Abstract
Identification of specific drivers of human cancer is required to instruct the development of targeted therapeutics. Here we demonstrate that CSNK1D is amplified and/or overexpressed in human breast tumors and that CK1δ is a vulnerability of human breast cancer subtypes overexpressing this kinase. Specifically, selective knockdown of CK1δ, or treatment with a highly selective and potent CK1δ inhibitor, triggers apoptosis of CK1δ-expressing breast tumor cells ex vivo, tumor regression in orthotopic models of triple negative breast cancer, including patient-derived xenografts, and tumor growth inhibition in HER2+ breast cancer models. We also show that Wnt/β-catenin signaling is a hallmark of human tumors overexpressing CK1δ, that disabling CK1δ blocks nuclear accumulation of β-catenin and T cell factor transcriptional activity, and that constitutively active β-catenin overrides the effects of inhibition or silencing of CK1δ. Thus, CK1δ inhibition represents a promising strategy for targeted treatment in human breast cancer with Wnt/β-catenin involvement.
INTRODUCTION
Breast cancer accounts for nearly one quarter of all cancer diagnoses and is the principal cause of cancer-related mortality in women worldwide (1, 2). Currently, treatment selection for breast cancer is based on pathological information and histological grade, and on the expression status of the estrogen (ER), progesterone (PR), and epidermal growth factor 2 (HER2/neu) receptors, where targeted treatments blocking receptor function have made improvement in overall survival (1, 3). Indeed expression of ER and/or PR is a good prognostic factor and predictive indicator for benefit from endocrine therapies, and although HER2 overexpression connotes adverse prognosis, patients greatly benefit from anti-HER2 targeted treatments (4, 5). In contrast, triple negative breast cancers (TNBC), defined by the absence of the ER and PR receptors and the lack of HER2 amplification, have no targeted treatment options, are highly aggressive, and exhibit poor prognosis (6, 7). Although breast cancer research has pioneered and highlights the clinical benefits of targeted treatments, further identification of drivers and associated signaling pathways, particularly for TNBC and HER2 breast cancers, is needed to instruct the development of targeted therapies, to extend disease-free survival, and to improve the lives of cancer patients.
Casein kinase-1 delta (CK1δ) and epsilon (CK1ε) are two highly related serine/threonine kinases known to regulate diverse cellular processes, including circadian rhythm, membrane trafficking, and the cytoskeleton, and both have been implicated in cancer (8–11). For example, myristolated CK1ε is sufficient to transform mammary epithelial cells in vitro, whereas expression of a dominant-negative mutant of CK1δ impairs SV40-induced mammary carcinogenesis in vivo (12). As kinases, CK1δ and CK1ε are eminently tractable for small molecule drug discovery. Nevertheless, the contribution of these kinases to human cancer is poorly understood and the non-selective nature of previously reported CK1δ/CK1ε inhibitors has impeded validation of these kinases as anti-cancer targets (9, 13–15). Indeed, pharmacological effects originally ascribed to inhibition of CK1δ/CK1ε are now known to be due to off-target actions of the non-selective inhibitors employed (13, 16). Thus, we sought to assess the functional role and potential clinical relevance of CK1δ and/or CK1ε as exploitable vulnerabilities in breast cancer.
Herein we report that CK1δ is a promising target for breast cancer therapeutics, and demonstrate the efficacy of a selective and potent small molecule inhibitor that is effective against breast cancer subtypes overexpressing CK1δ. Further, we demonstrate that CK1δ is frequently amplified and/or overexpressed in a subset of human breast cancers, across each of the major breast cancer subtypes, and that knockdown or inhibition of CK1δ provokes breast tumor regression in patient-derived and cell line orthotopic xenograft models of TNBC and HER2+ breast cancer. In addition, mechanistic studies establish that CK1δ activity is a driver of Wnt/β-catenin pathway activation in breast cancers, a molecular phenotype known to associate with poor prognosis in breast cancer patients.
