Abstract Abstract
Little is known about the physiologic determinants of 6-minute walk distance in idiopathic pulmonary fibrosis. We investigated the demographic, pulmonary function, echocardiographic, and hemodynamic determinants of 6-minute walk distance in patients with idiopathic pulmonary fibrosis evaluated for lung transplantation. We performed a cross-sectional analysis of 130 patients with idiopathic pulmonary fibrosis who completed a lung transplantation evaluation at the Hospital of the University of Pennsylvania between 2005 and 2010. Multivariable linear regression analysis was used to generate an explanatory model for 6-minute walk distance. After adjustment for age, sex, race, height, and weight, the presence of right ventricular dilation was associated with a decrease of 50.9 m (95% confidence interval [CI], 8.4–93.3) in 6-minute walk distance (). For each 200-mL reduction in forced vital capacity, the walk distance decreased by 15.0 m (95% CI, 9.0–21.1; ). For every increase of 1 Wood unit in pulmonary vascular resistance, the walk distance decreased by 17.3 m (95% CI, 5.1–29.5; ). Six-minute walk distance in idiopathic pulmonary fibrosis depends in part on circulatory impairment and the degree of restrictive lung disease. Future trials that target right ventricular morphology, pulmonary vascular resistance, and forced vital capacity may potentially improve exercise capacity in patients with idiopathic pulmonary fibrosis.
Keywords: exercise capacity, interstitial lung disease, right ventricular dilation
Idiopathic pulmonary fibrosis (IPF) is a progressive interstitial lung disease (ILD) associated with significant morbidity, including impairments in exertional tolerance, functional independence, and quality of life.1,2 Physiologic determinants of exercise capacity in patients with IPF are poorly understood. Proposed mechanisms for exercise limitation include inadequate oxygen delivery due to increased pulmonary vascular resistance (PVR), impaired augmentation of cardiac output, and diffusion limitation.2-4
The 6-minute walk distance (6MWD) is a reproducible and easily performed test that is associated with mortality in IPF.5-11 The 6MWD is also a component of the lung allocation score for consideration for lung transplantation.6,12 Because of its relationship with various patient-centered outcomes, clinical trials of therapies for patients with IPF have included the 6MWD as a primary or secondary end point. Understanding the physiologic determinants of 6MWD may help explain how certain therapeutic interventions affect 6MWD and may identify future therapeutic targets to improve quality of life and survival.
Unfortunately, no factor consistently predicts 6MWD across different cardiopulmonary syndromes, likely because of the heterogeneity of the physiologic factors that affect exercise.5,13-17 The clinical factors associated with 6MWD in IPF have been inconsistent between studies because of small sample sizes, inclusion of patients with a broad range of IPF severity, and lack of simultaneous echocardiography, spirometry, and right heart catheterization.5,6,11,16 Forced vital capacity (FVC), diffusing capacity for carbon monoxide (DLCO), and mean pulmonary artery pressure (mPAP) are all associated with 6MWD in IPF in different studies.5,16 We have previously shown that right ventricular (RV) dilation and dysfunction predict mortality among patients with IPF.18 However, little is known about the association between RV structure and function and exercise tolerance in patients with IPF.
We examined the demographic, pulmonary function, echocardiographic, and hemodynamic determinants of 6MWD in patients with IPF undergoing evaluation for lung transplantation. We hypothesized that RV morphology and PVR would be associated with 6MWD independent of other measures of lung function.
Methods
We conducted a cross-sectional analysis of a retrospective cohort of patients with IPF evaluated for lung transplantation at the Hospital of the University of Pennsylvania between 2005 and 2010. The University of Pennsylvania institutional review board approved this study (no. 813174).
Subjects
Patients were evaluated for inclusion if they presented to the Hospital of the University of Pennsylvania between 2005 and 2010 for lung transplantation evaluation for a diagnosis of IPF, pulmonary fibrosis, or ILD. Eligible patients were screened to ensure they fulfilled “definite” usual interstitial pneumonia (UIP) criteria by high-resolution computed tomography (CT) or “possible” UIP criteria by CT with “definite or probable” histopathologic UIP criteria using the 2011 American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines for IPF (reviewed by BNR-L).19 We excluded patients with other forms of diffuse parenchymal lung disease or coexisting rheumatologic disease.
Hemodynamic characteristics
Patients underwent a right heart catheterization (RHC) at rest (Swan-Ganz catheter, Edwards Lifesciences). For quality control, RHC tracings from a subset (68%) of the cohort were revaluated by an investigator (BNR-L) blinded to clinical and echocardiographic data.
