Abstract
Setting: The programmatic management of drug-resistant tuberculosis (TB) in Viet Nam has been rapidly scaled up since 2009.
Objectives: To document the annual numbers of patients enrolled for multidrug-resistant tuberculosis (MDR-TB) treatment during 2010–2014 and to determine characteristics and treatment outcomes of patients initiating treatment during 2010–2012.
Design: A retrospective cohort study using national reports and data from the national electronic data system for drug-resistant TB.
Results: The number of patients enrolled annually for MDR-TB treatment increased from 97 in 2010 to 1522 in 2014. The majority of patients were middle-aged men who had pulmonary disease and had failed a retreatment regimen; 77% had received ⩾2 courses of TB treatment. Favourable outcomes (cured and treatment completed) were attained in 73% of patients. Unfavourable outcomes included loss to follow-up (12.5%), death (8%) and failure (6.3%). Having had ⩾2 previous treatment courses and being human immunodeficiency virus-positive were associated with unfavourable outcomes.
Conclusion: Increasing numbers of patients are being treated for MDR-TB each year with good treatment outcomes under national programme management in Viet Nam. However, there is a need to increase case detection—currently at 30% of the estimated 5100 MDR-TB cases per year, reduce adverse outcomes and improve monitoring and evaluation.
Keywords: operational research, SORT IT, MDR-TB, programmatic management
Abstract
Contexte : La prise en charge de la tuberculose (TB) pharmacorésistante au Viet Nam a bénéficié d'une accélération considérable depuis 2009.
Objectifs : Documenter le nombre annuel de patients enrôlés pour un traitement de TB multirésistante (TB-MDR) entre 2010 et 2014, et déterminer les caractéristiques et les résultats du traitement des patients qui l'out commencé entre 2010 et 2012.
Schéma : Etude rétrospective de cohorte basée sur les rapports nationaux et les données du système national de données électroniques pour la TB pharmacorésistante.
Résultats : Le nombre de patients enrôlés chaque année pour traitement de TB-MDR a augmenté de 97 en 2010 à 1522 en 2014. La majorité des patients étaient des hommes d'âge moyen qui avaient une atteinte pulmonaire et chez qui un protocole de retraitement avait échoué ; 77% d'entre eux avaient reçu au moins deux traitements de TB. De bons résultats (guérison et achèvement du traitement) ont été obtenus chez 73% des patients. Les résultats défavorables incluaient les sujets perdus de vue (12,5%), les décès (8%) et les échecs (6,3%). Avoir eu plus de deux traitements préalables et être positif pour le virus de l'immunodéficience humaine étaient associés à des résultats défavorables.
Conclusion : Un nombre croissant de patients est traité pour TB-MDR chaque année dans le cadre du programme national de prise en charge au Viet Nam, avec de bons résultats. Cependant, il est nécessaire d'augmenter la détection des cas (actuellement seulement 30% des 5100 cas de TB-MDR estimés par an), de réduire la proportion de résultats défavorables et d'améliorer le suivi et l'évaluation.
Abstract
Marco de referencia: Desde el 2009, se ha ampliado rápidamente la escala del tratamiento de la tuberculosis (TB) farmacorresistente en el marco del programa nacional de Viet Nam.
Objetivos: Documentar el número de pacientes inscritos cada año en el tratamiento de la TB multidrogorresistente (TB-MDR) del 2010 al 2014 y determinar los desenlaces terapéuticos y sus características en los pacientes que iniciaron tratamiento del 2010 al 2012.
Método: Fue este un estudio retrospectivo de cohortes a partir de los registros y los datos del sistema electrónico nacional de datos sobre la TB farmacorresistente.
Resultados: El número de pacientes incorporados cada año al tratamiento de la TB-MDR aumentó de 97 en el 2010 a 1522 en el 2014. En su mayoría, se trató de hombres de mediana edad con afectación pulmonar, en quienes había fracasado una pauta de retratamiento y de los cuales el 77% había recibido dos o más ciclos de tratamiento antituberculoso. El 73% de pacientes alcanzó desenlaces favorables (curación y compleción del tratamiento). Los desenlaces desfavorables observados fueron la pérdida durante el seguimiento (12,5%), la muerte (8%) y el fracaso terapéutico (6,3%). El hecho de haber recibido dos o más ciclos de tratamiento antituberculoso y la positividad frente al virus de la inmunodeficiencia humana se asociaron con los desenlaces desfavorables.
Conclusión: Cada año, un mayor número de pacientes recibe tratamiento por TB-MDR en el marco del programa nacional contra la TB y alcanza desenlaces terapéuticos favorables en Viet Nam. Sin embargo, es preciso aumentar la detección de casos (que alcanza actualmente el 30% de los 5100 casos estimados de TB-MDR por año), disminuir los desenlaces desfavorables y mejorar el seguimiento y la evaluación.