RESULTS
CSNK1D is Amplified and/or Overexpressed in a Subset of Human Breast Cancers
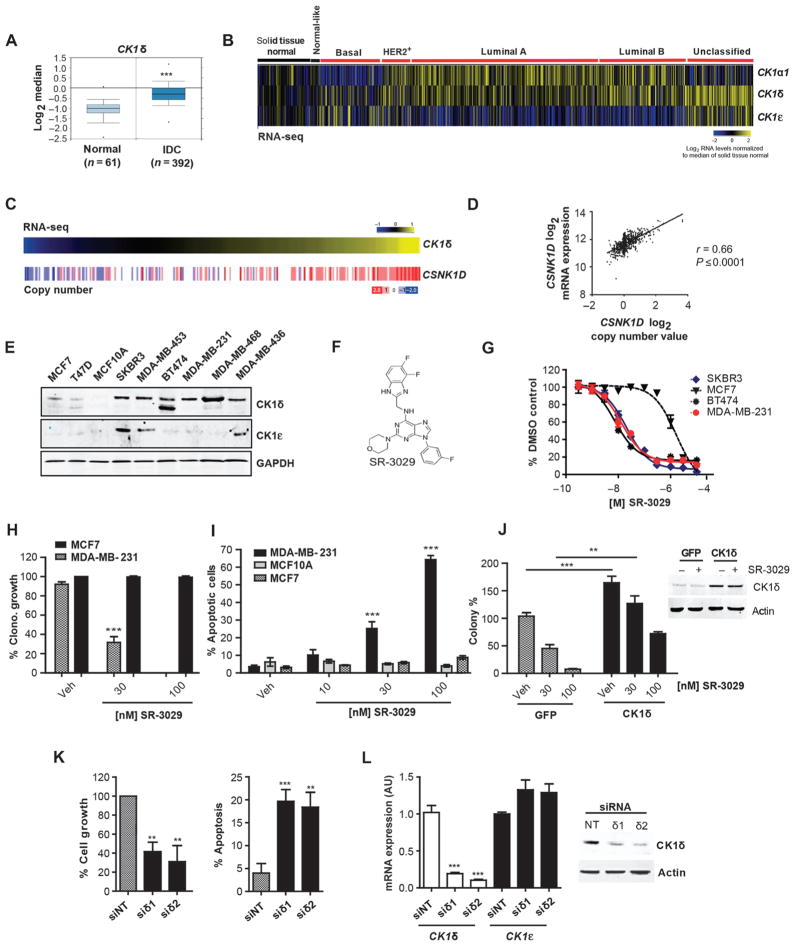
To assess the involvement of CK1δ and CK1ε in human breast cancer, we examined the expression of each isoform in human breast tumor specimens compared to normal mammary tissue. Analysis of the cancer genome atlas (TCGA) datasets revealed highly elevated expression of CK1δ (CSNK1D) in invasive breast carcinomas (Fig. 1A) and in an independent dataset (Fig. S1A) (17). Assessment of CK1 isoform expression across the four major breast cancer subtypes (Pam50 intrinsic classifications) (18) revealed that CK1δ is widely overexpressed within a subset of tumors across all major classes (Fig. 1B). In contrast, CK1ε expression is more restricted to the basal-like subclass (Fig. 1B) and is not associated with invasive breast carcinoma (Fig. S1B). Strikingly, gene copy number analysis (TCGA) revealed amplification (high- and low-level) of 17q25.3 involving the CSNK1D locus in over a third (36%) of human breast tumors, with higher frequencies of amplification in the luminal B and basal-like classes (Fig. S1C). Increased CSNK1D copy number significantly correlates with the expression of CK1δ transcripts (p value < 0.0001) (Table S1), with increased correlation observed within the HER2+, Basal-Like, and Luminal B subtypes compared to the Luminal A tumors (Fig. 1C and D, figure S1D, and tables S2–S5). Consistent with these findings, immunohistochemical analyses confirmed overexpression of CK1δ in human breast tumor specimens compared to normal breast tissue (Fig. S2), and CK1δ was overexpressed across a panel of human breast cancer cell lines (Fig. 1E). In contrast, high CK1ε expression was detected in only 3 of the breast cancer cell lines analyzed (Fig. 1E), and expression of both CK1 isoforms was low in immortal human MCF10A breast epithelial cells, as well as in the MCF7 and T47D ER+ breast cancer cells.
Fig. 1. CK1δ is a clinically relevant and effective target for select breast cancer subtypes.
(A) CK1δ mRNA expression in invasive ductal breast carcinomas (IDC) vs. adjacent normal tissue (***, p=6.78e–15). (B) Expression of CK1α1, CK1δ, and CK1ε across PAM50 breast cancer subtypes based on RNA-Seq data (n=972 tumor samples, 113 solid tissue normal). Log2 normalized read count (RSEM) is shown. (C) CSNK1D DNA copy number analysis in invasive breast carcinomas clustered according to CK1δ expression (n=303). Gene-level copy number estimates (GISTIC2 threshold) of −2 (dark blue), −1 (light blue), 0 (white), 1 (light red), 2 (dark red), representing homozygous deletion, single copy deletion, diploid normal copy, low-level copy number amplification, or high-level copy number amplification are shown. (D) Scatter plot of CSNK1D Log2 mRNA expression vs. Log2 copy number values (972 breast cancer patients). (E) CK1δ and CK1ε protein expression in indicated breast cancer cell lines and MCF10A mammary epithelial cells. (F) Chemical structure of SR-3029. (G) Anti-proliferative potency of SR-3029 in the indicated breast cancer cell lines. Data are plotted as % proliferation vs. vehicle (n=6). (H) Clonogenic growth and survival of the indicated cells in the presence of SR-3029 or vehicle (n=3; p=0.0008). (I) Percent apoptosis by PI /Annexin V FACS after 72 hours of treatment with indicated doses of SR-3029 (n=3; ***, left to right p=0.0007 and 0.0001). (J) Left, Clonogenic growth of MDA-MB-231 cells overexpressing CK1δ or GFP +/− SR-3029 (n=3; ***, p=0. 001, **, p=0.0035). Right, western blot confirming CK1δ overexpression +/− 30 nM SR-3029 at 48 hours. (K) Relative growth (left; n=3, siδ1; p=0.01, siδ2; p=0.003) and percent cell death by trypan blue dye exclusion (right; n=3, siδ1; p=0.01, siδ2 p=0.027) 5 days after transfection of MDA-MB-231 with non-targeting (NT) or CK1δ siRNAs. (L) qPCR data and immunoblot confirming knockdown of CK1δ but not CK1ε (n=3; ***, p<0.0001).