Echocardiography
Patients underwent transthoracic Doppler echocardiography, findings of which were interpreted by a physician (BNR-L) blinded to the clinical data and hemodynamic characteristics (Prosolv Cardio-Vascular, FUJIFILM Holdings America). A second physician blinded to the initial read, clinical data, and RHC tracings reviewed 41 (32%) of the echocardiograms for quality control. Left and right chamber volumes and systolic function were assessed according to the American Society of Echocardiography guidelines.20
Pulmonary function testing (PFT)
All patients underwent spirometry conducted according to the updated 2005 ATS/ERS guidelines.21 Lung volumes were measured using whole-body plethysmography, and DLCO was obtained by the single-breath technique. Standard normative equations were used for spirometry,22 lung volumes,23 and DLCO.24
Six-minute walk test
All patients underwent 6-minute walk testing indoors on a flat surface according to 2002 ATS guidelines10 with supplemental oxygen use. Arterial blood gas sampling was performed at rest with room air for all patients, except for two patients who underwent testing while receiving 2 or 6 L/min of oxygen.
Statistical analysis
Continuous variables were summarized with mean and standard deviation or median and interquartile range (IQR). Categorical variables were summarized by frequency and percentages. Differences in hemodynamic characteristics between those with and without RV dilation were assessed with unpaired t tests, Mann-Whitney U tests, or χ2 tests.
Bivariate analysis was used to evaluate the association of various demographic, pulmonary function, echocardiographic, and hemodynamic parameters selected a priori with 6MWD. Variables with P value ≤0.15 were considered for inclusion into the multivariable model. Interactions were assessed a priori between sex and covariates. If variables were collinear, the variable with the strongest correlation with 6MWD was included in multivariable analysis. Age, sex, race, height, and weight were forced into the multivariable analysis. We used purposeful selection of covariates to generate the final multivariable model. Individual variables were retained in the final multivariable regression model if the P value was <0.05 or their inclusion changed the β-coefficient of other predictors by >15%. RV dysfunction was evaluated in a multivariable model without PVR due to collinearity. Twenty complete data sets were generated with multiple imputation to account for missing data. Both the model excluding subjects with missing data (complete case analysis) and the model using multiply imputed data are presented. Postestimation model checking was performed to assess for normally distributed residuals and adequate model fit.
A P value <0.05 was used to indicate statistical significance. Statistical analyses were performed using STATA software version 12.0 (StataCorp, College Station, TX).
Results
During the study period, 787 patients were evaluated for lung transplantation at our center. Of those, 315 had a clinical diagnosis of IPF, pulmonary fibrosis, or ILD. A total of 153 met “definite” UIP criteria by high-resolution CT or “possible” UIP criteria by CT with “definite or probable” histopathologic UIP criteria using the 2011 ATS/ERS guidelines for IPF (reviewed by BNR-L).19 Of the 153 patients, 23 did not have Doppler echocardiogram, RHC, or 6MWD data available, leaving 130 patients in the final study sample (Fig. 1).
Figure 1.
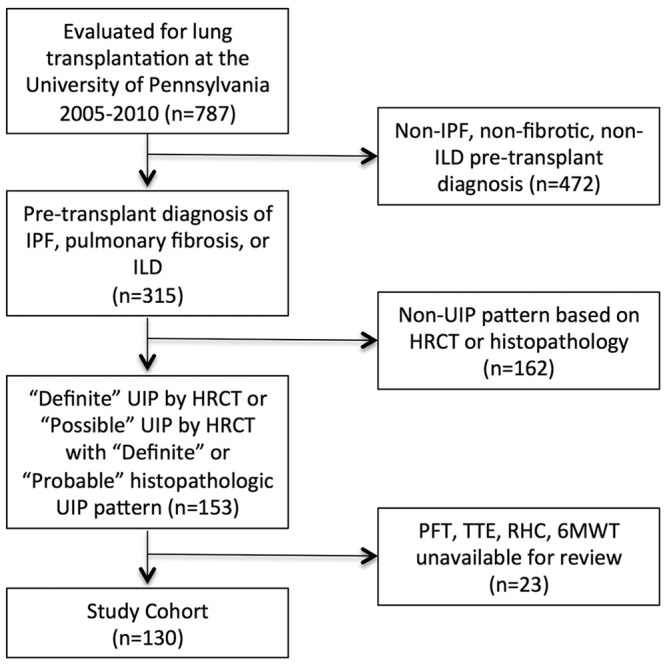
Study population. HRCT: high-resolution computed tomography; ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis; PFT: pulmonary function testing; RHC: right heart catheterization; TTE: transthoracic echocardiogram; UIP: usual interstitial pneumonia; 6MWT: 6-minute walk test.
