Abstract
Setting: The National Tuberculosis (TB) Programme in Viet Nam and Ho Chi Minh City (HCMC).
Objectives: To determine 1) at national level between 2011 and 2013, the relationship between human immunodeficiency virus (HIV) testing, uptake of TB-HIV interventions and adverse treatment outcomes among TB-HIV patients; and 2) in HCMC in 2013, patient characteristics associated with adverse outcomes.
Design: An ecological study reviewing aggregate nationwide data and a retrospective cohort review in HCMC.
Results: Nationwide, from 2011 to 2013, HIV testing increased in TB patients from 58% to 68% and antiretroviral therapy (ART) increased in TB-HIV patients from 54% to 63%. Adverse treatment outcomes in TB-HIV patients increased from 24% to 27%, largely due to transfer out (5–9% increase) and death. The Northern and Highland regions showed poor uptake of TB-HIV interventions. In HCMC, 303 (27%) of 1110 TB-HIV patients had adverse outcomes, with higher risks observed in those with previously treated TB, those diagnosed as HIV-positive before TB onset and those never placed on cotrimoxazole or ART.
Conclusion: Despite improving HIV testing rates and TB-HIV interventions, adverse outcomes in TB-HIV patients remain at about 26%. Characteristics predicting higher risk of adverse outcomes must be addressed if Viet Nam wishes to end the TB epidemic by 2030.
Keywords: operational research, SORT IT, adverse treatment outcomes, antiretroviral therapy, cotrimoxazole preventive therapy
Abstract
Contexte : Le programme national tuberculose (TB) au Viet Nam et à Ho Chi Minh ville (HCMC).
Objectifs : Déterminer 1) au niveau national entre 2011 et 2013, la relation entre le test pour le virus de l'immunodéficience humaine (VIH), l'utilisation des interventions TB-VIH et les mauvais résultats du traitement de la TB parmi les patients TB-VIH, et 2) à HCMC en 2013, les caractéristiques des patients associées à un mauvais résultat.
Schéma : Une étude écologique revoyant les données nationales agrégées et une étude de cohorte rétrospective dans HCMC.
Résultats : Au niveau national, le test VIH est passé de 58% à 68% chez les patients tuberculeux et le traitement antirétroviral (ART) est passé chez les patients TB-VIH de 54% à 63% entre 2011 et 2013. Les mauvais résultats du traitement chez les patients TB-VIH ont augmenté de 24% à 27%, largement à cause des transferts (de 5% à 9%) et des décès. Les régions du Nord et des Highlands ont montré une faible utilisation des interventions TB-VIH. A HCMC, 303 (27%) patients TB-VIH sur 1110 ont eu un mauvais résultat avec un risque plus élevé observé parmi ceux qui avaient déjà eu un traitement de TB, ceux dont le diagnostic de VIH précédait l'apparition de la TB et ceux qui n'avaient jamais bénéficié du traitement par cotrimoxazole et de l'ART.
Conclusion : En dépit de meilleurs taux de tests VIH et d'interventions TB-VIH, près de 26% des patients TB-VIH ont de mauvais résultats du traitement de la TB. Les facteurs de prédiction d'un risque plus élevé de mauvais résultats doivent être pris en compte si le Viet Nam souhaite mettre fin à l'épidémie de TB d'ici 2030.
Abstract
Marco de referencia: El Programa Nacional contra la Tuberculosis (TB) en Viet Nam y la ciudad de Ho Chi Minh.
Objetivos: 1) Definir a escala nacional la relación entre la aceptación de la prueba diagnóstica de la infección por el virus de la inmunodeficiencia humana (VIH), las intervenciones conjuntas contra la TB y el VIH y los desenlaces desfavorables del tratamiento antituberculoso en pacientes aquejados de esta coinfección, del 2011 y el 2013; y 2) determinar las características de los pacientes que se asocian con los desenlaces terapéuticos adversos en la ciudad de Ho Chi Minh.
Método: Se llevó a cabo un estudio ecológico, en el cual se examinaron los datos nacionales agregados y un estudio retrospectivo de cohortes en la ciudad de Ho Chi Minh.
