Abstract
Background and Aim
The American Association for the Study of Liver Disease (AASLD) recommends screening for esophageal varices (EV) by esophagoduodenoscopy (EGD) in patients with cirrhosis to guide decisions regarding primary prophylaxis for EV hemorrhage. We aimed to identify patient and facility factors associated with EV screening in veterans with hepatitis C (HCV)-associated cirrhosis.
Methods
This was a population-based cohort study. Veterans with HCV and newly diagnosed cirrhosis between 1/1/2004 and 12/31/2005 and followed until 12/31/2011 were included. The primary outcome was receipt of EGD within 1 year of cirrhosis diagnosis. Patient- and facility-level factors associated with EV screening were determined.
Results
A total of 4230 patients with HCV cirrhosis were identified. During median follow-up of 6.1 years (IQR: 4.0–8.0), 21.5 % developed a decompensating event, and 38.3 % died. Fifty-four percent received an EGD, and 33.8 % had an EGD within guidelines. Median time from cirrhosis diagnosis to EGD was 72 days (IQR: 12–176). Factors independently associated with receipt of EV screening were a decompensation event (OR 1.16, CI 1.01–1.32) and gastroenterology/hepatology clinic access (OR 2.1, CI 1.73–2.46), whereas cardiovascular (OR 0.81, CI 0.69–0.95), mental health (OR 0.79, CI 0.68–0.91), and respiratory (OR 0.85, CI 0.72–0.99) comorbidities were associated with reduced likelihood of EV screening.
Conclusion
EV screening per AASLD guidelines occurs in only one-third of patients. This missed opportunity was strongly associated with access to gastroenterology/hepatology specialty care. Additionally, providers may be relying on clinical cues (i.e., decompensation) to prompt referral for endoscopy suggesting education to improve compliance with guidelines is needed.
Keywords: Variceal screening, Cirrhosis, Hepatitis C, Endoscopy
Introduction
Esophageal variceal bleeding (EVB) is a devastating complication of cirrhosis with an in-hospital mortality rate of almost 15 % [1, 2]. Approximately 50 % of patients at the time of diagnosis of cirrhosis will have esophageal varices (EV) with the strongest predictors of EVB being the presence of endoscopic high-risk stigmata (large varices or red wale marks) in addition to underlying advanced liver dysfunction (Child-Turcotte-Pugh class B or C) [3, 4]. Multiple randomized studies have shown an almost 50 % decrease in the risk of first EVB in individuals with high-risk EV receiving primary prophylaxis with either non-selective beta-blockade or esophageal variceal band ligation [5–7]. This has led to recommendations to screen for EV by esophagogastroduodenoscopy (EGD) in patients with cirrhosis [8]. The American Association for the Study of Liver Disease (AASLD) clinical practice guidelines recommend that all patients with cirrhosis receive an EGD at the time of cirrhosis diagnosis in order to screen for EV and document the presence or absence of high-risk stigmata to allow clinicians to make evidence-based decisions regarding primary prophylaxis against EVB [4].
To date, there have been no population-based cohort studies evaluating whether EV screening guidelines are being adhered to in routine clinical practice of patients with cirrhosis. In the early 2000’s analysis of the Clinical Outcomes Research Initiative (CORI) database, a prospective cohort of patients from 68 practices (academic, community, and VHA) in 27 states established that 1 % of all EGDs were performed for EV screening and that the proportion for this indication was increasing over time [9]. However, the prevalence of cirrhosis in the underlying population was unknown, limiting the ability to comment on the proportion of the cirrhotic population receiving routine EV screening per guidelines. Furthermore, few studies have identified patient-related factors associated with the absence of EV screening. A recent study conducted in three Veterans Health Administration hospitals revealed that the majority of patients not receiving EV screening lacked a documented reason, but access to a gastroenterologist or being seen in an academic facility was associated with receiving recommended variceal care [10].