Characteristics of the study sample are shown in Tables 1 and 2. The median age was 60 years (IQR, 55–63 years). The study population was mostly male (73.1%), white (81.5%), and former smokers (73.1%). The mean FVC percentage predicted (FVC%) was , and the median DLCO% was 36% (IQR, 31%–49%). Sixteen patients (12.3%) had FVC% >70%. Eleven subjects (8.5%) were prescribed pulmonary vasodilators. Eighty percent of our cohort required supplemental oxygen during their 6-minute walk test at a median flow rate of 6 L/min (IQR, 2–10 L/min). The mean 6MWD was m. Of the 130 patients, 67 (51.5%) underwent transplantation.
Table 1.
Characteristics of the cohort
| Characteristic | Patients (n = 130) |
|---|---|
| Age, years, median (IQR) | 60 (55–63) |
| Male sex | 95 (73.1) |
| Race/ethnicity | |
| White | 106 (81.5) |
| Black | 10 (7.7) |
| Other | 14 (10.8) |
| BMI, mean ± SD | 29.0 ± 5.31 |
| Past smoker | 95 (73.1) |
| Supplemental O2 at rest, L, median (IQR) | 3 (0–5) |
| Use of pulmonary vasodilators | 11 (8.5) |
| Pulmonary function test result | |
| FVC, L, mean ± SD | 2.2 ± 0.8 |
| FEV1, L, mean ± SD | 1.8 ± 0.6 |
| FEV1/FVC ratio, median (IQR) | 85.5 (81–90) |
| TLC, L, mean ± SD | 3.6 ± 0.9a |
| DLCO, mL/min/mmHg, median (IQR) | 9.3 (7.1–12.7)b |
| Six-minute walk test | |
| Total distance, m, mean ± SD | 379.1 ± 127.6 |
| Oxygen use during walk test | 104 (80.0) |
| Supplemental oxygen during walk test, L/min, median (IQR) | 6 (2–10) |
| Nadir oxygen saturation while receiving supplemental oxygen, %, mean ± SD | 88.3 ± 5.2 |
Data are no. (%) of patients, unless otherwise indicated. BMI: body mass index, calculated as weight in kilograms divided by the square of height in meters; DLCO: diffusing capacity of the lung for carbon monoxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; IQR: interquartile range; SD: standard deviation; TLC: total lung capacity.
n = 124.
n = 104.
Table 2.
Echocardiographic and hemodynamic characteristics of the cohort
| Finding | Patients |
|---|---|
| Echocardiography | |
| Presence of right atrial dilation (n = 125) | 51 (40.8) |
| Presence of RV dilation (n = 127) | 56 (44.1) |
| Presence of moderate to severe RV dysfunction (n = 128) | 12 (9.4) |
| TAPSE, cm, mean ± SD (n = 129) | 2.0 ± 0.5 |
| RV systolic pressure, mmHg, median (IQR) (n = 93) | 42 (34–54) |
| Ratio of RV to LV internal diameter, median (IQR) (n = 127) | 0.9 (0.7–1.1) |
| Left ventricular ejection fraction, %, median (IQR) (n = 130) | 65 (60–70) |
| Presence of diastolic dysfunction (n = 101) | 69 (68.3) |
| E/A, median (IQR) (n = 126) | 0.9 (0.7–1.1) |
| Presence of tricuspid regurgitation (n = 130) | 62 (47.7) |
| Presence of mitral regurgitation (n = 129) | 36 (27.9) |
| Right heart catheterization | |
| Heart rate, beats per minute, median (IQR) (n = 130) | 76.5 (66–86) |
| Right atrial pressure, mmHg, mean ± SD (n = 128) | 5.4 ± 4.5 |
| Mean PAP, mmHg, median (IQR) (n = 130) | 23 (19–30) |
| Pulmonary capillary wedge pressure, mmHg, mean ± SD (n = 129) | 10.3 ± 5.6 |
| Left ventricular end-diastolic pressure, mmHg, mean (IQR) (n = 122) | 11.5 (8–16) |
| Pulmonary vascular resistance, Wood units, median (IQR) (n = 126) | 3.1 (2.5–4.3) |
| Systemic vascular resistance, Wood units, median (IQR) (n = 125) | 14.7 (12–18.1) |
| Cardiac output, L/min, median (IQR) (n = 126) | 5.6 (4.7–6.4) |
| Cardiac index, L/min/m2, mean ± SD (n = 126) | 2.9 ± 0.6 |
| Presence of PHa (n = 129) | 38 (29.2) |
Data are no. (%) of patients unless otherwise indicated. E/A: early to late mitral inflow ventricular filling velocities; IQR: interquartile range; LV: left ventricle; PAP: pulmonary artery pressure; PH: pulmonary hypertension; RV: right ventricle; SD: standard deviation; TAPSE: tricuspid annular plane systolic excursion.