Resultados: A escala nacional, del 2011 al 2013 se observó un aumento de 58% a 68% en la práctica de la prueba del VIH a los pacientes con diagnóstico de TB y de 54% a 63% en la administración del tratamiento antirretrovírico (ART) a los pacientes coinfectados. Los desenlaces terapéuticos desfavorables en los pacientes coinfectados aumentaron de 24% a 27%, debido sobre todo a las transferencias a otros centros (de 5% a 9%) y a los fallecimientos. En los regions del norte y Highland se observó una baja aceptación de las intervenciones conjuntas contra la TB y el VIH. En la ciudad de Ho Chi Minh, 303 de los 1110 pacientes coinfectados (27%) presentaron desenlaces adversos y el riesgo fue mayor en los pacientes con antecedente de tratamiento antituberculoso, en pacientes cuyo diagnóstico de infección por el VIH precedió el comienzo de la TB y en los pacientes que nunca se inscribieron al tratamiento con cotrimoxazol o ART.
Conclusión: Pese a los progresos en la práctica de la prueba diagnóstica de la infección por el VIH y las intervenciones conjuntas contra la TB y el VIH, la tasa de desenlaces terapéuticos desfavorables alcanza un 26% en los pacientes coinfectados. Es preciso abordar los factores que favorecen el mayor riesgo de desenlaces adversos, si se desea poner fin a la epidemia de TB en el 2030 en Viet Nam.
One of the most important threats to the successful global control of tuberculosis (TB) is human immunodeficiency virus (HIV) associated TB (TB-HIV). In 2014, there were an estimated 9.6 million patients with active TB, of whom 1.2 million (12%) were co-infected with HIV.1 Of these co-infected patients, 390 000 (33%) died because of TB,1 an unacceptable mortality rate given that TB can be successfully cured with standard anti-tuberculosis treatment regimens and HIV can be treated with antiretroviral therapy (ART).
In March 2012, the World Health Organization (WHO) released an updated policy document on collaborative TB-HIV activities to reduce the burden of dual disease, focusing on 1) mechanisms for delivering integrated TB and HIV services; 2) reducing the burden of TB in people living with HIV by early ART and the ‘Three Is’, i.e., Intensified TB case finding, Isoniazid preventive therapy and Infection control at all clinical encounters; and 3) reducing the burden of HIV in patients with presumptive or diagnosed TB through a strategy of HIV testing and cotrimoxazole preventive therapy (CPT) and ART for those found to be HIV-infected.2
Viet Nam is one of the 22 high-burden TB countries, with TB prevalence estimated in 2014 at 198 per 100 000 population and incidence at 140/100 000.1 There is also a high burden of HIV: in 2014 there were an estimated 250 000 people living with HIV (PLHIV), constituting 0.5% of the adult population aged 15–49 years.3 As a result, there is a sizeable burden of TB-HIV. Rates of HIV testing and collaborative TB-HIV activities have been gradually improving in Viet Nam over the last few years, but despite this, treatment outcomes in HIV-infected TB patients are poor, with less than 75% successfully completing treatment compared with overall treatment success rates of nearly 90% for all new and relapse TB cases registered nationally.1
Ho Chi Minh City (HCMC) is the main problem area. In 2013, 16% of all notified TB cases and about one third of all TB-HIV patients in Viet Nam were from HCMC, and these TB-HIV patients had a low treatment success rate of about 65% (source: National Tuberculosis Control Programme [NTP], based on 1553 TB-HIV patients reported in the aggregate database for HCMC in 2013). A major contributor to poor treatment outcomes is high case fatality, but this adverse outcome and its associated factors have not been formally studied. A survey in HCMC in 1998–2000 found a case fatality rate of 30% in TB-HIV patients.4 Case fatality and other adverse treatment outcomes may be higher in patients with smear-negative TB and recurrent TB,5 and this again has not been formally studied. The NTP needs to better understand how TB-HIV collaborative activities are implemented in the whole country and its different regions, including HCMC, and, in individual co-infected patients, how these might relate to TB treatment outcomes.
This study was planned with two specific objectives: 1) to describe the relationship between HIV testing among TB patients, and CPT and ART uptake and TB treatment outcomes among TB-HIV patients at national level in Viet Nam between 2011 and 2013; and 2) to determine the association between demographic and clinical features in patients with TB-HIV and adverse TB treatment outcomes in HCMC in 2013.
METHODS
Study design
The study assessed TB-HIV collaborative activities and treatment outcomes through 1) a review of aggregate nationwide data (ecological design), and 2) a retrospective record review of individual patients registered in HCMC (cohort design).