A Potent, Highly Specific CK1δ/CK1ε Inhibitor Selectively Inhibits Breast Cancer Cell Growth and Survival
We recently reported initial structure activity relationships of a series of small molecule dual inhibitors of CK1δ and CK1ε (16). Our most advanced lead, SR-3029 (Fig. 1F), is an ATP competitive inhibitor with exceptional potency and selectivity, and is therefore well-suited for use as a small molecule probe of CK1δ/CK1ε biology. Cell proliferation assays revealed that cell types overexpressing CK1δ are extremely sensitive to CK1δ/CK1ε inhibition, with EC50s in the low nanomolar range (5–70 nM). In contrast, MCF7 and T47D breast cancer cells and the MCF10A cell line, which all express low amounts of CK1δ, were 2–3 orders of magnitude less sensitive to SR-3029 (Fig. 1G, Table S6). This selectivity for breast cancer cells overexpressing CK1δ was confirmed in clonogenic growth assays, where SR-3029 completely blocked clonogenic growth and survival of MDA-MB-231 cells, but had no effect on the colony-forming capacity of MCF7 breast cancer cells (Fig. 1H). FACS analysis established that SR-3029 treatment selectively triggered rapid apoptosis of CK1δ overexpressing breast cancer cells (Fig. 1I).
We determined whether the anti-cancer effects of SR-3029 were due to on-target inhibition of CK1δ and/or CK1ε. First, forced overexpression of CK1δ augmented the clonogenic growth of MDA-MB-231 cells but not GFP-expressing controls and was sufficient to rescue the growth-inhibitory effects of SR-3029 (Fig. 1J). Second, simultaneous knockdown of CK1δ and CK1ε or silencing of CK1δ alone triggered apoptosis and impaired growth of MDA-MB-231 cells (Fig. 1K and L, and fig. S3A and B). This effect was specific in that expression of siRNA-resistant CK1δ transgene effectively rescued the growth-inhibitory effects of CK1δ silencing (Fig. S3C). Knockdown of CK1ε alone had no effect on MDA-MB-231 cell growth and survival (Fig. S3D, E). Finally, we investigated whether there were any potential off-target effects of our inhibitor. SR-3029 inhibits only 1% of the kinome and has a weaker affinity for three kinases in addition to CK1δ and CK1ε (CDK4, MYLK4, and FLT3) (16). Of these, only FLT3 is a common (and weak) off-target of SR-3029 and its close analogues SR-2890 and SR-1277, which all show similar anti-proliferative activities against human cancer cells (16), including MDA-MB-231 cells (Fig. S4). In contrast, MDA-MB-231 cells were insensitive to AC220, a low nanomolar inhibitor of FLT3 and FLT3 mutants (Fig. S4). Collectively, these findings establish CK1δ as a biologically relevant target of SR-3029 that is required for the growth and survival of certain breast cancer cells.
Silencing or Inhibition of CK1δ Provokes Potent Anti-Tumor Effects in vivo
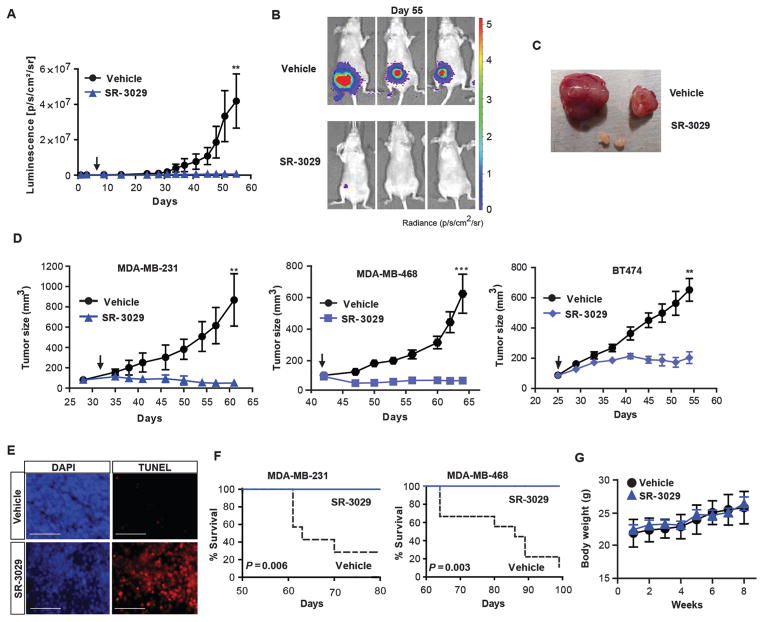
SR-3029 exhibits pharmacokinetic properties amenable for in vivo studies (16). Given this, and the apparent addiction of CK1δ-overexpressing breast cancer cells to this kinase, we tested the efficacy of SR-3029 in preclinical tumor models. MDA-MB-231 TNBC cells engineered to express firefly luciferase were engrafted into the mammary fat pads of immunodeficient nude mice, and treatment began seven days later. As a single agent, SR-3029 (20 mg/kg daily i.p.) markedly impaired tumor growth (Fig. 2A, B). Only 2 recipients subjected to SR-3029 therapy developed tumors, and these were strikingly small in size compared to those that arose in vehicle-treated mice (Fig. 2B, C). Next, orthotopic MDA-MB-231, MDA-MB-468 (TNBC), SKBR3 and BT474 (HER2+) tumor xenografts were allowed to reach an average size of 100 mm3 before treatment. Daily i.p. administration of SR-3029 (20 mg/kg) markedly inhibited tumor growth in all xenograft models (Fig. 2D and S5). Regression of aggressive MDA-MB-231 and MDA-MB-468 tumors was associated with fulminant apoptosis (Fig. S5A and 2E) and with a significant increase in lifespan (Fig. 2F) (p=0.006). Moreover, analysis of body weight, blood chemistry, and tissue integrity by histochemistry revealed that long-term daily dosing with SR-3029 (48 days) is well tolerated, with no overt toxicity (Fig. 2G, table S7, and fig. S6).