Defined as mean PAP ≥25 and pulmonary capillary wedge pressure <15.
Among the 56 subjects with RV dilation, 22 had moderate to severe RV dilation. Thirty-eight patients (29.2%) had pulmonary hypertension (PH) defined as mPAP ≥25 mmHg and pulmonary capillary wedge pressure (PCWP) <15 mmHg. The median mPAP in those with and without PH was 30 mmHg (IQR, 27–33 mmHg) and 21 mmHg (IQR, 18–24 mmHg), respectively (). Compared with subjects without RV dilation, those with RV dilation had a significantly higher PVR, higher mPAP, and shorter 6MWD (Table 3). Among the 52 patients with normal PVR (≤3 Wood units [WU]) at rest, 13 had mild RV dilation and 5 had moderate RV dilation.
Table 3.
Pulmonary function test and hemodynamic data stratified by the presence of right ventricular (RV) dilation on echocardiogram
| Variable | No RV dilation (n = 71) |
RV dilation (n = 56) |
Pa |
|---|---|---|---|
| Age, years | 60 (56–63) | 59 (53.5–63.0) | 0.64 |
| Male sex, no. (%) of patients | 47 (66.2) | 45 (80.4) | 0.08 |
| LVEF, % | 65 (60–70) | 65 (60–70) | 0.74 |
| FVC, % predicted, mean ± SD | 52.9 ± 14.9 | 50.3 ± 15.9 | 0.35 |
| FEV1, % predicted, mean ± SD | 57.7 ± 15.5 | 54.9 ± 17.3 | 0.34 |
| TLC, % predicted | 58 (51–64) (n = 69) | 57 (49–63.5) (n = 52) | 0.41 |
| DLCO, mL/min/mmHg | 9.2 (7.1–13.4) (n = 58) | 9.5 (7.1–11.5) (n = 44) | 0.42 |
| PASP, mmHg | 34 (29–41) | 40 (34–49) | 0.001 |
| Mean PAP, mmHg | 22 (17–26) | 25.5 (20.5–31.5) | 0.002 |
| PVR, Wood units | 3 (2.2–3.9) (n = 70) | 3.4 (2.8–5.3) (n = 53) | 0.001 |
| PCWP, mmHg | 10 (8–13) (n = 70) | 8.5 (6.5–14.0) | 0.61 |
| Cardiac output, L/min, mean ± SD | 5.6 ± 1.3 (n = 70) | 5.6 ± 1.4 (n = 53) | 0.88 |
| Cardiac index, L/min/m2, mean ± SD | 2.9 ± 0.5 (n = 70) | 2.8 ± 0.6 (n = 53) | 0.60 |
| Abnormal RV function, no. (%) of patients | 4 (5.6) | 29 (52.7) (n = 55) | <0.001 |
| Six-minute walk distance, m, mean ± SD | 411.4 ± 116.3 | 345.6 ± 128.4 | 0.003 |
| Nadir oxygen saturation, %, mean ± SD | 88.9 ± 5.8 | 87.3 ± 4.1 | 0.06 |
Data are median (interquartile range) unless otherwise indicated. DLCO: diffusing capacity of the lung for carbon monoxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; LVEF: left ventricular ejection fraction; PAP: pulmonary artery pressure; PASP: pulmonary artery systolic pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; SD: standard deviation; TLC: total lung capacity.
Fisher exact test or χ2 test for categorical variables; Student t test with equal variance for normally distributed continuous variables; Mann-Whitney U test for nonnormally distributed continuous variables.
The median time between RHC and Doppler echocardiogram was 24 hours (IQR, 24–48 hours), and the median time between RHC and PFTs was 48 hours (IQR, 24–72 hours). To ensure accurate identification of subjects with IPF for inclusion into our study, 32 (10%) of the 315 subjects who had a diagnosis of ILD, IPF, or pulmonary fibrosis were independently reviewed for a diagnosis of IPF (SMK). An interobserver κ of 1.0 demonstrated perfect agreement for the diagnosis of IPF between the two observers. The intraclass correlation coefficients (ICCs) between two independent interpretations of the echocardiograms ranged from 0.70 to 0.90 (depending on the measure). The ICCs for hemodynamic parameters in the 92 patients with RHC (68%), blindly reinterpreted and compared with the clinical reports, ranged from 0.95 to 0.99. Because of the very strong agreement, the clinical report data were used in the analysis.