Tuberculosis (TB) remains one of the most highly prevalent infectious diseases worldwide, with high mortality and morbidity. The emergence of drug-resistant TB has threatened global efforts to eliminate the disease.1 Viet Nam is a high TB burden country, with associated high rates of multidrug-resistant TB (MDR-TB, defined as TB resistant to at least both isoniazid [INH] and rifampicin [RMP]). In 2013, of an estimated 130 000 new TB cases nationally, 102 196 were notified.1 The Fourth National Drug Resistance Survey (DRS) in 2011 showed an MDR-TB rate of 4% among new cases and 23% among retreatment cases.2 Applying the DRS rates to all notified TB cases in 2013, the number of MDR-TB cases was estimated at 5100 per year.1
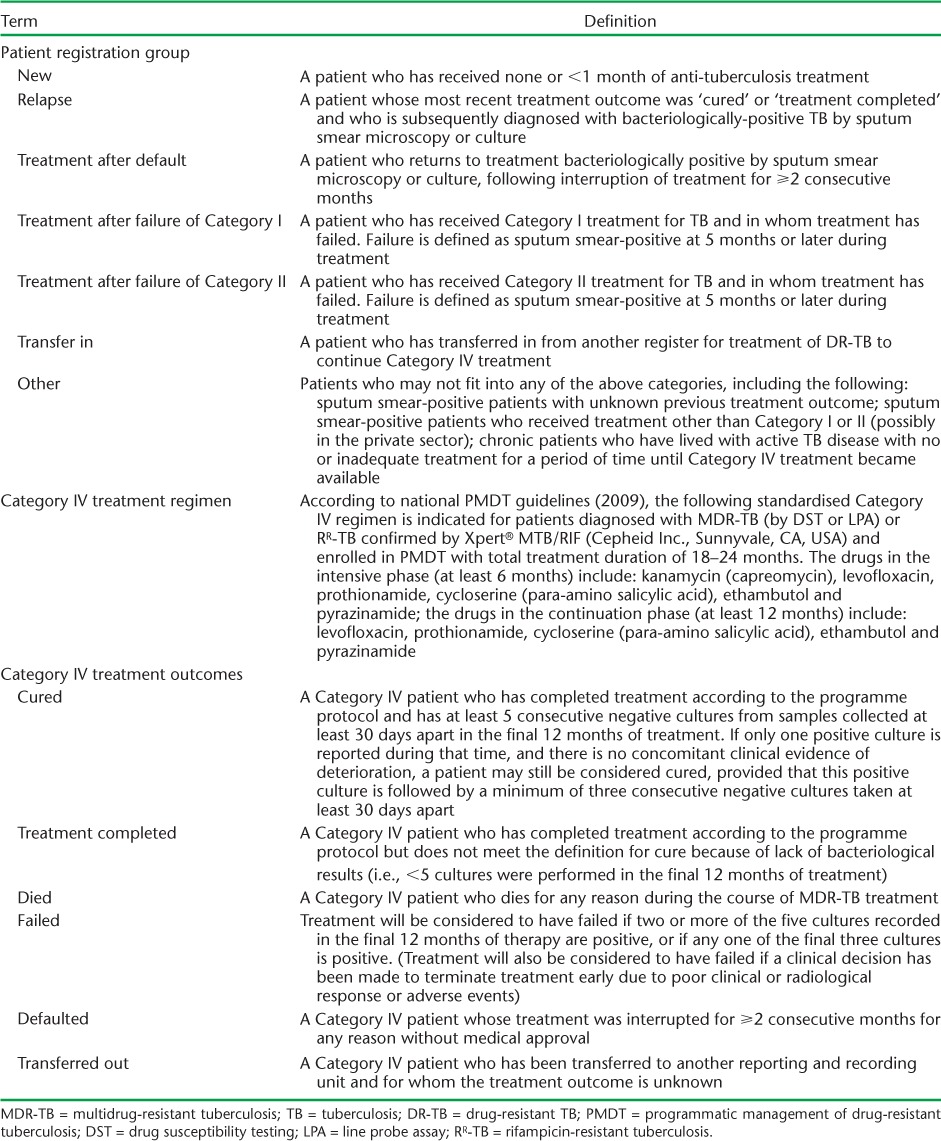
With support from the Global Fund (Geneva, Switzerland), the Viet Nam National TB Control Programme (NTP) implemented Programmatic Management of Drug-resistant TB (PMDT) in 2009 to detect and enrol MDR-TB patients for treatment with quality-assured second-line drugs (SLDs) provided through the Green Light Committee mechanism of the World Health Organization (WHO) and the Stop TB Partnership.3 Following WHO and national guidelines, a standardised Category IV regimen was indicated for those diagnosed with MDR-TB (Table 1).4,5 For the first 101 patients enrolled in PMDT in 2009 at one treatment centre only, treatment outcomes were promising, with 73% treatment success (source: Viet Nam NTP Report), substantially higher than that achieved globally during the same period.1
TABLE 1.