Fig. 2. Inhibition of CK1δ/CK1ε impairs orthotopic breast tumor growth in vivo.
(A) Effects of CK1δ/CK1ε inhibitors on growth and establishment of MDA-MB-231-luc tumors monitored by luminescence intensity over time. Mice were treated once per day with SR-3029 or vehicle (10:10:80, DMSO:Tween-80:Water) at 20 mg/kg by i.p. injection. Arrow indicates start of treatment (n=8 for each cohort; **, p=0.01). (B) Tumor size by luminescence and (C) comparison of gross tumor size at day 55. Representative tumors are shown. (D) Growth curves of indicated TNBC tumor models in mice treated with vehicle (black line) or SR-3029 (blue line) as above. Arrows indicate timing of first dose (n=8–10 for each cohort; **, p=0.01; ***, p=0.0008). (E) TUNEL staining on serial sections of vehicle and SR-3029 treated MDA-MB-231 tumors (representative images are shown) (scale bar=200 μm). (F) Kaplan-Meier survival curves corresponding to studies shown in (D) (p value calculated by log-rank test). (G) Body weight of mice treated daily with SR-3029 (blue line) or vehicle (black line) was monitored for 8 weeks (n=8–10).
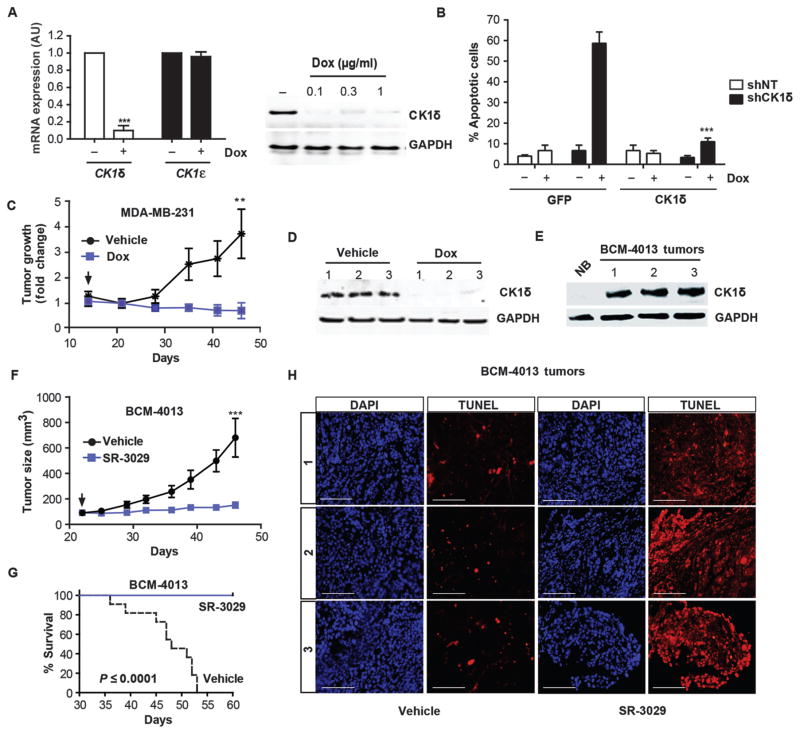
To confirm that inhibitory effects of SR-3029 on tumor growth in vivo were due to on-target inhibition of CK1δ, MDA-MB-231 cells were engineered to stably express a doxycycline (Dox)-inducible shRNA directed against CK1δ (MDA-MB-231-shCK1δ). Treatment of these cells with Dox ex vivo resulted in efficient and selective knockdown of CK1δ and rapid apoptosis/cell growth inhibition. This effect was rescued by exogenous expression of a shRNA-resistant CK1δ transgene (Fig. 3A, B, and S7). Following establishment of orthotopic xenografts of MDA-MB-231-shCK1δ tumors (> 100 mm3), administration of Dox and inducible knockdown of CK1δ resulted in a marked suppression of tumor growth, consistent with results obtained with SR-3029 (Fig. 3C, D). We also assessed the effects of SR-3029 on growth of a primary patient-derived xenograft (PDX) model, BCM-4013, an aggressive basal-like invasive ductal carcinoma, which had limited response to treatment with dasatinib and docetaxel. Histological and molecular profiling of this stably passaged PDX recapitulated many features of the primary tumor, including response to treatment (19). After expansion of this model in vivo, we established orthotopic tumors, tested them for expression of CK1δ (Fig. 3E), and performed efficacy studies. In accord with breast cancer cell line models, administration of SR-3029 (20 mg/kg daily i.p.) significantly inhibited the growth of these PDX tumors and triggered tumor cell apoptosis (Fig. 3F–H) (p=0.0002). These studies support the notion that CK1δ expression predicts sensitivity to SR-3029 and indicate that CK1δ is an efficacious breast cancer target with potential relevance to human disease.