Setting
General setting
Viet Nam is an Asian country of wide topographical diversity, with mountainous, mid-land and lowland areas and a coastal strip. The total population is 91 million, with 28% living in urban areas. Administratively, Viet Nam has eight regions, 63 provinces, 687 cities and districts and 11 035 communes (Figure 1). HCMC, a city in the South-East region of Viet Nam, has a population of 8 million, making it the most populous metropolitan area in the country.
FIGURE 1.
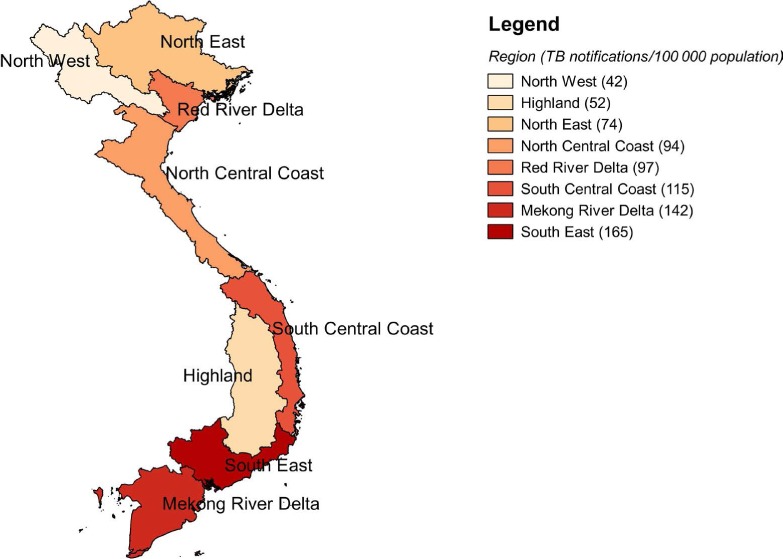
Map of Viet Nam showing the eight regions and TB case notification rates in 2013. TB = tuberculosis.
Study setting
TB-HIV collaborative activities started under the NTP in 2007 in 10 provinces, including HCMC. By Febru-ary 2014, 53 provinces were reporting on the implementation of TB-HIV collaborative activities. The roles of the TB and HIV programmes in service delivery for co-infected patients are clearly described in the national guidelines.6–8 In brief, PLHIV diagnosed during the study period were eligible for ART based on the WHO 2010 guidelines of a CD4 cell count <350 cells/μl or being at WHO clinical stage 3 or 4.9 All PLHIV are screened for TB at each clinic visit, and if TB is suspected they are referred for TB investigations in line with NTP guidelines. For all TB patients registered in NTP clinics, an enquiry is made about HIV status, and if the status is unknown or previously negative, provider-initiated HIV testing and counselling is undertaken. According to national guidelines all TB-HIV patients are eligible for CPT and ART simultaneously with anti-tuberculosis treatment.
All patients are monitored during treatment using standard outcome definitions in line with WHO recommendations.10 The main monitoring tools are paper-based treatment cards and registers, with the data then single-entered into an electronic web-based system (the Viet Nam Information Management Electronic System [VITIMES]).
Study population
For the review of aggregate nationwide data, the study population included all TB patients registered in Viet Nam between 2011 and 2013. For the record review of individual-level data, the study population included all TB-HIV patients registered in HCMC in 2013.
Data variables, sources of data and data collection
For the nationwide aggregate data, variables included year, region, TB patients stratified by all TB types and new smear-positive pulmonary tuberculosis (PTB), HIV testing among TB patients, HIV-positive status, and CPT uptake, ART uptake and anti-tuberculosis treatment outcomes in those with TB-HIV. For the record review of individual-level data, variables included age, sex, TB type and category, HIV testing, CPT initiation, ART initiation and TB treatment outcomes. Adverse treatment outcomes were defined as death, failure, loss to follow-up and transfer out combined. The data source was VITIMES. Data were collected between March and November 2015.
Analysis and statistics
The data were extracted into a Microsoft Office Excel spreadsheet (Microsoft Corp, Redmond, WA, USA) and imported and analysed in EpiData analysis software (version 2.2.2.183, EpiData Association, Odense, Denmark). For aggregate data, frequencies and proportions were analysed for HIV testing, HIV-positive status and CPT and ART uptake. For individual patient data, the association between demographic and clinical factors and adverse treatment outcomes was summarised using relative risk (RR) and 95% confidence interval (CI).