Since the pilot study in 2009, PMDT has been scaled up rapidly in Viet Nam. However, no formal detailed evaluation has been undertaken of PMDT performance and treatment outcomes to assess whether the initial high success rate of the pilot study has been achieved on a larger scale and under routine programme conditions. Such an evaluation could help guide the NTP to further improve MDR-TB care and treatment in Viet Nam. The findings from the evaluation might potentially be useful for other countries attempting to reach the Global Plan target of at least 75% MDR-TB treatment success.1
The aim of this study was therefore to evaluate treatment outcomes and related factors in patients enrolled in PMDT in Viet Nam. The first objective was to document the annual numbers of patients enrolled in Category IV treatment at the national level during 2010–2014. Other objectives were to determine, among all patients enrolled for Category IV treatment during 2010–2012, 1) their demographic and clinical characteristics, 2) Mycobacterium tuberculosis culture results at 6 months after starting treatment, and 3) end-of-treatment outcomes and factors associated with an unfavourable treatment outcome.
METHODS
Study design
This was a retrospective cohort study using national reports and record reviews.
General setting
Viet Nam is a South-East Asian country with a population of 91.6 million and a gross national income of US$5030 per capita.6 TB is a major public health problem in Viet Nam, which is one of the world's 22 high-burden countries for TB and one of the 27 high-burden MDR-TB countries.1 Within the NTP, TB diagnosis and treatment services are free of charge, including for MDR-TB.7
Programme management of TB and MDR-TB
TB control based on the Stop TB strategy has been implemented throughout Viet Nam since 1995, with case finding, registration, standardised treatment and monitoring and evaluation carried out in accordance with WHO guidelines.7,8 The programme is structured and organised through several levels: central, provincial, district and commune. Specific health care personnel are responsible for TB control at each level.7
To tackle the problem of emergent MDR-TB, PMDT was initiated in Viet Nam in 2009 at a single treatment centre. By the end of 2014, PMDT had expanded to 10 main treatment centres covering 41 of the 63 provinces and about 80% of the national population. The number of provinces providing MDR-TB services expanded to 6 by 2010, to 20 by 2011, to 35 in 2012–2013, and to 41 by 2014. By this time, approximately 4000 patients had been cumulatively enrolled in MDR-TB treatment. The national policy for MDR-TB diagnosis among patients with suspected MDR-TB (treatment failure, relapse and loss to follow-up [LTFU] among previously treated cases) was initially based on traditional culture and drug susceptibility testing (DST) and the line probe assay (LPA). However, at the end of 2012, a national decision was made to use and scale up the Xpert® MTB/RIF (Cepheid Inc, Sunnyvale, CA, USA) assay as the first-line investigation for identifying RMP-resistant TB (RR-TB). Once diagnosed, patients with drug-resistant TB (including MDR-TB confirmed by DST or LPA and RR-TB confirmed by Xpert) are registered in PMDT and started on Category IV treatment according to WHO and national PMDT guidelines.4,5 Patients were also assessed for resistance to SLDs on an ad hoc basis if they had previously been treated with SLDs or had been contacts of patients with extensively drug-resistant TB.
The definitions of the patients' registration groups, the Category IV treatment regimen and treatment outcomes are shown in Table 1. Monitoring and evaluation is performed through standardised paper-based and electronic recording and reporting systems. eTB Manager, the Viet Nam national electronic data system for drug-resistant TB, is used as the data source at the NTP's Central Unit.
Study settings
In addition to the 10 MDR-TB treatment centres established throughout the country, 19 treatment sites and 12 satellite sites were also established and linked to these centres so that PMDT services could be decentralised. The satellite sites refer patients with suspected MDR-TB to the centres or treatment sites and provide follow-up treatment for patients with confirmed MDR-TB and RR-TB.
Study population
The study included all patients enrolled for Category IV treatment under PMDT in Viet Nam from 1 January 2010 to 31 December 2014, and the characteristics and treatment outcomes of those enrolled between 2010 and 2012.