The Veterans Health Administration (VHA) with 174,302 infected with hepatitis C (HCV) is the largest single provider of HCV care in the nation [11]. Of these, 42,582 (24 %) have cirrhosis and sequelae of end-stage liver disease (e.g., hepatocellular carcinoma), and these complications are rising over time. Given the rising burden of HCV cirrhosis in the USA and veteran populations, the aims of this study were to identify the proportion of US veterans with newly diagnosed HCV-related cirrhosis who received EV screening per AASLD guidelines and to identify facility- and patient-related factors associated with EV screening with the goal of identifying potentially modifiable factors likely to yield changes in EV screening adherence.
Methods
Data Sources and Cohort Definition
We created a national cohort of HCV-infected veterans with a new diagnosis of cirrhosis made between 1/1/2004 and 12/31/2005 and followed until 12/31/2011. Included patients had continuous care within the VHA defined as having any visit within 2 years before and after date of cirrhosis diagnosis as well as at least one primary care visit 1 year before and after cirrhosis diagnosis and those who were alive for at least 2 years after cohort entry. We used the VHA HCV Clinical Case Registry, a national dataset of hepatitis C viremic confirmed veterans [12]. The HCV Clinical Case Registry data elements included demographics as well as outpatient and inpatient pharmacy data, laboratory data, and diagnoses and procedure codes. Cirrhosis was identified by one inpatient and/or two outpatient International Classification of Diseases 9th Revision (ICD-9) diagnostic codes (occurring on different dates) of cirrhosis (571.2, 571.5, 571.6) or of liver decompensation [13]. Liver decompensation was defined by ascites (789.5), hepatic encephalopathy (348.3, 572.2, 572.8), hepatorenal syndrome (572.4), viral hepatitis with coma (070.2, 070.4, 070.44, 070.6, 070.71), or hepatocellular carcinoma (155.0) [14]. Patients who did not have continuous care with the VHA, had an EGD greater than 1 year before cirrhosis diagnosis, had history of variceal bleeding greater than 1 month before cirrhosis diagnosis, had received transjugular intrahepatic portosystemic shunt or liver transplantation, were excluded (Fig. 1).
Fig. 1.
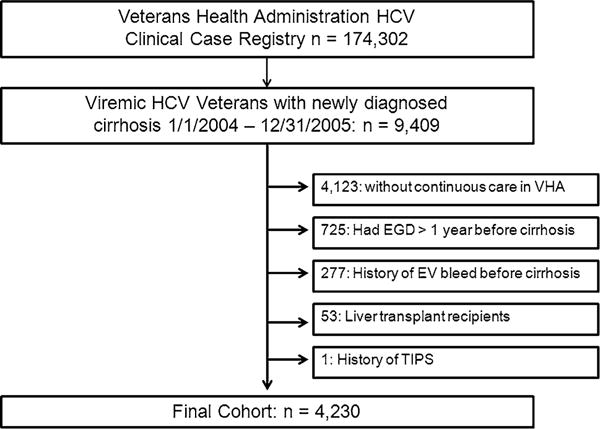
Cohort definition flow diagram. HCV hepatitis C, VHA Veterans Health Administration, EGD esophagoduodenoscopy, EV esophageal varices, TIPS transhepatic portosystemic shunt
Definition of Outcome Variables
Our primary outcome variable, screening EGD within AASLD guidelines, was defined as the receipt of an outpatient current procedural terminology (CPT) code for EGD (43200, 43217, 43219, 43234, 43235, 43201, 43259, 43244, 43205) within 1 year of cirrhosis diagnosis. The use of CPT codes for EGD in patients with cirrhosis in the VHA has been shown to have a positive predictive value of 90 % and a negative predictive value of 96 % [10]. Although the wording of the AASLD guidelines state an EGD should be performed “at the time of cirrhosis diagnosis,” we chose a 1-year interval to account for the time required for referral, consultation, and scheduling of the procedure [4]. We considered those individuals who had an EGD within 1 year prior to their cirrhosis diagnosis date to have also been screened as per guidelines since, in clinical practice, an EGD would have been unlikely repeated if done just before the diagnosis. Further, the EGD may have led to the diagnosis of cirrhosis if varices or portal hypertensive gastropathy was found during the endoscopy. The indication for the EGD was not examined. EGD outside of guidelines was defined as any outpatient CPT EGD code performed outside of the 1-year interval during the follow-up period. Receipt of an EGD within 30 days of a variceal bleeding event was not considered as a screening procedure given the temporal proximity to the bleeding event.