Fig. 3. Silencing or inhibition of CK1δ provokes breast tumor regression and blocks growth of PDX breast models.
(A) Left, qPCR data confirming efficient knockdown of CK1δ, but not CK1ε after 72 hours of treatment of MDA-MB-231 shCK1δ-expressing cells with Dox (0.3 μg/ml) (n=3; ***, p=0.0003). Right, corresponding CK1δ protein expression. (B) Cells expressing Dox-inducible CK1δ or non-targeting (NT) shRNA were treated with 1 μg/ml Dox for 72 hours and transfected with vectors expressing CK1δ or GFP cDNA resistant to shRNA. After a further 72 hours, percent cell death was measured by trypan blue dye exclusion in MDA-MB-231-shCK1δ (black) and cells expressing non-targeting shRNAs (NT, white) (n=4; ***, p=0.0001). (C) Growth of orthotopic MDA-MB-231-shCK1δ tumors in mice +/− Dox (administered ad lib in chow, 200 mg/kg), as monitored by caliper measurements (n=8 for each cohort; **, p=0.006). Arrow indicates addition of Dox. (D) CK1δ knockdown in tumor tissue isolated from three independent mice, 7 days after Dox administration began. (E) Immunoblot comparing CK1δ expression in extracts of normal human breast or three independent BMC-4013 PDX tumors. (F) Growth curves of BCM-4013 PDX tumors in mice treated with vehicle (black line) or SR-3029 (blue line). Arrow indicates timing of first dose (n=12 for each cohort; ***, p=0.0002). (G) Kaplan-Meier survival curve corresponding to studies shown in (F) (p value calculated using log-rank test). (H) TUNEL staining on serial sections of vehicle and SR-3029 treated BMC-4013 tumors (representative images are shown) (scale bar=200 μm).
Wnt/β-catenin Signaling is a Hallmark of CK1δ-Expressing Breast Cancers
To define pathways regulated by CK1δ in human breast cancer and to identify potential biomarkers for CK1δ inhibition, we analyzed TCGA patient tumor datasets for gene signatures associated with CK1δ overexpression. We identified 612 genes whose expression significantly correlated with CK1δ expression (fold change > 2, p value < 0.05) (fig. S8A and B), and Ingenuity Pathway Analysis (IPA) indicated marked overlap with genes of the canonical Wnt pathway, including Wnt/β-catenin targets (CCND1), Wnt pathway components (FZD9), and endogenous modulators of the pathway (WNT3, WNT9A, and SFRP1) (Secreted frizzled-related protein 1) (Fig. 4A and fig. S8C). Although activated Wnt/β-catenin signaling is associated with poor clinical outcome, pathway-activating mutations typical of other cancer types are rare in breast cancer (20, 21). Our findings suggested that CK1δ is an important activator of the Wnt pathway in human breast tumorigenesis and that genes regulated by this pathway could potentially serve as biomarkers required for further preclinical and clinical development of CK1δ inhibitors.