Data collection
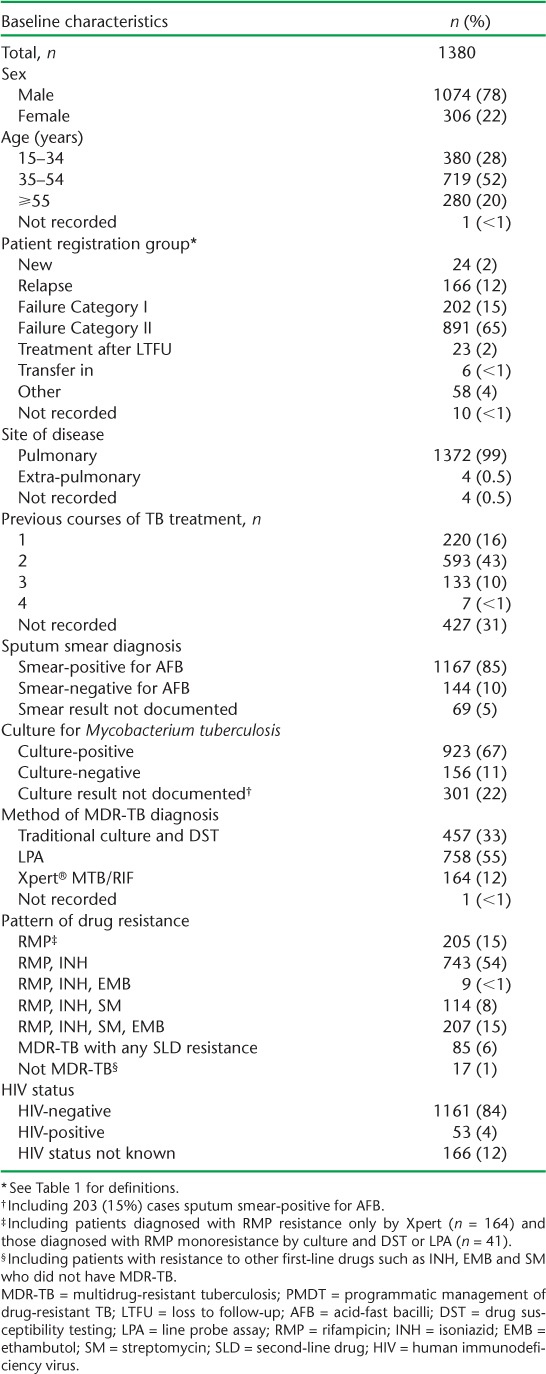
Aggregate data from the national quarterly reports were used to derive the numbers of cases enrolled for Category IV treatment from 2010 to 2014. For those enrolled between 2010 and 2012, patient characteristics and treatment outcomes included patient age, sex, registration group, site of disease, number of previous courses of TB treatment, method of diagnosis, DST results, human immunodeficiency virus (HIV) status, sputum culture results at baseline and 6 months after treatment initiation and treatment outcomes (Table 2). Based on the WHO and national guidelines, Category IV treatment outcomes were further grouped as favourable (cured or treatment completed) or unfavourable (failed, died, LTFU or transferred out).9 All data were retrieved from the Viet Nam eTB Manager at the NTP Central Unit.
TABLE 2.
Characteristics of all patients enrolled for MDR-TB treatment in PMDT, Viet Nam, 2010–2012
Data analyses
The data used in the study were collected between January and March 2015 and single-entered into EpiData, version 3.1 (EpiData Association, Odense, Denmark). Frequencies and proportions were used to summarise categorical variables and medians and interquartile ranges were used to summarise continuous variables. The χ2 test (and χ2 test for trend) was used to assess significant associations between exposure and outcome variables. Unadjusted relative risks (RR) and 95% confidence intervals (CIs) were calculated to establish measures of association between risk factors and treatment outcomes. Levels of significance were set at 5%. Data analyses were performed using OpenEpi version 3.03a (Open Source Epidemiologic Statistics for Public Health, Atlanta, GA, USA).
Ethics approval
This study was approved by the Viet Nam NTP. Ethics approval from the Ethics Committee of the Viet Nam National Lung Hospital (Hanoi, Viet Nam) was not required since the study used routine NTP data. Ethics approval for the study was obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. As the study was based on a record review, informed consent from patients was not necessary.
RESULTS
Trends and characteristics of patients enrolled for MDR-TB treatment
The number of patients enrolled annually for MDR-TB treatment in national PMDT in Viet Nam increased from 97 in 2010 to 1522 in 2014 (Figure).
FIGURE.