Ethics approval
Permission to carry out the study was obtained from the NTP, Viet Nam. Ethics approval was obtained from the Ethics Advisory Group (EAG) of the International Union against Tuberculosis and Lung Disease (The Union), Paris, France. As the study was based on already collected routine data, the EAG waived the need for informed patient consent.
RESULTS
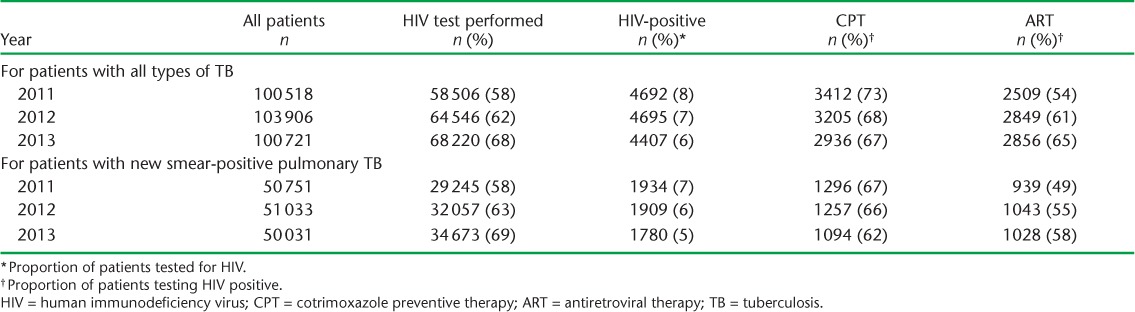
The annual results of HIV testing, HIV-positive status and CPT and ART uptake in TB patients found to be HIV-positive are shown in Table 1. Results were fairly similar for patients with all types of TB and those with new smear-positive PTB. Over the 3 years, the annual number of notified TB cases remained stable, the proportion tested for HIV gradually increased while the proportion found to be HIV-positive gradually decreased. For those found to be HIV-positive, there was a small gradual decrease in CPT uptake and an increase in ART uptake, resulting in about 65% of patients receiving both types of medication in 2013.
TABLE 1.
HIV testing, HIV positivity, CPT and ART status in patients registered for TB each year, Viet Nam, 2011–2013
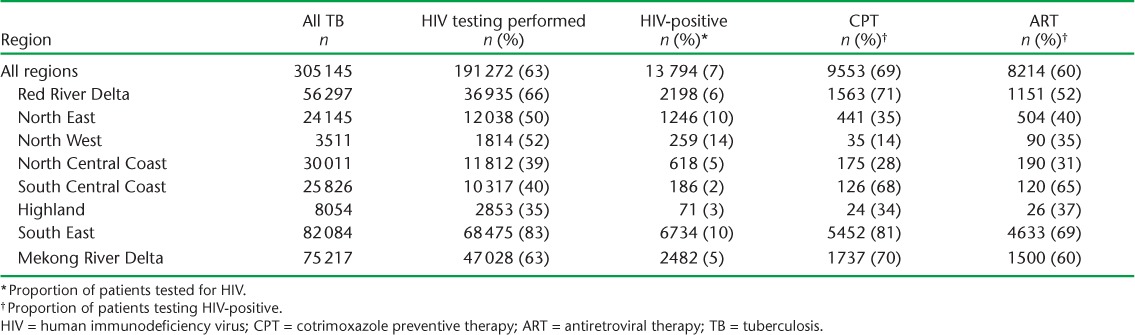
The results of HIV testing, HIV-positive status and CPT and ART uptake for the 3 years combined for patients with all types of TB in each region are shown in Table 2. There were variations between regions. In the South-East region, where the number of cases of all types of TB was the highest, the highest rates of HIV testing and uptake of CPT and ART were observed among the 10% of patients found to be HIV-positive. In contrast, the Highland region had the lowest HIV testing rate, with poor uptake of CPT and ART. Similar poor rates of testing and HIV interventions were found in the three Northern regions. These results were replicated for patients with new smear-positive PTB (data not shown).
TABLE 2.
HIV testing, HIV positivity, CPT and ART status in patients registered for all types of TB in each region, Viet Nam, 2011–2013
Treatment outcomes for patients registered annually in Viet Nam with TB-HIV are shown in Table 3. For patients with all types of TB and new smear-positive PTB, treatment success varied between 69% and 76%, with a small decrease occurring annually during the 3 years. The main adverse outcomes were death and transfer out, with a slight decrease in deaths and an increase in transfers out during the 3 years.