Predictors of 6MWD
Table E1 (63.1KB, pdf) shows the bivariate analysis of demographic and PFT variables with 6MWD. Lower FVC, forced expiratory volume in 1 second, total lung capacity, DLCO, and resting partial pressure of oxygen in arterial blood (PaO2) were all associated with significantly shorter 6MWD. The presence of right arterial (RA) dilation or RV dilation was associated with significantly shorter 6MWD (Table E2 (63.1KB, pdf) ), whereas the presence of tricuspid regurgitation, mitral regurgitation, or any RV dysfunction were not. However, the presence of moderate to severe RV dysfunction (), compared with no or mild RV dysfunction, was associated with a 99.7 m (95% CI, 26.0–173.5; ) shorter 6MWD. Higher RV∶LV diameter was also associated with a significantly shorter 6MWD. Higher mPAP and PVR (Table E2 (63.1KB, pdf) ; Fig. 2) on RHC were significantly associated with lower 6MWD; however, cardiac output, cardiac index, and PCWP were not.
Figure 2.
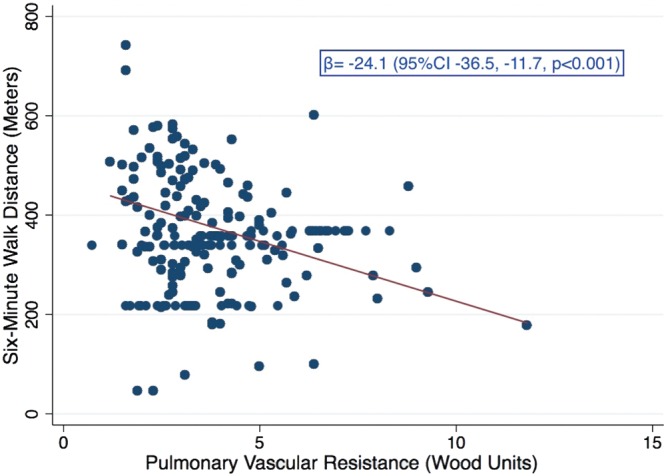
Bivariate regression of pulmonary vascular resistance and 6-minute walk distance. CI: confidence interval.
Multivariable regression with multiple imputation is shown in Table 4. After adjustment for age, sex, race, height, and weight, the presence of RV dilation was associated with a 50.9 m (95% CI, 8.4–93.3) shorter 6MWD (). For each 200-mL reduction in FVC, 6MWD decreased by 15.0 m (95% CI, 9.0–21.1; ). For every 1-WU increase in PVR, 6MWD decreased by 17.3 m (95% CI, 5.1–29.5; ). As sex, race, height, and weight were included in the model, use of indexed parameters was not required. Inclusion of PaO2 decreased the β coefficients for both PVR (from −17.3 to −11.7) and RV dilation (from −50.9 to −37.8) by >15%. If moderate to severe RV dysfunction was substituted for PVR in the model without PaO2, the presence of moderate to severe RV dysfunction and RV dilation were independently associated with a lower 6MWD (moderate to severe RV dysfunction [95% CI, −166.3 to −20.6]; ; RV dilation [95% CI, −99.5 to −15.1]; ).
Table 4.
Multivariable regression coefficients using multiple imputation
| Without PaO2 (n = 130) |
With PaO2 (n = 130) |
|||
|---|---|---|---|---|
| Variable | β (95% CI) | P | β (95% CI) | P |
| Age, years | −2.6 (−5.6 to 0.5) | 0.10 | −2.4 (−5.4 to 0.5) | 0.11 |
| Male sex | 44.8 (−14.4 to 104.1) | 0.14 | 54.3 (−2.7 to 111.4) | 0.06 |
| Race | ||||
| White | Ref | … | Ref | … |
| Black | −39.1 (−119.3 to 41.1) | 0.34 | −34.3 (−113.2 to 44.5) | 0.39 |
| Other | −21.2 (−85.8 to 43.5) | 0.52 | −33.9 (−97.4 to 29.6) | 0.29 |
| Height, per 10-cm increase | −35.6 (−66.7 to −4.6) | 0.03 | −31.2 (−60.9 to −1.4) | 0.04 |
| Weight, per 1-kg increase | −0.6 (−2.0 to 0.7) | 0.35 | −0.1 (−1.4 to 1.2) | 0.87 |
| FVC, per 200-mL decrease | −15.0 (−21.1 to −9.0) | <0.001 | −11.9 (−18.0 to −5.9) | <0.001 |
| Presence of RV dilation | −50.9 (−93.3 to −8.4) | 0.02 | −37.8 (−79.1 to 3.5) | 0.08 |
| PVR, per 1-WU increase | −17.3 (−29.5 to −5.1) | 0.006 | −11.7 (−24.9 to 1.5) | 0.08 |
| PaO2, per 10-mmHg decrease | … | … | −19.3 (−30.6 to −8.0) | 0.001 |
CI: confidence interval; FVC: forced vital capacity; PaO2: partial pressure of oxygen in arterial blood; PVR: pulmonary vascular resistance; RV: right ventricular; WU: Wood unit.