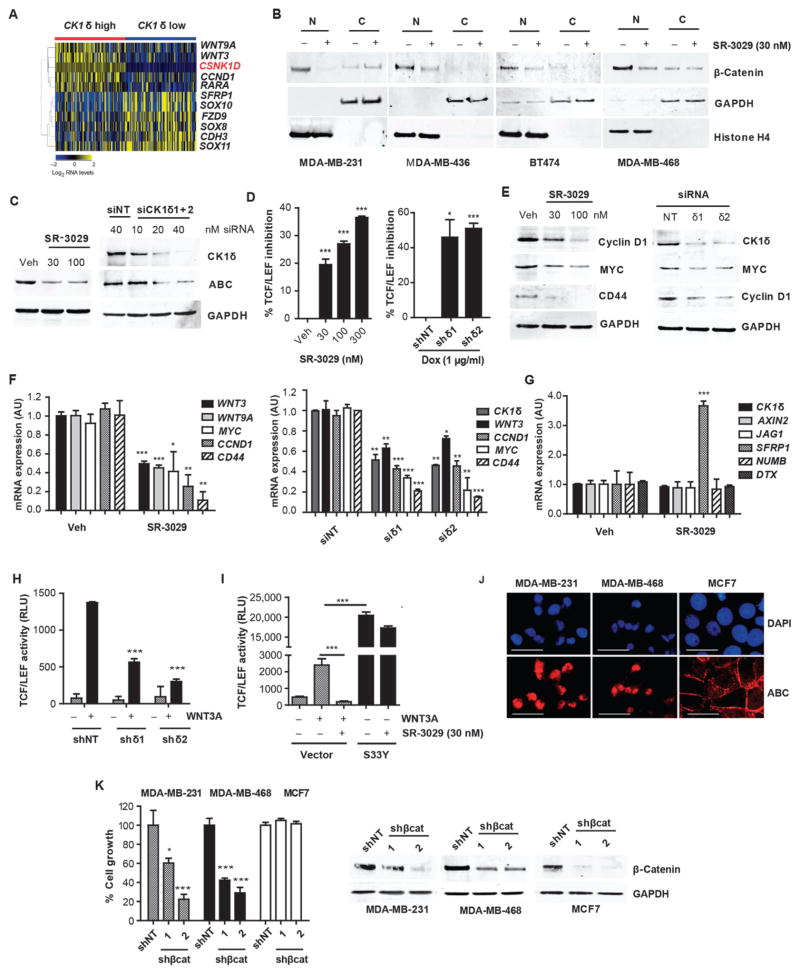
Fig. 4. Modulation of the Wnt/β-catenin pathway is a biomarker for CK1δ activity and inhibition.
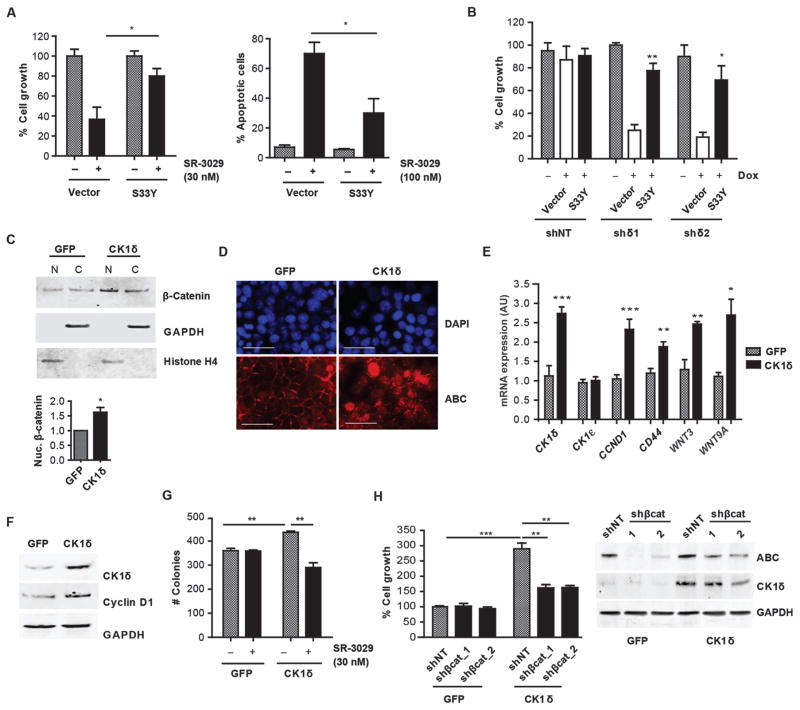
(A) Wnt pathway genes significantly enriched in CK1δ-overexpressing human breast tumors (fold change > 2, p value < 0.05) (red is CK1δ gene). (B) Effect of SR-3029 (+) or vehicle (−) treatment (18 hours, 30 nM) on nuclear vs. cytoplasmic β-catenin in the indicated breast cancer cell lines. (C) Expression of active β-catenin (ABC) in MDA-MB-231 cells after 18 hours of treatment with SR-3029 or vehicle or after transfection with CK1δ siRNAs (harvested at 48 hours). (D) Inhibition of TCF-dependent luciferase activity in MDA-MB-231 cells treated with increasing doses of SR-3029 for 6 hours or after 5 days of treatment with 1 μg/ml Dox to activate expression of indicated shRNAs (n=3; *, p=0.013; ***, p=0.0002). (E) Effect of CK1δ inhibition (left, 24 hours of treatment with 30 or 100 nM SR-3029) or knockdown (right, 48 hours after transfection) on expression of indicated proteins and (F) mRNAs by immunoblot or qPCR, respectively (n=3). (G) Expression of indicated mRNAs 24 hours after treatment with 100 nM SR-3029 (n=3; *, p<0.05; **, p<0.01; ***, p<0.001; SFRP1 p=0.0003; WNT3 p=0.001; WNT9A p=0.0007; MYC p=0.0271; CCND1 p=0.0054; CD44 p=0.0071). (H) TCF-dependent luciferase activity in HEK293T cells +/− CK1δ shRNA expression induced with 1 μg/ml Dox for 72 hours followed by +/− 3 hours of treatment with 1 μg/ml WNT3A (***, p=7.23E-05 and 5.57E-06 left to right). (I) HEK293T cells stably expressing the TCF-dependent luciferase reporter were transfected with a control vector or a constitutively active (nuclear) mutant of β-catenin (S33Y) and incubated overnight +/− SR-3029 before addition of recombinant WNT3A for 3 hours (representative of 3 independent experiments is shown; ***, p= 0.0004 (left) and 0.0002 (right)). (J) Immunostaining for ABC expression (scale bar=200 μm). (K) Relative growth 4 days after infection of indicated cell lines with lentiviruses expressing either non-targeting (NT) or β-catenin shRNAs (n=3; Left to right; *, p=0.05, ***, p=0.0009, ***, p=0.001, ***, p=0.001) and corresponding western blots (right panel).
