiation linear in continuous dose + modification by age at exposure | 65.05 | 57 | .033† |
| Radiation linear in continuous dose + modification by time since exposure | 68.74 | 57 | .359† |
NOTE. Radiation dosed in Gy; chemotherapeutic drugs dosed in milligrams per meter squared.
Abbreviations: df, degrees of freedom; LRT, likelihood ratio test; PNET, primitive neuroectodermal tumor.
LRT P value relative to null model.
LRT P value relative to model with radiation fitted as a linear trend in continuous dose.
LRT P value relative to model with radiation and intrathecal methotrexate each fitted as a linear trend in continuous dose.
Footnotes
Written on behalf of the British Childhood Cancer Survivor Study Group.
Supported in part by Cancer Research UK; the Kay Kendall Leukaemia Fund; the Department of Health and Scottish Ministers; and Contract No. FP6-036465 from the European Commission (M.P.L.).
Funding sources have had no involvement in the study design, the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the paper for publication. The views expressed in this publication are those of the authors and not necessarily those of our funders.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Aliki J. Taylor, Michael M. Hawkins
Financial support: Aliki J. Taylor, Michael M. Hawkins
Administrative support: Aliki J. Taylor, Emma R. Lancashire
Provision of study materials or patients: Aliki J. Taylor, David L. Winter, Charles A. Stiller, Clare Frobisher, Emma R. Lancashire, Raoul C. Reulen, Michael M. Hawkins
Collection and assembly of data: Aliki J. Taylor, Mark P. Little, David L. Winter, Elaine Sugden, David W. Ellison, Charles A. Stiller, Marilyn Stovall, Clare Frobisher, Emma R. Lancashire, Raoul C. Reulen,Michael M. Hawkins
Data analysis and interpretation: Aliki J. Taylor, Mark P. Little, David L. Winter, Elaine Sugden, David W. Ellison, Charles A. Stiller, Marilyn Stovall, Clare Frobisher, Emma R. Lancashire, Raoul C. Reulen,Michael M. Hawkins
Manuscript writing: Aliki J. Taylor, Mark P. Little, David L. Winter, Elaine Sugden, David W. Ellison, Charles A. Stiller, Marilyn Stovall, Clare Frobisher, Emma R. Lancashire, Raoul C. Reulen,Michael M. Hawkins
Final approval of manuscript: Aliki J. Taylor, Mark P. Little, David L. Winter, Elaine Sugden, David W. Ellison, Charles A. Stiller, Marilyn Stovall, Clare Frobisher, Emma R. Lancashire, Raoul C. Reulen,Michael M. Hawkins
REFERENCES
- 1.Inskip PD Ries LAG Cohen RJ, etal: Curtis RE, Freedman DM, Ron E, et al.New malignancies following childhood cancer New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000 465–482,2006. NIH Bethesda, MD: National Cancer Institute; NIH publication 05-5302 [Google Scholar]
- 2.Jenkinson HC Hawkins MM Stiller CA, etal: Long-term population-based risks of second malignant neoplasms after childhood cancer in Britain Br J Cancer 91:1905–1910,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neglia JP Robison LL Stovall M, etal: New Primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the childhood cancer survivor study J Natl Cancer Inst 98:1528–1537,2006 [DOI] [PubMed] [Google Scholar]
- 4.Little MP de Vathaire F Shamsaldin A, etal: Risks of brain tumour following treatment for cancer in childhood: Modification by genetic factors, radiotherapy and chemotherapy Int J Cancer 78:269–275,1998 [DOI] [PubMed] [Google Scholar]
- 5.Goshen Y Stark B Kornreich L, etal: High incidence of meningioma in cranial irradiated survivors of childhood acute lymphoblastic leukemia Pediatr Blood Cancer 49:294–297,2007 [DOI] [PubMed] [Google Scholar]
- 6.Ron E Modan B Boice JD Jr, etal: Tumors of the brain and nervous system after radiotherapy in childhood N Engl J Med 319:1033–1039,1988 [DOI] [PubMed] [Google Scholar]
- 7.United Nations Scientific Committee on the Effects of Atomic Radiation. New York, NY: United Nations; 2008. UNSCEAR 2006 Report to the General Assembly, With Scientific Annexes: Effects of Ionizing Radiation—Epidemiological studies of radiation and cancer. [Google Scholar]
- 8.Taylor AJ Frobisher C Ellison DW, etal: Survival After second primary neoplasms of the brain or spinal cord in survivors of childhood cancer: Results from the British Childhood Cancer Survivor Study J Clin Oncol 27:5781–5787,2009 [DOI] [PubMed] [Google Scholar]
- 9.Preston DL Ron E Yonehara S, etal: Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure J Natl Cancer Inst 94:1555–1563,2002 [DOI] [PubMed] [Google Scholar]
- 10.Relling MV Rubnitz JE Rivera GK, etal: High incidence of secondary brain tumours after radiotherapy and antimetabolites Lancet 354:34–39,1999 [DOI] [PubMed] [Google Scholar]
- 11.Hawkins MM Lancashire ER Winter DL, etal: The British Childhood Cancer Survivor Study: Objectives, methods, population structure, response rates and initial descriptive information Pediatr Blood Cancer 50:1018–1025,2008 [DOI] [PubMed] [Google Scholar]
- 12.Fritz A Percy C Jack A, etal: International Classification of Diseases for Oncology ICD-O (ed 3) 2000. Geneva, Switzerland: World Health Organization [Google Scholar]
- 13.Tucker MA Meadows AT Boice JD Jr, etal: Leukemia after therapy with alkylating agents for childhood cancer J Natl Cancer Inst 78:459–464,1987 [PubMed] [Google Scholar]
- 14.Gooley TA Leisenring W Crowley J, etal: Estimation of failure probabilities in the presence of competing risks: New representations of old estimators Stat Med 18:695–706,1999 [DOI] [PubMed] [Google Scholar]
- 15.College Station, TX: Statacorp; 2009. Statacorp: Stata Statistical Software: Release 10. [Google Scholar]
- 16.Sadetzki S Chetrit A Freedman L, etal: Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis Radiat Res 163:424–432,2005 [DOI] [PubMed] [Google Scholar]
- 17.Flint-Richter P, Sadetzki S: Genetic predisposition for the development of radiation-associated meningioma: An epidemiological study Lancet Oncol 8:403–410,2007 [DOI] [PubMed] [Google Scholar]
- 18.Fontana M Stanton C Pompili A, etal: Late multifocal gliomas in adolescents previously treated for acute lymphoblastic leukemia Cancer 60:1510–1518,1987 [DOI] [PubMed] [Google Scholar]
- 19.Walter AW Hancock ML Pui CH, etal: Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children's Research Hospital J Clin Oncol 16:3761–3767,1998 [DOI] [PubMed] [Google Scholar]
- 20.Kwong YL, Yeung DY, Chan JC: Intrathecal chemotherapy for hematologic malignancies: Drugs and toxicities Ann Hematol 88:193–201,2009 [DOI] [PubMed] [Google Scholar]