Multivariable regression using complete case analysis is provided in the Appendix (Table E3) (63.1KB, pdf) . The effect of each variable on 6MWD as measured by the β coefficient estimate was similar between the complete case analysis and the multivariable model created using multiple imputation data with the exception of height and black race.
Discussion
We have identified several determinants of 6MWD in patients with IPF undergoing evaluation for lung transplantation, including RV dilation and dysfunction, PVR, and FVC. Our study confirms that exercise limitation in IPF is multifactorial in nature with contribution from circulatory impairment and respiratory mechanics. This study is unique in its incorporation of hemodynamics and detailed echocardiographic assessment of RV morphology and function. This is the first study, to our knowledge, to demonstrate that RV morphology on transthoracic echocardiogram (TTE) has a significant and independent association with 6MWD.
In our study, higher PVR and the presence of RV dilation were independently associated with shorter 6MWD. Taken together, this likely reflects an impact of RV afterload and circulatory impairment on exercise capacity. Pulmonary vascular remodeling of the small and large vessels in IPF occurs via hypoxia-dependent and hypoxia-independent mechanisms.25-28 These changes increase PVR and decrease compliance, both of which contribute to RV afterload.28 The effect of higher resting PVR and the presence of RV dilation on 6MWD in our study could both be mediated through limitation in RV flow due to higher afterload. PVR may partially reflect the afterload encountered by the RV at rest, whereas RV dilation may indirectly reflect exertional afterload. Several case studies have documented transient RV dilation during exercise in patients with PH.29,30 Recurrent transient increases in afterload during exercise or nocturnal desaturation in patients with IPF may lead to changes in RV morphology over time that may not be captured by resting hemodynamic measurements. In a study of 65 patients with ILD including IPF without PH at rest, the mPAP increased with exercise from 21.7 to 45.3 mmHg.31 In a study of 25 patients with ILD, 7 had PH based on resting hemodynamic characteristics, and another 8 demonstrated abnormal pulmonary vascular response on the basis of an elevated mPAP indexed to cardiac output on invasive cardiopulmonary exercise testing.32 Such increases in mPAP during exercise or sleep may lead to subclinical increased RV wall stress, resulting in RV dilation and impaired exercise tolerance. PaO2 attenuated the association of 6MWD with PVR and RV dilation, suggesting that hypoxemia may be a mediator or confounder of this relationship. The prevalence of RV dilation in our cohort was higher than in previous studies,33 which may be due to our study population of patients undergoing lung transplantation evaluation.
The presence of PH with normal left-sided filling pressure did not demonstrate a significant association on bivariate analysis with 6MWD, as it has in other studies.16,25,26 Resting PH itself may not accurately reflect increases in afterload that occur in the setting of exercise, during sleep, or over time. Degani-Costa and colleagues demonstrated abnormal vascular response to exercise in 28% of their ILD cohort with normal resting hemodynamics.32 RV function was relatively preserved in our cohort as assessed by cardiac output, stroke volume, and RA pressure. The presence of moderate to severe RV dysfunction was associated with a shorter 6MWD. After adjusting for RV dysfunction, the presence of RV dilation remained independently associated with 6MWD and suggests that RV chamber size may provide additional insight into RV function beyond qualitative measures on resting TTE. RV dilation may reflect remodeling, increased RV wall stress or tension, and possible impaired ventricular-arterial coupling as a result of elevated RV afterload, all affecting exercise performance.34,35
Lower FVC was also independently associated with lower 6MWD, consistent with earlier studies,6,36,37 and suggests that the degree of restriction plays a role in exertional tolerance. Progressive fibrosis in IPF leads to ventilation/perfusion mismatch and reduced diffusion capacity. In our cohort, DLCO was significantly associated with 6MWD on bivariate analysis, a finding similar to those of previous studies.11,38 However, on multivariable analysis, DLCO did not maintain significance after adjustment for PVR, RV dilation, and FVC.
Our study has some limitations. The 6-minute walk test was continued with supplemental oxygen if the oxygen saturation decreased below 80%, reflecting the “real world” function of patients with IPF. Earlier studies have terminated the walk test if the oxygen saturation fell below a certain cutoff point, which may falsely truncate the distance and limit generalizability, because patients with IPF use oxygen during exertion in their daily lives.5,6,38 By allowing the subjects to use supplemental oxygen, our study was able to capture the factors that limit exercise capacity beyond oxygenation. Despite the variable use of supplemental oxygen during the 6-minute walk test in other studies, 6MWD has been repeatedly shown to be a reproducible and strong predictor of survival.5,6,38,39 This suggests that the use of supplemental oxygen does not impact on the inferences drawn from the distance.