Definition of Patient and Facility Factors
We captured patient demographics [age and race/ethnicity (Caucasian, African-American, Hispanic and Unknown), obesity (body mass index ≥30)] and medical and psychiatric comorbidities [cardiac, renal failure (end-stage renal failure, stage-five chronic kidney disease, or hemodialysis), diabetes, psychiatric history, respiratory (including chronic obstructive pulmonary disease), human immunodeficiency syndrome (HIV) and substance use] identified using the Agency for Healthcare Research and Quality ICD-9 codes (supplementary table). The HCV Clinical Case Registry was merged with the Planning Systems Support Group geocoded file containing urban, rural, or highly rural categorization of patient residence [15, 16]. Urban residents are defined as anyone living in a US-Census-defined urbanized area. Rural residents include anyone not defined as urban. Highly rural residents live in counties with average population density of fewer than seven civilians per square mile [15]. Gastroenterology (GI)/hepatology clinic access was defined as at least one GI or hepatology clinic visit after cirrhosis diagnosis and before screening EGD (in those receiving EGD).
Ethics Statement
The HCV Clinical Case Registry is managed by the VHA Center for Quality Management in Public Health. Request for data download is reviewed by Center for Quality Management in Public Health staff, and upon local site investigator IRB approval, requested data elements are made available for download in de-identified form with each patient assigned a national random number identifier. The data were linked to a geo-coded file using this random number identifier. All downloaded data are stored on secured VHA servers and accessible only to approved study investigators and staff. This study was approved by the University of California, San Francisco Institutional Review Board and the San Francisco Veterans Affairs Research and Development Committee. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and the principles of Good Clinical Practice.
Statistical Analysis
Statistical comparisons of means, medians, and proportions of baseline cohort characteristics between patients receiving screening EGD within 1 year as compared to those receiving screening EGD greater than 1 year or not at all were calculated using ANOVA and Chi-square tests, respectively. Facility- and patient-level factors associated with EV screening within 1 year of cirrhosis diagnosis were examined using univariate and multivariate logistic regression. We adjusted for patient age and race in every model. Variables with P values <0.15 in univariate analysis were evaluated in the multivariate model. We evaluated interactions between (1) time to decompensation and receipt of EGD and (2) age and comorbid illness. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
Cohort Description (Table 1)
Table 1.
Baseline patient characteristics
| Characteristic | All patients (n = 4230) | EGD within 1 year (n = 1431)a | EGD longer than 1 year or no EGD (n = 2799)a | P value** |
|---|---|---|---|---|
| Age at cirrhosis diagnosis, years, median (IQR) | 54.4 (50.3–57.1) | 54.2 (50.2–57.1) | 54.5 (50.3–57.2) | 0.61 |
| Male, no (%) | 4147 (98.0) | 1405 (98.2) | 2742 (98.0) | 0.63 |
| Race/ethnicity, no (%)b | <0.0001 | |||
| White, Non-Hispanic | 2481 (66.2) | 909 (71.0) | 1572 (63.8) | |