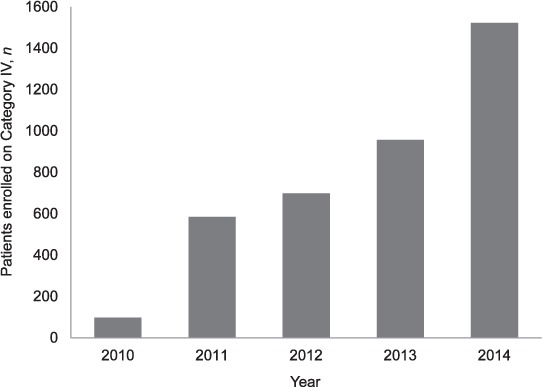
Number of patients enrolled for MDR-TB treatment in PMDT, Viet Nam, 2010–2014. Category IV = treatment for MDR-TB; MDR-TB = multidrug-resistant tuberculosis; PMDT = programmatic management of drug-resistant tuberculosis.
The characteristics of patients enrolled for MDR-TB treatment between 2010 and 2012 are shown in Table 2. The majority of the patients were male, and the median age (interquartile range) was 44 (33–53) years. Most patients had been diagnosed with pulmonary disease; the predominant category was failure of Category II retreatment regimen. Information on the number of previous courses of TB treatment was available for about 70% of patients; 77% of these had received ⩾2 previous courses of treatment. About 85% of patients were sputum smear-positive for acid-fast bacilli and 67% were culture-positive for Mycobacterium tuberculosis. The proportions of patients diagnosed with MDR-TB using culture and DST, LPA and Xpert were respectively 33%, 55% and 12%. There were 1073 (78%) patients with MDR-TB on its own or associated with resistance to other first-line anti-tuberculosis drugs, 205 (15%) with resistance only confirmed for RMP (due to diagnosis by Xpert only or because of true RMP monoresistance) and 85 (6%) with additional resistance to one of the SLDs. Seventeen patients who did not have MDR-TB but were resistant to INH, ethambutol or streptomycin, alone or in combination, were placed on Category IV treatment. HIV testing was performed among 1214 (88%) patients: of these, 53 (4%) were HIV-infected, of whom 25 (47%) were known to be on antiretroviral therapy (ART), with or without cotrimoxazole.
Interim outcome at 6 months
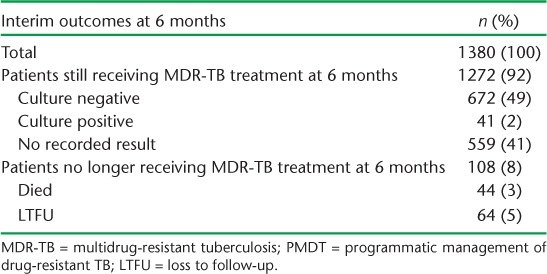
Interim outcomes at 6 months for all patients enrolled between 2010 and 2012 are shown in Table 3. Of the 92% of patients who were still receiving MDR-TB treatment at 6 months, approximately 40% had no recorded sputum culture result. Among the 713 patients in whom culture had been performed, 94% were culture negative.
TABLE 3.
Interim outcomes at 6 months for all patients enrolled for MDR-TB treatment in PMDT, Viet Nam, 2010–2012
Final treatment outcomes and factors associated with unfavourable outcomes
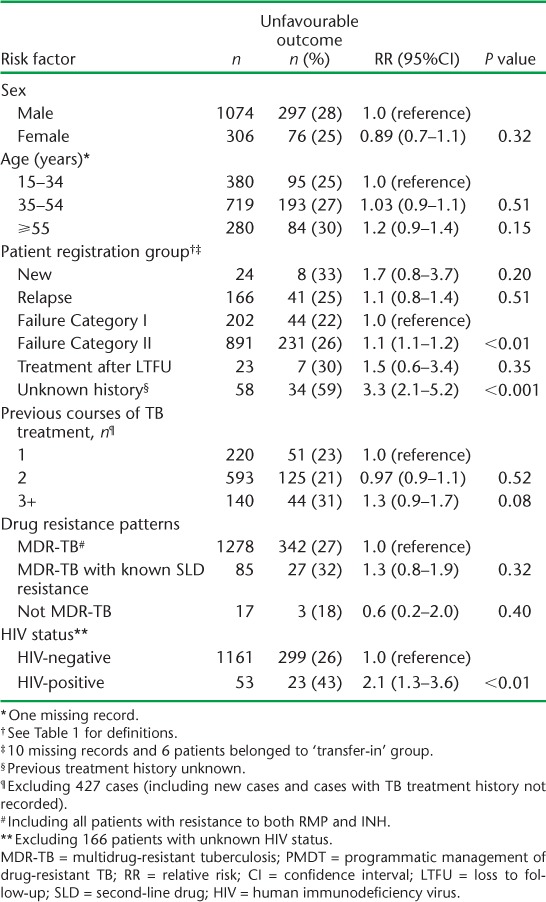
Final treatment outcomes of patients enrolled in each annual cohort and in total from 2010 to 2012 are shown in Table 4. For all patents combined, a favourable outcome was achieved in 73%. The proportion achieving a favourable outcome showed a non-significant decrease over the 3 years, from 78% in 2010 to 72% in 2012, as the numbers of patients increased (χ2 test for trend = 1.48, P = 0.22). The most common unfavourable outcome was LTFU, followed by death and failure. Table 5 shows factors associated with an unfavourable outcome. The risk of an unfavourable outcome was higher among patients failing Category II treatment (RR 1.1, 95%CI 1.1–1.2) and those with an unknown previous treatment history (RR 3.3, 95%CI 2.1–5.2), compared with those having failed Category I treatment. HIV-positive patients had a higher risk of an unfavourable outcome compared with non-HIV-infected patients. In HIV-positive patients, 17 (61%) of the 28 who were not on ART had an unfavourable outcome, significantly more than the 6/25 (24%) patients on ART (RR 2.5, 95%CI 1.2–5.4, P < 0.01) (data not shown in the table).