All tests were not always performed on the same day, potentially introducing variability. However, 75% of subjects had their RHC and Doppler echocardiogram completed within 2 days of each other, making significant clinical changes between assessments unlikely. No information regarding skeletal strength, frailty, or reason for 6MWD termination was available for analysis. Studies of patients evaluated for lung transplantation may have selection bias. Although lung transplantation evaluation is recommended for all patients with IPF,19 the lower median age of our population (likely constrained by the transplantation referral) may limit generalizability to older patients with IPF. Factors determining exercise limitation may differ in those patients who are not referred for evaluation for transplantation.
Our findings stress the importance of pulmonary vascular function and RV structural changes in determining 6MWD in IPF, in addition to the well-known lung structural changes. A recent study of sildenafil in IPF showed the greatest effect in patients with changes in RV morphology.40 The effect of PVR and RV dilation on 6MWD may in part be mediated by transient or sustained impairment in oxygenation. Understanding the etiology of exercise limitation on 6MWT in patients in IPF may offer a potential target to increase exercise capacity and improve quality of life.
Acknowledgments
Some of the results of this study were presented in abstract and poster form at the 2014 American Thoracic Society International Conference, May 16–21, San Diego, California.
Source of Support: National Institutes of Health (NIH) grants T32 HL-007891 (MKP) and K24 HL-103844 (SMK).
Conflict of Interest: MKP, BNR-L, MK, and JL have no conflicts of interest. SMK reports grants to his institution from the NIH, Actelion, United Therapeutics, Gilead, Lung Biotech, Pfizer, Ikaria, Pulmonary Hypertension Association, Merck, GeNO, and Bayer. SMK also reports personal fees from Insmed and European Respiratory Journal and nonfinancial support from the American College of Clinical Pharmacy and the American Thoracic Society.
Supplements
Appendix (63.1KB, pdf)
References
- 1.Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183(4):431–440. [DOI] [PubMed]
- 2.Harris-Eze AO, Sridhar G, Clemens RE, Gallagher CG, Marciniuk DD. Oxygen improves maximal exercise performance in interstitial lung disease. Am J Respir Crit Care Med 1994;150(6 Pt 1):1616–1622. [DOI] [PubMed]
- 3.Holland AE. Exercise limitation in interstitial lung disease: mechanisms, significance and therapeutic options. Chron Respir Dis 2010;7(2):101–111. [DOI] [PubMed]
- 4.Hansen JE, Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest 1996;109(6):1566–1576. [DOI] [PubMed]
- 5.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med 2011;183(9):1231–1237. [DOI] [PubMed]
- 6.Lederer DJ, Arcasoy SM, Wilt JS, D’Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174(6):659–664. [DOI] [PMC free article] [PubMed]
- 7.Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med 2006;100(10):1734–1741. [DOI] [PubMed]
- 8.du Bois RM, Albera C, Bradford WZ, Costabel U, Leff JA, Noble PW, Sahn SA, et al. 6-Minute walk test distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J 2014;43(5):1421–1429. [DOI] [PubMed]
- 9.Cahalin L, Pappagianopoulos P, Prevost S, Wain J, Ginns L. The relationship of the 6-min walk test to maximal oxygen consumption in transplant candidates with end-stage lung disease. Chest 1995;108(2):452–459. [DOI] [PubMed]
- 10.American Thoracic Society (ATS) Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166(1):111–117. [DOI] [PubMed]
- 11.Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171(10):1150–1157. [DOI] [PubMed]
- 12.Davis SQ, Garrity ER Jr. Organ allocation in lung transplant. Chest 2007;132(5):1646–1651. [DOI] [PubMed]
- 13.Reesink HJ, van der Plas MN, Verhey NE, van Steenwijk RP, Kloek JJ, Bresser P. Six-minute walk distance as parameter of functional outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2007;133(2):510–516. [DOI] [PubMed]
- 14.Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, Nakanishi N, Miyatake K. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension: comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000;161(2 Pt 1):487–492. [DOI] [PubMed]
- 15.Rovedder PM, Ziegler B, Pasin LR, Rampon G, Pinotti AF, de Tarso Roth Dalcin P, Menna-Barreto SS. Doppler echocardiogram, oxygen saturation and submaximum capacity of exercise in patients with cystic fibrosis. J Cyst Fibros 2007;6(4):277–283. [DOI] [PubMed]