TABLE 3.
TB treatment outcomes among patients with TB-HIV, Viet Nam, 2011–2013 *
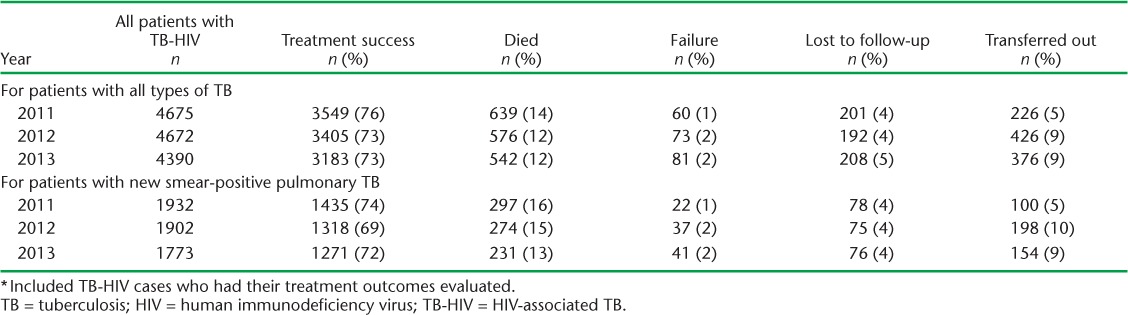
Figure 2 shows CPT and ART uptake and adverse treatment outcomes in TB-HIV patients in each region. The Highland region had poor uptake of HIV interventions and high rates of adverse outcomes (27%), which over the 3 years comprised death at 18% and transfer out at 7%, with annual rates being fairly similar. The South-East region had a generally good uptake of CPT and ART. However, this region had the highest rate of adverse outcomes (31%), which over the 3 years comprised death at 13% and transfer out at 11%, with annual deaths decreasing from 15% to 13% and annual transfers out increasing from 6% to 13%.
FIGURE 2.
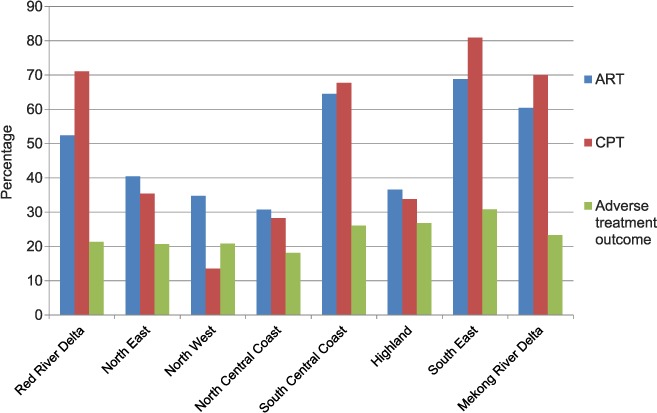
Uptake of ART and CPT and adverse treatment outcome in TB-HIV patients by region in Viet Nam over a 3-year period between 2011 and 2013 (n = 13 737). ART = antiretroviral therapy; CPT = cotrimoxazole preventive therapy; TB = tuberculosis; HIV = human immunodeficiency virus.
In 2013, there were 1553 TB-HIV patients registered in the aggregate database in HCMC, of whom 1273 (82%) were documented in the VITIMES individual case base data file. Of these, no treatment outcomes were recorded for 163 (12.8%). Treatment outcomes (including loss to follow-up) were therefore reported in 1110 individual patients. Adverse treatment outcomes amongst these 1110 TB-HIV patients in relation to patient characteristics are shown in Table 4. Overall, 303 (27%) patients had an adverse outcome, of whom 113 (10%) died, 38 (3%) failed treatment, 75 (7%) defaulted or were lost to follow-up and 77 (7%) transferred out to another facility, with no reported treatment outcome. Patients with previously treated TB and those diagnosed HIV-positive before the onset of TB had higher risks of adverse outcomes. Overall, those who were not on CPT or ART had a higher risk of adverse outcome compared with those on CPT and ART. Among those diagnosed HIV-positive before the onset of TB and initiated on CPT/ART, the risk of adverse outcomes was higher in those starting CPT/ART before the diagnosis of TB.