| Black | 798 (21.3) | 213 (16.6) | 585 (23.7) | |
| Hispanic | 434 (11.6) | 147 (11.5) | 287 (11.6) | |
| Asian/Pacific Islander | 32 (0.9) | 11 (0.9) | 21 (0.9) | |
| Living in rural or high rural area at cohort entry, no (%) | 1277 (32.6) | 492 (34.4) | 885 (31.6) | 0.07 |
| Comorbidity at cohort entry, no (%) | ||||
| Cardiovascular | 3280 (77.5) | 1074 (75.1) | 2206 (78.8) | 0.006 |
| Obesity | 1050 (24.8) | 342 (23.9) | 708 (25.3) | 0.32 |
| Mental health diagnosis | 3022 (71.4) | 983 (68.7) | 2039 (72.9) | 0.005 |
| Renal failure | 717 (17.0) | 246 (17.2) | 471 (16.8) | 0.76 |
| Diabetes | 1752 (41.4) | 620 (43.3) | 1132 (40.4) | 0.07 |
| Substance use | 3497 (82.7) | 1173 (82.0) | 2324 (83.0) | 0.39 |
| Respiratory | 1029 (24.3) | 326 (22.8) | 703 (25.1) | 0.09 |
| Human immunodeficiency syndrome | 102 (2.4) | 26 (1.8) | 76 (2.7) | 0.07 |
| Gastroenterology or hepatology clinic visit, no (%) | 3471 (82.1) | 1228 (85.5) | 2100 (75) | <0.0001 |
| Decompensated cirrhosis at cohort entry, no (%) | 1883 (44.5) | 665 (45.8) | 1228 (43.9) | 0.24 |
| Developed decompensationc during follow-upd, no (%) | 909 (21.5) | 365 (47.6) | 544 (34.6) | <0.001 |
| Death during follow-up, no (%) | 1618 (38.3) | 568 (39.7) | 1050 (37.5) | 0.17 |
EGD esophagogastroduodenoscopy
P values were calculated from comparison of characteristics of patients with EGD within 1 year vs. Longer than 1 year or No EGD. Chisquare test for category variables and ANOVA for continuous variables unless otherwise marked
Based on time from cirrhosis diagnosis
Race/ethnicity data missing in 485 (11.5 %) patients
Defined as development of hepatic encephalopathy, ascites, hepatorenal syndrome, viral hepatitis with coma or hepatocellular carcinoma after cohort entry
Among those with compensated cirrhosis at cohort entry (n = 2347)
A total of 4230 patients with chronic HCV and newly diagnosed cirrhosis were identified and followed for a median of 6.1 years (IQR: 4.0–8.0 years). Median age at cirrhosis diagnosis was 54.4 years (IQR: 50.3–57.1 years), 4147 (98.0 %) were male, 2481 (66.2 %) were non-Hispanic White, and 1883 (44.5 %) presented with decompensation as their first diagnosis of cirrhosis. A total of 2284 (54.0 %) of patients received an EGD; 1431 (33.8 %) of patients received an EGD within 1 year with a median time from cirrhosis diagnosis to EGD of 72 days (IQR: 12–176 days). A total of 909 (21.5 %) patients progressed to decompensation and 1618 (38.3 %) died during study follow-up.
Baseline Patient Characteristics Based on Screening EGD (Table 1)
Compared to patients receiving screening EGDs greater than 1 year or not at all, patients receiving screening EGDs within 1 year were more frequently White, Non-Hispanic (71.0 vs. 63.8 %, P<0.001) and less frequently had cardiovascular (75.1 vs. 78.8 %, P = 0.006) and mental health (68.7 vs. 72.9 % P = 0.005) comorbidities. The majority (85.8 %) of patients who received a screening EGD per AASLD guidelines had been seen previously in a GI or hepatology clinic. Among those with compensated cirrhosis at cohort entry, just under half (47.0 %) of patients who received a screening EGD per AASLD guidelines developed decompensated disease prior to receipt of EGD.
Association Between Patient and Facility Factors and Receipt of Screening EGD per AASLD Guidelines (Tables 2, 3)
Table 2.