- 16.Minai OA, Santacruz JF, Alster JM, Budev MM, McCarthy K. Impact of pulmonary hemodynamics on 6-min walk test in idiopathic pulmonary fibrosis. Respir Med 2012;106(11):1613–1621. [DOI] [PubMed]
- 17.Mak VH, Bugler JR, Roberts CM, Spiro SG. Effect of arterial oxygen desaturation on six minute walk distance, perceived effort, and perceived breathlessness in patients with airflow limitation. Thorax 1993;48(1):33–38. [DOI] [PMC free article] [PubMed]
- 18.Rivera-Lebron BN, Forfia PR, Kreider M, Lee JC, Holmes JH, Kawut SM. Echocardiographic and hemodynamic predictors of mortality in idiopathic pulmonary fibrosis. Chest 2013;144(2):564–570. [DOI] [PMC free article] [PubMed]
- 19.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, et al.; ATS ERS JRS ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183(6):788–824. [DOI] [PMC free article] [PubMed]
- 20.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18(12):1440–1463. [DOI] [PubMed]
- 21.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26(5):948–968. [DOI] [PubMed]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159(1):179–187. [DOI] [PubMed]
- 23.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed]
- 24.Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state: predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis 1983;127(3):270–277. [DOI] [PubMed]
- 25.Glaser S, Noga O, Koch B, Opitz CF, Schmidt B, Temmesfeld B, Dorr M, Ewert R, Schaper C. Impact of pulmonary hypertension on gas exchange and exercise capacity in patients with pulmonary fibrosis. Respir Med 2009;103(2):317–324. [DOI] [PubMed]
- 26.Glaser S, Obst A, Koch B, Henkel B, Grieger A, Felix SB, Halank M, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis: the predictive value of exercise capacity and gas exchange efficiency. PLoS ONE 2013;8(6):e65643. [DOI] [PMC free article] [PubMed]
- 27.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest 2007;132(3):998–1006. [DOI] [PubMed]
- 28.Naeije R. Physiology of the pulmonary circulation and the right heart. Cur Hypertens Rep 2013;15(6):623–631. [DOI] [PubMed]
- 29.Cotrim C, Loureiro MJ, Miranda R, Almeida S, Almeida AR, Simoes O, Cordeiro P, Carrageta M. Should right ventricle dilatation during exercise have clinical implications in patients with chronic thromboembolic pulmonary hypertension? Case report. Cardiovasc Ultrasound 2007;5:50. [DOI] [PMC free article] [PubMed]
- 30.Cotrim C, Loureiro MJ, Miranda R, Lohmann C, Simoes O, Cordeiro P, Fernandes R, et al. Right ventricular dilatation during exercise: a new sign? Rev Port Cardiol 2007;26(9):939–940. [PubMed]
- 31.Weitzenblum E, Ehrhart M, Rasaholinjanahary J, Hirth C. Pulmonary hemodynamics in idiopathic pulmonary fibrosis and other interstitial pulmonary diseases. Respiration 1983;44(2):118–127. [DOI] [PubMed]
- 32.Degani-Costa LH, Levarge B, Digumarthy SR, Eisman AS, Harris RS, Lewis GD. Pulmonary vascular response patterns during exercise in interstitial lung disease. Eur Respir J 2015;46(3):738–749. [DOI] [PubMed]
- 33.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 2005;128(4):2393–2399. [DOI] [PubMed]
- 34.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009;135(3):794–804. [DOI] [PubMed]
- 35.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006;114(17):1883–1891. [DOI] [PubMed]
- 36.Garin MC, Highland KB, Silver RM, Strange C. Limitations to the 6-minute walk test in interstitial lung disease and pulmonary hypertension in scleroderma. J Rheumatol 2009;36(2):330–336. [DOI] [PubMed]
- 37.Mura M, Ferretti A, Ferro O, Zompatori M, Cavalli A, Schiavina M, Fabbri M. Functional predictors of exertional dyspnea, 6-min walking distance and HRCT fibrosis score in idiopathic pulmonary fibrosis. Respiration 2006;73(4):495–502. [DOI] [PubMed]
- 38.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med 2006;174(7):803–809. [DOI] [PMC free article] [PubMed]
- 39.Hook JL, Arcasoy SM, Zemmel D, Bartels MN, Kawut SM, Lederer DJ. Titrated oxygen requirement and prognostication in idiopathic pulmonary fibrosis. Eur Respir J 2012;39(2):359–365. [DOI] [PMC free article] [PubMed]
- 40.Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB, Anstrom KJ, Martinez FJ, IPFnet Investigators. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest 2013;143(6):1699–1708. [DOI] [PMC free article] [PubMed]