The role of CK1δ and CK1ε in development and disease has been attributed to both Wnt-dependent and independent roles (10, 15, 22, 23). Moreover, the requirement for CK1δ/CK1ε in canonical pathway activation is controversial, with both cell type and context specificities (13, 22). We therefore assessed the effects of CK1δ inhibition on β-catenin activity in CK1δ-expressing breast cancer cells. Activation of Wnt signaling results in the stabilization and nuclear translocation of β-catenin, which together with TCF/LEF transcription factors induces the expression of downstream target genes associated with breast cancer tumorigenesis (24–26). Treatment of CK1δ-overexpressing breast cancer cell lines MDA-MB-231, MDA-MB-436, MDA-MB-468, and BT474 with SR-3029 markedly reduced expression of the active, nuclear pool of β-catenin (Fig. 4B). Further, CK1δ knockdown or SR-3029 treatment reduced the unphosphorylated, active form of β-catenin (ABC, Fig. 4C) and markedly reduced endogenous β-catenin/TCF transcriptional activity, as measured in MDA-MB-231 cells stably expressing a TCF-dependent luciferase reporter (Fig. 4D). Accordingly, selective knockdown of CK1δ or inhibition by SR-3029 repressed the expression of endogenous β-catenin/TCF target genes, including CCND1, MYC, CD44, as well as WNT3 and WNT9A, which were all associated with CK1δ expression in human tumors (Fig. 4E and F). Moreover, SR-3029 treatment markedly increased the expression of the endogenous Wnt antagonist SFRP1, yet had no effect on Notch pathway mRNAs (JAG1, NUMB, DTX) (Fig. 4G), an additional pathway strongly implicated in breast cancer pathogenesis (27).
To test if inhibition of CK1δ/CK1ε is sufficient to switch off canonical Wnt signaling in response to Wnt ligands, we generated HEK293T cells stably expressing a TCF-dependent luciferase reporter. As predicted, Wnt-3a-directed induction of the TCF reporter was abolished by treatment with SR-3029 or CK1δ knockdown (Fig 4H and I). Further, forced expression of a constitutively active (nuclear) mutant of β-catenin (S33Y) (28) increased TCF reporter activity, and this was refractory to the inhibitory effects of SR-3029 (Fig. 4I). Thus, inhibition of CK1δ is sufficient to block activated β-catenin signaling in human breast cancer cells and Wnt-inducible activation of the pathway through canonical signaling.
To assess the consequence of impaired Wnt/β-catenin signaling on the tumorigenic growth of human breast cancer cell subtypes that are sensitive to CK1δ inhibition, we expressed β-catenin shRNAs in MDA-MB-231 and MDA-MB-468 cells. Each of these cell types expressed nuclear β-catenin (Fig. 4J) and depended on β-catenin for sustained cell growth and survival (Fig. 4K). Conversely, MCF7 cells, which express little to no nuclear β-catenin, were insensitive to β-catenin knockdown, consistent with their low expression of CK1δ and relative insensitivity to SR-3029 (Fig. 4K).
To more directly assess the role of impaired β-catenin signaling and the anti-tumor effects of targeting CK1δ, we used two constitutively active β-catenin mutants. Forced expression of β-catenin-S33Y or the NH2-terminal constitutively active mutant (β-catenin N90) was sufficient to rescue the growth inhibitory and apoptotic effects of either SR-3029 or CK1δ knockdown in MDA-MB-231 cells (Fig. 5A and B, fig. S9). Thus, CK1δ controls β-catenin activity, which is necessary for breast cancer cell growth and survival.
Fig. 5. CK1δ is a necessary and sufficient driver of Wnt/β-catenin signaling in human breast cancer.