Univariate associations between patient and facility factors and receipt of screening EGD within 1 year of cirrhosis diagnosis
| Patient and facility factor | OR (95 % CI) | P value |
|---|---|---|
| Age at cirrhosis diagnosis, per year | 0.99 (0.99–1.00) | 0.30 |
| Male (vs. female) | 1.12 (0.70–1.79) | 0.63 |
| Race/ethnicitya | ||
| White, Non-Hispanic | Ref. | Ref. |
| Black | 0.63 (0.53–0.75) | <0.0001 |
| Hispanic | 0.89 (0.71–1.10) | 0.27 |
| Asian/Pacific Islander | 0.91 (0.43–1.89) | 0.79 |
| Living in rural or high rural (vs. urban) area at cohort entry | 0.88 (0.77–1.01) | 0.07 |
| Comorbidity (vs. no comorbidity) at cohort entry | ||
| Cardiovascular | 0.81 (0.70–0.94) | 0.006 |
| Obesity | 0.93 (0.80–1.08) | 0.32 |
| Mental health diagnosis | 0.82 (0.71–0.94) | 0.005 |
| Renal failure | 1.03 (0.87–1.22) | 0.77 |
| Diabetes | 1.13 (0.99–1.28) | 0.07 |
| Substance use | 0.93 (0.79–1.10) | 0.39 |
| Respiratory | 0.88 (0.76–1.02) | 0.09 |
| Human immunodeficiency syndrome | 0.66 (0.42–1.04) | 0.07 |
| Gastroenterology or hepatology clinic visit before EGD | 2.01 (1.70–2.39) | <0.0001 |
| Decompensated cirrhosis at cohort entry or during follow-upb | 2.12 (1.85–2.43) | <0.0001 |
EGD esophagogastroduodenoscopy
Race/ethnicity data missing in 485 (11.5 %) patients
Defined as development of hepatic encephalopathy, ascites, hepatorenal syndrome, viral hepatitis with coma, or hepatocellular carcinoma after cohort entry
Table 3.
Multivariate associations between patient and facility factors and receipt of screening EGD within 1 year of cirrhosis diagnosis
| Patient and facility factora | OR (95 % CI) | P value |
|---|---|---|
| Comorbidity (vs. no comorbidity) at cohort entry | ||
| Cardiovascular | 0.81 (0.69–0.95) | 0.01 |
| Mental health diagnosis | 0.79 (0.68–0.91) | 0.001 |
| Respiratory | 0.85 (0.72–0.99) | 0.041 |
| Gastroenterology or hepatology clinic visit before EGD | 2.07 (1.73–2.46) | <0.0001 |
| Decompensated cirrhosis at cohort entry or during follow-upb | 1.16 (1.01–1.32) | 0.03 |
EGD esophagogastroduodenoscopy
Model also adjusted for age at cirrhosis diagnosis, race/ethnicity, diabetes, HIV, renal failure, and living in rural, or high rural area at cohort entry
Defined as development of hepatic encephalopathy, ascites, hepatorenal syndrome, viral hepatitis with coma, or hepatocellular carcinoma after cohort entry
In univariate analysis, GI or hepatology clinic visit before screening EGD (OR 2.01, 95 % CI 1.70–2.39) and ever had a decompensation event (OR 2.12, 95 % CI 1.85–2.43) were positively associated with receiving EV screening within 1 year, while Black race (OR 0.63, 95 % CI 0.53–0.75), comorbidities including cardiovascular (OR 0.81, 95 % CI 0.70–0.94), and mental health diagnosis (OR 0.82, 95 % CI 0.71–0.94) were negatively associated with receiving EV screening within 1 year. In multivariate analysis, GI or hepatology clinic visit before screening EGD (OR 2.07, 95 % CI 1.73–2.46) and a decompensation event (OR 1.16, 95 % CI 1.01–1.32) were positively associated with receiving EV screening within 1 year, while cardiovascular (OR 0.81, 95 % CI 0.69–0.95), respiratory (OR 0.85, 95 % CI 0.72–0.99), and mental health (OR 0.79, 95 % CI 0.68–0.91) comorbidities were negatively associated.