TABLE 4.
Characteristics associated with adverse TB treatment outcomes among patients with TB-HIV registered in Ho Chi Minh City, South-East region, Viet Nam, 2013
DISCUSSION
There were a number of important programmatic findings from this nationwide study in Viet Nam. Despite a gradual increase in HIV testing and CPT/ART uptake in co-infected patients from 2011 to 2013, the proportion of patients receiving TB-HIV interventions was still below the 100% recommended by the WHO.2 Overall, less than 75% of TB-HIV patients were successfully treated; the main adverse outcomes were death and transfer out, with the latter outcome increasing between 2011 and 2013. There were some marked regional variations within the country: TB-HIV collaborative activities and associated treatment outcomes were worse in the mountainous regions, which are largely inhabited by poor people and minority groups for whom health services may be poor.11 These findings are not unique to Viet Nam; other countries such as Nepal experience similar difficulties with health care provision amongst those living in the hills and mountains.12
This retrospective cohort study in TB-HIV patients in HCMC showed that the main factors associated with adverse treatment outcomes were recurrent TB and, not surprisingly, the absence of CPT and ART. We have no information in our study on the profile of drug resistance, but those with recurrent TB may have had a higher risk of drug resistance, which in PLHIV compromises treatment outcomes.13,14 Adjunctive CPT reduces morbidity and mortality in co-infected patients before, during and after anti-tuberculosis treatment, and this effect is maintained even in the presence of ART.15 Providing ART significantly transforms the prognosis of co-infected TB patients in whom there is a substantial reduction in mortality risk.16 We do not know the precise reasons why PLHIV were not on ART in our study, but those diagnosed HIV-positive before the onset of TB may not have fulfilled the eligibility criteria for starting on ART, while those diagnosed HIV-positive after the onset of TB may have progressed rapidly to death before ART was started.
Patients who were diagnosed HIV-positive before the diagnosis of TB had an increased risk of adverse outcomes compared with those diagnosed HIV-positive after TB; in the former group, starting CPT and ART before anti-tuberculosis treatment was associated with an increased risk of adverse outcomes. These findings have been previously reported elsewhere.17–20 One of the reasons for poor outcomes in those starting ART and CPT may be lower CD4 cell counts in this group; however, we have no programmatic data to confirm or refute this hypothesis. Other reasons for poor outcomes may include the unmasking of previously subclinical TB, difficulties and delays encountered in diagnosing TB in severely immunocompromised patients and paradoxical worsening of disease associated with immune reconstitution inflammatory syndrome.
The strengths of this study were its conduct under routine programme conditions, the nationwide coverage with the aggregate data and the large number of patients registered in HCMC, making the findings representative for the country. The study also adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for conducting and reporting on observational studies.21
There were some limitations. First, there were missing data in the VITIMES case base data file and some important variables, such as baseline CD4 counts, continuation of CPT and ART after initiation and time to events, were not yet recorded in this electronic database. The missing data in the case base data file meant that we had different numbers of registered patients and different proportions with treatment outcomes in HCMC from aggregate data (65% treatment success) and from individual data (73% treatment success). We also had nearly 13% in the VITIMES data file with no treatment outcomes recorded—the cells were blank—and we removed these from the denominator as we had no information about whether these patients had completed treatment or had an adverse outcome. As experience with the electronic database grows, these challenges will hopefully resolve. Second, we had no information in the VITIMES database on HIV-negative TB patients, and this may have skewed overall treatment outcomes. Third, our sample population with adverse outcomes (303 patients) was relatively small, and we therefore did not have sufficient power to distinguish between specific adverse outcomes and risk factors. Finally, the time trend of the study was restricted due to VITIMES starting only in 2011 (and in HCMC only for 1 year).
This study has some important programmatic implications. First, the NTP needs to make improvements in the VITIMES database to ensure that aggregate and individual data match, that all variables are always entered and that other variables that may provide useful programmatic information, as discussed earlier, are included.