TABLE 4.
Treatment outcomes for all patients enrolled for MDR-TB treatment in PMDT in Viet Nam, 2010–2012
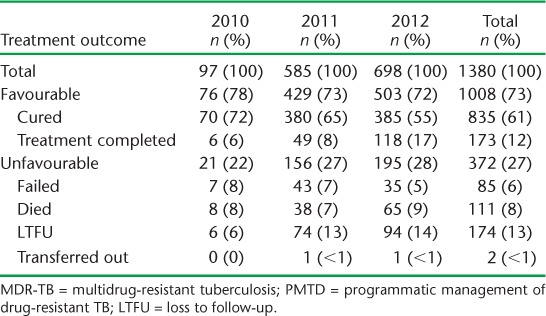
TABLE 5.
Risk factors associated with unfavourable treatment outcomes in all patients enrolled for MDR-TB treatment in PMDT, Viet Nam, 2010–2012
DISCUSSION
This is the first formal and detailed evaluation on the performance and outcomes of expanded PMDT in Viet Nam. Over the 5-year period from 2010 to 2014, the number of patients enrolled for MDR-TB treatment increased considerably. The majority of these patients were male and middle-aged, mirroring the general sociodemographic characteristics of TB patients in Viet Nam.10 Many patients had already received >2 previous courses of anti-tuberculosis treatment, and 33% had failed a retreatment regimen, indicating a pressing need to identify drug resistance much earlier. Nonetheless, treatment outcomes were encouraging. For those with documented results, a high proportion had negative sputum cultures at 6 months of treatment. The treatment success rate of 73% in the combined 2010–2012 cohort is comparable to the 2009 pilot study and higher than the global average of 48% reported in 2011.11 LTFU was the main unfavourable treatment outcome. This could be improved by shortening the length of the treatment regimens, which is being assessed in several countries, including Viet Nam.12,13 Unsurprisingly, those who failed the retreatment regimen, had an unknown history of previous TB treatment, or were HIV-infected and not on ART, had a higher risk of an unfavourable outcome.
A major strength of our study is that we used countrywide data with large numbers of patients enrolled for anti-tuberculosis treatment. We also followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines and sound ethical principles for conducting and reporting in this observational study.14,15 The main limitation was related to unrecorded data in the electronic monitoring system, especially on the number of patients evaluated at baseline for SLD resistance, the use of incentives and enablers, and the sputum culture results at baseline and follow-up. This last deficiency compromised our ability to evaluate 6-month treatment outcomes in annual cohorts of patients enrolled for treatment. There was also a discrepancy between the number of patients enrolled for MDR-TB treatment during 2010–2012 in the aggregate quarterly report data compared with that in the eTB Manager data; this may have been due to differences in dates between registration and start of treatment at the end of the year in the two reporting systems.