Discussion
This is the first large population-based cohort study to look at the practice of EV screening in patients with newly diagnosed cirrhosis. We found that only one-third of all veterans with HCV cirrhosis received an EGD within a year of diagnosis as per the recommendations of practice guidelines. Further, 46 % of patients with cirrhosis never received an EGD after a median follow-up of 6 years. We were able to identify that a GI/hepatology visit and lack of cardiovascular, respiratory, and psychiatric comorbidities were associated with receiving EV screening within 1 year of cirrhosis diagnosis that may allow for the development of educational strategies aimed to increase EV screening uptake by clinicians caring for patients with cirrhosis.
This low rate of adherence to EV screening guidelines in patients with cirrhosis has been shown in two smaller studies. In one study, conducted within the VHA, investigators found that only 107/441 (24.3 %) of veterans with cirrhosis received an EGD within 1 year of cirrhosis diagnosis [10]. Similarly, a recent population-based cross-sectional study in an insured non-VHA population revealed that <20 % of patients with cirrhosis received an EGD within 1 year [17]. One potential explanation for the low EV screening rate may be a lack of awareness among the care providers of the value of EV screening in the cirrhotic population. This is supported by our finding that access to a GI/hepatology specialist was associated with receiving EV screening as this group of providers are those most likely to be aware of the practice recommendations and able to facilitate access to endoscopy. This is also supported by work from investigators at the Cleveland Clinic who reviewed their experience with screening 179 patients with cirrhosis for EV recruited from their tertiary care liver clinic and found that 94 % received an EGD [18]. Further, it has been shown that having been seen by a GI specialist was associated with higher quality of care indices for the management of EV [10].
Another possible explanation for the lack of screening EGDs in the cirrhotic population may be that clinicians are using other clinical or laboratory clues to determine those individuals most likely to have high-risk varices. This is suggested by our observation that the development of a decompensation event during follow-up prompted referral for EGD. Clinicians may also be using other noninvasive clinical tests (i.e., platelet count, aminotransferase-to-platelet ratio index, platelet count-to-spleen size ratio, or model for end-stage liver disease score) to determine which patients with cirrhosis are more likely to have EV. This approach is suboptimal as these clinical tests have been consistently shown to have poor sensitivity and specificity in identifying patients likely to have EV on screening [19–21]. While spleen stiffness and the combination of liver stiffness, spleen size, and platelet counts have shown improvements in the ability to identify patients with high-risk varices, they are not yet ready for use in routine clinical practice [19, 22]. As such, EGD remains the recommended screening modality. An additional clinical consideration is that providers may be substituting non-selective beta-blocker without EGD as a prophylactic strategy. While potentially a cost-effective strategy [23], prophylactic beta-blockade is not without its risks, especially in patients with refractory ascites [24] or a history of spontaneous bacterial peritonitis [25] and should not be pursued unless indicated by EGD findings. Further, the use of non-selective beta-blockers does not prevent the subsequent development of EV. Unfortunately, we were not able to explore this consideration in our administrative data set.
As EGD is an invasive procedure requiring conscious sedation, it is not unexpected that individuals with a history of cardiovascular and respiratory comorbidities were less likely to receive a screening EGD. We suspect that this may be related to concerns from clinicians that the risks of the EGD may outweigh the benefit in these populations, in spite of the overall low complication rate of 0.5 % [26–28]. Further, several studies have suggested that EGD performed for active bleeding within 30 days of myocardial infarction can be completed safely without significant added risk [29, 30]. Conversely, these groups of patients may be the least likely to tolerate an EVB due to their underlying cardiac and respiratory disease and consequently may derive more benefit from the prevention of an EVB then those with normal cardiac and lung function. Our results also suggest that EGD was not routinely performed in those with a history of mental illness. The rationale for this is less obvious but does not appear to be related to a lack of clinical follow-up (data not shown). However, we hypothesize that it maybe related to less adherence to medical recommendations in this population as this has been shown in previous studies [31]. Regardless, these groups of patients and their providers could be targeted for efforts to increase referral for EGD or may be a population in which the use and development of noninvasive means to detect high-risk EV would be beneficial.