Second, the NTP needs to increase its rates of HIV testing and uptake of CPT and ART in the Northern regions and the Highlands so that the country can move rapidly to 100% coverage. Sustainable Development Goal 3.3 aims to end the epidemics of AIDS and TB by 2030,22 and concerted efforts will be needed nationally with TB-HIV collaborative activities if these objectives are to be realised at the country level. Linked to this, the NTP also needs to reduce its transfer-out rates and ensure that treatment outcome data are available and shared between transferring out and transferring in centres. This can be achieved by encouraging greater cooperation between different TB officers.23,24
Third, mortality rates must be reduced. PLHIV should be started earlier on ART. The 2013 WHO ART guidelines moved the CD4 cell count threshold for starting ART up to 500 cells/μl,25 and rapid advice issued by the WHO in September 2015 recommended that all persons with HIV start ART, regardless of CD4 cell count and WHO clinical stage.26 There is strong evidence that this approach reduces mortality,27,28 and these guidelines should pave the way for future countrywide implementation. Closer attention is also needed to provide rapid and more accurate diagnosis of TB in patients with HIV, especially those who are sick and immunocompromised. The use of Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) at point of care in both sputum and urine, combined with measurement of urine lipoarabinomannan (LAM), holds promise, and this combined diagnostic approach needs to be considered within a programmatic context.29,30
In conclusion, this study shows that HIV testing rates and TB-HIV collaborative activities are gradually improving in Viet Nam, although these all fall well below 100%, especially in the Northern and Highland regions. Treatment success is 74%, with adverse outcomes particularly increased in those not taking CPT and ART and in those developing TB after these interventions have been started. The Viet Nam NTP needs to address these issues if the country wishes to end the TB epidemic by 2030.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Médecins sans Frontières (MSF). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by The Union South-East Asia Regional Office, New Delhi, India; the Centre for Operational Research, The Union, Paris, France; the Operational Research Unit (LUXOR), MSF, Brussels Operational Centre, Luxembourg; the School of Public Health, Post Graduate Institute of Medical Education and Research, Chandigarh, India; and the Department of Preventive and Social Medicine, Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India. We especially thank V C Thanh, statistics staff of the Viet Nam National Tuberculosis Control Programme, for her kind assistance in extracting the data from VITIMES and providing the data for the authors.
The SORT IT programme was funded by the Department for International Development, London, UK. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report 2015. Geneva, Switzerland: WHO; 2015. WHO/HTM/TB/2015.22. [Google Scholar]
- 2.World Health Organization. WHO policy on collaborative TB/HIV activities. Guidelines for national programmes and other stakeholders. Geneva, Switzerland: WHO; 2012. WHO/HTM/TB/2012.1; WHO/HIV/2012.1. [PubMed] [Google Scholar]
- 3.UNAIDS. Viet Nam. HIV and AIDS estimates (2014) Geneva, Switzerland: UNAIDS; 2014. http://www.unaids.org/en/regionscountries/countries/vietnam Accessed February 2016. [Google Scholar]
- 4.Quy H T, Cobelens F G J, Lan N T N, Buu T N, Lambregts C S, Borgdorff M W. Treatment outcomes by drug resistance and HIV status among tuberculosis patients in Ho Chi Minh City, Viet Nam. Int J Tuberc Lung Dis. 2006;10:45–51. [PubMed] [Google Scholar]
- 5.Harries A D, Hargreaves N J, Kemp J et al. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet. 2001;357:1519–1523. doi: 10.1016/S0140-6736(00)04639-0. [DOI] [PubMed] [Google Scholar]
- 6.Viet Nam Ministry of Health. [Decision on guidance issuance of intensified TB case finding and isoniazid preventive therapy for PLWHIV] Hanoi, Viet Nam: Ministry of Health; 2012. [Vietnamese] [Google Scholar]
- 7.Viet Nam Ministry of Health. [Decision 4139/QD-BYT on revision of HIV/AIDS diagnosis and treatment guidance] Hanoi, Viet Nam: Ministry of Health; 2011. http://thuvienphapluat.vn/archive/Quyet-dinh/Quyet-dinh-4139-QD-BYT-sua-doi-Huong-dan-chan-doan-va-dieu-tri-HIV-AIDS-vb131843t17.aspx Accessed February 2016 [Vietnamese] [Google Scholar]
- 8.Viet Nam Ministry of Health. [Decision on issuance of collaborative algorithm between HIV/AIDS and Tuberculosis programmes which are under National Health Target Programmes] Hanoi, Viet Nam: Ministry of Health; 2012. [Vietnamese] [Google Scholar]