Despite the impressive increase in numbers of MDR-TB patients enrolled each year, in 2014 PMDT had only reached 30% of the estimated 5100 MDR-TB patients in the country. Possible reasons for this include the fact that PMDT does not yet have 100% countrywide coverage, new TB patients (of whom 4% have MDR-TB) are not being prioritised for Xpert screening due to limited resources, there is insufficient TB screening capacity in peripheral facilities, and patients diagnosed and managed in the private sector are not being reported.16 These deficiencies aside, Viet Nam's PMDT has performed well over the past 5 years in terms of expanding service coverage and maintaining high treatment success rates, and Viet Nam is one of only five high MDR-TB burden countries worldwide to achieve a success rate of ⩾70%.1
In the Western Pacific region, which has a similar MDR-TB epidemiology to Viet Nam, the 2011 MDR-TB cohorts achieved a treatment success of only 52%.17 Results also vary by country in areas outside the Western Pacific region. Both Israel and the Dominican Republic, which have low MDR-TB burdens, recently reported treatment success rates of respectively 71% and 74%,18,19 while South Africa, which has a large MDR-TB burden associated with a high prevalence of HIV, achieved a treatment success rate of only 44%.20
This study has several programmatic implications. First, Viet Nam needs to increase its MDR-TB case detection rate. This will be possible once full national coverage of PMDT is achieved, which is expected by 2018 given current expansion. Within the PMDT, additional resources are also needed to increase drug resistance screening of new patients by traditional culture and DST, LPA or Xpert. There is also a need for better collaboration with the private sector to ensure that patients outside PMDT are diagnosed, treated and evaluated according to standardised guidelines.21 The current public-private mix initiative for drug-susceptible TB must also incorporate drug-resistant TB.
Second, there is a need for earlier identification of patients with MDR-TB and better knowledge about SLD resistance. Currently, 33% of MDR-TB patients are diagnosed after two or more previous courses of anti-tuberculosis treatment, and this diagnostic delay threatens successful treatment outcomes. Drug resistance must be investigated and identified in individuals who have failed Category I treatment and/or are still sputum smear-positive at 2 or 5 months after starting their first course of Category II treatment. Systems for transporting sputum specimens from peripheral health facilities to laboratories equipped to diagnose drug resistance often function poorly in low- and middle-income countries. Thus, efforts to improve transportation and deal with logistical challenges are needed.22–24 Earlier diagnosis of MDR-TB should lead to improved treatment outcomes and reduced TB transmission in the community.
Resistance to SLDs also worsens treatment outcomes. Viet Nam needs to increase its laboratory capacity to measure SLD resistance patterns as a systematic policy rather than performing DST only among patients with a previous history of SLD treatment. The inclusion of new drugs such as delamanid and bedaquiline in more effective regimens also needs to be considered. These measures should be included in a comprehensive PMDT package to ensure quality service as numbers enrolling for treatment increase over the years.
Third, HIV is associated with poor treatment outcomes in MDR-TB patients, especially in those not on ART. All HIV-infected TB patients should start ART and cotrimoxazole as early as possible, as these interventions improve treatment success and reduce mortality.25,26
Finally, more attention needs to be paid to monitoring and evaluation and ensuring that all data pertaining to baseline characteristics, follow-up sputum culture results and treatment outcomes are entered into paper-based registers and electronic data systems. In this regard, our study demonstrates the potential benefit of countrywide deployment of an electronic-based monitoring system for MDR-TB. This provides not only real-time case-based information but also a systematic evaluation of overall performance from the NTP perspective.
In conclusion, this study indicates favourable national expansion of the PMDT in Viet Nam, and shows that even as patient numbers increase, good treatment outcomes, equal to some of the best observed in other programmes around the world, can be achieved. There are areas for improvement, and this study has identified several, but if tackled well, this should lead to improved programme performance.
Acknowledgments
The authors thank the provincial NTP staff from all the PMDT sites in Viet Nam for their routine data collection and entry to the MDR-TB electronic system; and Management Sciences for Health (Arlington, VA, USA), the United States Agency for International Development (Washington, DC, USA), the KNCV Tuberculosis Foundation (The Hague, The Netherlands) and the Global Fund (Geneva, Switzerland), for their support with the installation and deployment of eTB Manager.
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union), Paris, France and Médecins sans Frontières (MSF) Brussels Operational Centre, Luxembourg. The specific SORT IT programme that resulted in this publication was jointly developed and implemented by: the Centre for Operational Research, The Union; the Operational Research Unit (LUXOR), MSF; The Union South-East Asia Regional Office, New Delhi, India; and the Centre for International Health, University of Bergen, Bergen, Norway.