There are several limitations to our study. First, due to the nature of administrative datasets, misclassification bias could be present in the study, specifically related to the administrative coding algorithms used for hepatic encephalopathy, as they have not been well validated. Additionally, we were not able to account for the severity of an individual’s comorbid illness (i.e., severity of chronic heart and lung diseases) due to the use of ICD codes and this could have influenced providers’ decisions to recommend EGD. Further, the generalizability of these findings may not be directly translatable beyond the VHA population. Secondly, we are unable to examine provider motivation and decision making for EGD referral, and we were unable to account for those individuals in whom an EGD was recommended for EV screening and was declined by the patient or was done at a facility outside of the VHA. Third, we were unable to look at the association between receipt of EGD and the outcome of EV bleeding as the dataset did not allow for the identification of veterans who received primary prophylaxis, a key variable which would need to be adjusted for in a model looking at this outcome. Finally, a large proportion of individuals in our cohort first presented with decompensated disease (44.5 %) and may not be representative of the majority of outpatient practices that may consist primarily of patients with well compensated cirrhosis. However, this proportion is similar to other populations based studies and is reflective of late diagnosis of advanced liver disease [32].
Variceal bleeding is a frequent and serious complication of cirrhosis and efforts to reduce mortality from this complication are important. As initiation and type of variceal bleeding prophylaxis is dependent upon EGD results, efforts to increase the uptake of EV screening by EGD could lead to better outcomes for individuals at high risk of variceal hemorrhage. As we identified that access to GI/Hepatology care was associated with EV screening, improving linkage to specialists would likely result in improved adherence to screening guidelines. Additionally, educational tools or best practice alerts for primary care providers caring for patients with cirrhosis may be a strategy to improve adherence to EV screening and could facilitate referral to GI/hepatology providers. Our results also support the need for further validation of noninvasive testing—such as liver and spleen stiffness [19, 22]—for high-risk EV in patients with cirrhosis and, if determined to be clinically useful, may provide another method to increase EV screening and/or provide primary prophylaxis in the absence of EV screening in the cirrhotic population.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. David Goldberg for his expertise and suggestions regarding the VA CCR. This study was supported in part by the Canadian Association for the Study of the Liver (CASL)/Merck Clinical Hepatology Fellowship (JAF). This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2 TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10620-015-3865-8) contains supplementary material, which is available to authorized users.
Conflict of interest The authors have no conflicts to disclose.
References
- 1.El-Serag HB, Everhart JE. Improved survival after variceal hemorrhage over an 11-year period in the Department of Veterans Affairs. Am J Gastroenterol. 2000;95:3566–3573. doi: 10.1111/j.1572-0241.2000.03376.x. [DOI] [PubMed] [Google Scholar]
- 2.Carbonell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652–659. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645–663. doi: 10.1016/s1089-3261(05)70186-0. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W, et al. Practice Guidelines Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 5.Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Beta-adrenergic antagonists in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Hepatology. 1997;25:63–70. doi: 10.1053/jhep.1997.v25.pm0008985266. [DOI] [PubMed] [Google Scholar]
- 6.Pascal JP, Cales P. Propranolol in the prevention of first upper gastrointestinal tract hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med. 1987;317:856–861. doi: 10.1056/NEJM198710013171403. [DOI] [PubMed] [Google Scholar]
- 7.Khuroo MS, Khuroo NS, Farahat KL, Khuroo YS, Sofi AA, Dahab ST. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther. 2005;21:347–361. doi: 10.1111/j.1365-2036.2005.02346.x. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332–354. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 9.Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc. 2007;65:82–88. doi: 10.1016/j.gie.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan PM, Kramer JR, El-Serag HB, Asch SM, et al. The quality of care provided to patients with varices in the department of Veterans Affairs. Am J Gastroenterol. 2014;109:934–940. doi: 10.1038/ajg.2013.487. [DOI] [PubMed] [Google Scholar]