- 9.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach. 2010 revision. Geneva, Switzerland: WHO; 2010. [PubMed] [Google Scholar]
- 10.World Health Organization. Treatment of tuberculosis guidelines. 4th ed. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB. 2009.420. [PubMed] [Google Scholar]
- 11.Chinh N, Huyen T, Phan N. Barriers in accessing TB control in Sonla and Gialai, Viet Nam. Saarbrücken, Germany: Lambert Academic Publishing; 2015. pp. 1–77. [Google Scholar]
- 12.Mishra P, Hansen E H, Sabroe S, Kafle K K. Socio-economic status and adherence to tuberculosis treatment: a case-control study in a district of Nepal. Int J Tuberc Lung Dis. 2005;9:1134–1139. [PubMed] [Google Scholar]
- 13.Wells C D, Cegielski J P, Nelson L J et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl.):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 14.Isaakidis P, Casas E C, Das M, Tseretopoulou X, Ntzani E E, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:969–978. doi: 10.5588/ijtld.15.0123. [DOI] [PubMed] [Google Scholar]
- 15.Suthar A B, Vitoria M A, Nagata J M et al. Co-trimoxazole prophylaxis in adults, including pregnant women, with HIV: a systematic review and me-ta-analysis. Lancet HIV. 2015;2:e137–e150. doi: 10.1016/S2352-3018(15)00005-3. [DOI] [PubMed] [Google Scholar]
- 16.Lawn S D, Meintjes G, McIlleron H, Harries A D, Wood R. Management of HIV-associated tuberculosis in resource-limited settings: a state-of-the-art review. BMC Med. 2013;11:253. doi: 10.1186/1741-7015-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J K-L, Bong C-N, Chen S C-C et al. Outcomes in HIV-infected patients who develop tuberculosis after starting antiretroviral treatment in Malawi. Int J Tuberc Lung Dis. 2008;12:692–694. [PubMed] [Google Scholar]
- 18.Koenig S P, Riviere C, Leger P et al. High mortality among patients with AIDS who received a diagnosis of tuberculosis in the first 3 months of antiretroviral therapy. Clin Infect Dis. 2009;48:829–831. doi: 10.1086/597098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes C P, Wehbe F H, McGowan C C et al. Duration of anti-tuberculosis therapy and timing of antiretroviral therapy initiation: association with mortality in HIV-related tuberculosis. PLOS ONE. 2013;8:e74057. doi: 10.1371/journal.pone.0074057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferroussier O, Dlodlo R A, Capo-Chichi D et al. Results of rapid and successful integration of HIV diagnosis and care into tuberculosis services in Benin. Int J Tuberc Lung Dis. 2013;17:1405–1410. doi: 10.5588/ijtld.12.0593. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman D G, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.United Nations. Transforming our world: the 2030 agenda for sustainable development. New York, NY, USA: United Nations Sustainable Development Knowledge Platform; 2015. https://sustainabledevelopment.un.org/post2015/transformingourworld Accessed February 2016. [Google Scholar]
- 23.Meijnen S, Weismuller M M, Claessens N J M, Kwanjana J H, Salaniponi F M, Harries A D. Outcome of patients with tuberculosis who transfer between reporting units in Malawi. Int J Tuberc Lung Dis. 2002;6:666–671. [PubMed] [Google Scholar]
- 24.Takarinda K C, Harries A D, Mutasa-Apollo T, Sandy C, Mugurungi O. Characteristics and treatment outcomes of tuberculosis patients who ‘transfer-in’ to health facilities in Harare city, Zimbabwe: a descriptive cross-sectional study. BMC Public Health. 2012;12:981. doi: 10.1186/1471-2458-12-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendation for a public health approach. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 26.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO; 2015. [PubMed] [Google Scholar]
- 27.Lundgren J D, Babiker A G, Gordin F et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danel C, Moh R, Gabillard D et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 29.Kerkhoff A D, Wood R, Vogt M, Lawn S D. Prognostic value of a quantitative analysis of lipoarabinomannan in urine from patients with HIV-associated tuberculosis. PLOS ONE. 2014;9:e103285. doi: 10.1371/journal.pone.0103285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawn S D, Kerkhoff A D, Burton R et al. Rapid microbiological screening for tuberculosis in HIV-positive patients on the first day of acute hospital admission by systematic testing of urine samples using Xpert MTB/RIF: a prospective cohort in South Africa. BMC Med. 2015;3:192. doi: 10.1186/s12916-015-0432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