The programme was supported and funded by Bloomberg Philanthropies, New York, NY, USA; The Union; MSF; the Department for International Development, London, UK; and the WHO, Geneva, Switzerland. La Fondation Veuve Emile Metz-Tesch, Luxembourg, supported the open access publication costs. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
In accordance with the WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report 2014. Geneva, Switzerland: WHO; 2014. WHO/HTM/TB/2014.08. [Google Scholar]
- 2.Nhung N V, Hoa N B, Sy D N, Hennig C M, Dean A S. The fourth national anti-tuberculosis drug resistance survey in Viet Nam. Int J Tuberc Lung Dis. 2015;19:670–675. doi: 10.5588/ijtld.14.0785. [DOI] [PubMed] [Google Scholar]
- 3.Marais B, Munez N, Quelapio M I, Hennig C, Khanh P. PMDT Monitoring Mission Report, Viet Nam. 12–16 March 2012. Hanoi, Viet Nam: Viet Nam National Tuberculosis Control Programme; 2012. [Google Scholar]
- 4.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis, Emergency update 2008. Geneva, Switzerland: WHO; 2008. WHO/HTM/TB/2008.402. [Google Scholar]
- 5.Viet Nam National Tuberculosis Control Programme. Guidelines for the management and treatment of MDR-TB. Hanoi, Viet Nam: Viet Nam NTP; 2009. [Google Scholar]
- 6.World Health Organization. World health statistics 2015. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 7.Fujiwara P I, Hennig C, Hu D M End term evaluation National Tuberculosis Control Program of Viet Nam 2007–2011. Hanoi, Viet Nam: Viet Nam National Tuberculosis Control Programme; 2011. http://www.wpro.who.int/vietnam/topics/tuberculosis/2012_01_19_viet_nam_end_development_plan_report_2007_2011.pdf. [Google Scholar]
- 8.World Health Organization. Treatment of tuberculosis guidelines. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2009.420. [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines for the programmatic management of multidrug-resistant tuberculosis. Geneva, Switzerland: WHO; 2011. [PubMed] [Google Scholar]
- 10.Hoa N B, Sy D N, Nhung N V, Tiemersma E W, Borgdorff M W, Cobelens F G. National survey of tuberculosis prevalence in Viet Nam. Bull World Health Organ. 2010;88:273–280. doi: 10.2471/BLT.09.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: WHO; 2015. WHO/HTM/TB/2014.11. http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf?ua=1&ua=1 Accessed January 2016. [PubMed] [Google Scholar]
- 12.Van Deun A, Maug A K J, Salim M A H et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 13.Aung K J M, Van D, eun A, Declercq E et al. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18:1180–1187. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 14.Edginton M, Enarson D, Zachariah R et al. Why ethics is indispensable for good-quality operational research. Public Health Action. 2012;2:21–22. doi: 10.5588/pha.12.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang T T T, Nguyen N V, Dinh S N et al. Challenges in detection and treatment of multidrug resistant tuberculosis patients in Viet Nam. BMC Public Health. 2015;15:980. doi: 10.1186/s12889-015-2338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam T, Marais B J, Nhung N V et al. Western Pacific Regional Green Light Committee: progress and way forward. Int J Infect Dis. 2015;32:161–165. doi: 10.1016/j.ijid.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mor Z, Goldblatt D, Cedar N, Rorman E, Chemtob D. Drug-resistant tuberculosis in Israel: risk factors and treatment outcomes. Int J Tuberc Lung Dis. 2014;18:1195–1201. doi: 10.5588/ijtld.14.0192. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez M, Monedero I, Caminero J A et al. Successful management of multidrug-resistant tuberculosis under programme conditions in the Dominican Republic. Int J Tuberc Lung Dis. 2013;17:520–525. doi: 10.5588/ijtld.12.0481. [DOI] [PubMed] [Google Scholar]
- 20.Brust J C M, Gandhi N R, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis. 2010;14:413–419. [PMC free article] [PubMed] [Google Scholar]
- 21.Hoa N B, Khanh P H, Chinh N V, Hennig CM. Prescription patterns and treatment outcomes of MDR-TB patients treated within and outside the National Tuberculosis Programme in Pham Ngoc Thach hospital, Viet Nam. Trop Med Int Health. 2014;19:1076–1081. doi: 10.1111/tmi.12347. [DOI] [PubMed] [Google Scholar]
- 22.Tharu M B, Harries A D, Goel S et al. Screening retreatment tuberculosis patients for drug resistance in mid-west Nepal: how well are we doing? Public Health Action. 2014;1:14–18. doi: 10.5588/pha.13.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harries A D, Michongwe J, Nyirenda T E et al. Using a bus service for transporting sputum specimens to the Central Reference Laboratory: effect on the routine TB culture service in Malawi. Int J Tuberc Lung Dis. 2004;8:204–210. [PubMed] [Google Scholar]
- 24.Chadha S S, Sharath B N, Reddy K et al. Operational challenges in diagnosing multi-drug resistant TB and initiating treatment in Andhra Pradesh, India. PLOS ONE. 2011;6:e26659. doi: 10.1371/journal.pone.0026659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi N R, Shah N S, Andrews J R et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 26.Palacios E, Franke M, Muñoz M et al. HIV-positive patients treated for multidrug-resistant tuberculosis: clinical outcomes in the HAART era. Int J Tuberc Lung Dis. 2012;16:348–354. doi: 10.5588/ijtld.11.0473. [DOI] [PubMed] [Google Scholar]