- 11.Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88–96. doi: 10.1002/hep.20502. [DOI] [PubMed] [Google Scholar]
- 12.Backus LI, Gavrilov S, Loomis TP, Halloran JP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16:775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg D, Lewis J, Halpern S, Weiner M, et al. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21:765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rongey C, Shen H, Hamilton N, Backus LI, Asch SM, Knight S. Impact of rural residence and health system structure on quality of liver care. PLoS One. 2013;8:e84826. doi: 10.1371/journal.pone.0084826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West AN, Lee RE, Shambaugh-Miller MD, Bair BD, et al. Defining “rural” for veterans’ health care planning. J Rural Health. 2010;26:301–309. doi: 10.1111/j.1748-0361.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 17.Waghray A, Waghray N, Kyprianou A, Menon KVN. Variceal screening in cirrhotic patients. Hepatology. 2014;60(S1) Abstract 1594. [Google Scholar]
- 18.Moodley J, Lopez R, Carey W. Compliance with practice guidelines and risk of a first esophageal variceal hemorrhage in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010;8:703–708. doi: 10.1016/j.cgh.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Berzigotti A, Seijo S, Arena U, Abraldes JG, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102–111. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Zambam de Mattos A, Alves de Mattos A, Daros LF, Musskopf MI. Aspartate aminotransferase-to-platelet ratio index (APRI) for the non-invasive prediction of esophageal varices. Ann Hepatol. 2013;12:810–814. [PubMed] [Google Scholar]
- 21.Tafarel JR, Tolentino LH, Correa LM, Bonilha DR, et al. Prediction of esophageal varices in hepatic cirrhosis by noninvasive markers. Eur J Gastroenterol Hepatol. 2011;23:754–758. doi: 10.1097/MEG.0b013e3283488a88. [DOI] [PubMed] [Google Scholar]
- 22.Takuma Y, Nouso K, Morimoto Y, Tomokuni J, et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144:92–101. doi: 10.1053/j.gastro.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Saab S, DeRosa V, Nieto J, Durazo F, Han S, Roth B. Costs and clinical outcomes of primary prophylaxis of variceal bleeding in patients with hepatic cirrhosis: a decision analytic model. Am J Gastroenterol. 2003;98:763–770. doi: 10.1111/j.1572-0241.2003.07392.x. [DOI] [PubMed] [Google Scholar]
- 24.Serste T, Melot C, Francoz C, Durand F, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017–1022. doi: 10.1002/hep.23775. [DOI] [PubMed] [Google Scholar]
- 25.Mandorfer M, Bota S, Schwabl P, Bucsics T, et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680–1690. doi: 10.1053/j.gastro.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Arrowsmith JB, Gerstman BB, Fleischer DE, Benjamin SB. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37:421–427. doi: 10.1016/s0016-5107(91)70773-6. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PA, Campbell DR, Antonson CW, Weston AP, Shuler FN, Lozoff RD. Complications associated with endoscopic band ligation of esophageal varices. Gastrointest Endosc. 1993;39:181–185. doi: 10.1016/s0016-5107(93)70064-4. [DOI] [PubMed] [Google Scholar]
- 28.Daneshmend TK, Bell GD, Logan RF. Sedation for upper gastrointestinal endoscopy: results of a nationwide survey. Gut. 1991;32:12–15. doi: 10.1136/gut.32.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim RG, Cobell WJ, Theivanayagam S, Kilgore TW, et al. Endoscopy after acute myocardial infarction: an evaluation of safety. South Med J. 2013;106:545–549. doi: 10.1097/SMJ.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 30.Spier BJ, Said A, Moncher K, Pfau PR. Safety of endoscopy after myocardial infarction based on cardiovascular risk categories: a retrospective analysis of 135 patients at a tertiary referral medical center. J Clin Gastroenterol. 2007;41:462–467. doi: 10.1097/01.mcg.0000225624.91791.fa. [DOI] [PubMed] [Google Scholar]
- 31.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 32.Ratib S, Fleming KM, Crooks CJ, Aithal GP, West J. 1 and 5 year survival estimates for people with cirrhosis of the liver in England, 1998–2009: a large population study. J Hepatol. 2014;60:282–289. doi: 10.1016/j.jhep.2013.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