(A) Cell growth (left) and apoptosis (right) measured after 72 hours +/− SR-3029 in MDA-MB-231 cells transfected with an empty vector or β-catenin-S33Y (n=4; *, p=0.05). (B) MDA-MB-231-shCK1δ cells treated with Dox (4 days, 1 μg/ml) were transfected with an empty vector or β-catenin-S33Y, and cell number was measured after 72 hours (n=3: ***, p=0.001). (C) Expression of nuclear and cytoplasmic β-catenin in MCF7 cells engineered to overexpress CK1δ or GFP. Bottom, quantification of nuclear β-catenin expression normalized to Histone H4 (n=3; *, p=0.02). (D) Immunostaining for ABC in MCF7 cells overexpressing CK1δ or GFP (scale bar=200 μm). (E) qPCR analysis of β-catenin targets in MCF7-CK1δ vs. MCF7-GFP cells (n=3; **, p=0.01; ***, p=0.001). (F) Immunoblot confirming CK1δ overexpression and increased cyclin D1. (G) Effect of SR-3029 on clonogenic growth of MCF7 cells overexpressing CK1δ vs. GFP (n=6; Left to right; **, p=0.01, **, p=0.002). (H) Growth of MCF7-CK1δ and MCF7-GFP cells 4 days after infection with β-catenin shRNA lentiviruses (n=3, Left to right; ***, p=0.0006; **, p=0.004, **, p=0.001). Right panel, immunoblot showing CK1δ overexpression and knockdown of β-catenin.
MCF7 breast cancer cells express a low amount of CK1δ (Fig. 1E), have reduced expression of active (nuclear) β-catenin compared to MDA-MB-231 cells (Fig. S10A), are refractory to SR-3029 (Fig. 1G), and have limited tumorigenic potential relative to other human breast cancer cells (29–31). MCF7 cells engineered to overexpress CK1δ displayed increased expression of nuclear β-catenin (Fig. 5C, D) and downstream Wnt target genes, including CCND1, CD44, WNT3, and WNT9A (Fig. 5E, F). Further, forced overexpression of CK1δ potentiated the clonogenic growth of MCF7 cells and sensitized them to SR-3029 in both short-term and long-term growth assays (Fig. 5G and Fig. S10B). Knockdown of β-catenin was sufficient to impair exogenous CK1δ-driven MCF7 cell growth (Fig. 5H), confirming a critical mechanistic role for the Wnt/β-catenin pathway in the growth-promoting activity of CK1δ.
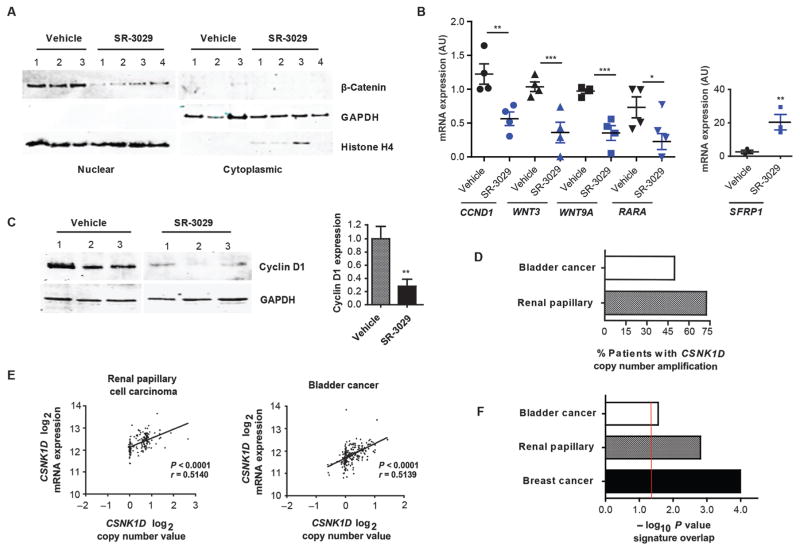
To assess if CK1δ inhibition impairs Wnt/β-catenin signaling in vivo and if modulation of this pathway represents a predictive biomarker, MDA-MB-231 tumors isolated from mice treated for 7 days with 20 mg/kg SR-3029 or with vehicle (once daily, i.p administration) were analyzed for markers of activated β-catenin signaling. Expression of nuclear β-catenin was markedly reduced in tumors derived from SR-3029-treated mice compared to vehicle-treated controls (Fig. 6A), and this was associated with decreases in CCND1, WNT3, WNT9A, and RARA mRNA transcripts, as well as with a marked increase in expression of SFRP1 transcripts (Fig. 6B). Moreover, down-regulation of Cyclin D1 protein was observed in tumors derived from SR-3029-treated mice as well as in those that had undergone Dox-inducible silencing of CK1δ (Fig. 6C and fig. S11). Finally, because Wnt/β-catenin signaling is known to play a key role in the homeostasis of regenerating tissues, we assessed the effects of long-term SR-3029 treatment on the integrity of the small intestine. H&E staining revealed no gross morphological defects, and TUNEL staining failed to detect evidence of apoptosis (fig. S12), in contrast to that observed in corresponding tumors (Fig. 2E).
Fig. 6. CK1δ is a driver of Wnt/β-catenin signaling in vivo.
(A) Expression of nuclear and cytoplasmic β-catenin and (B) the indicated mRNAs, in MDA-MB-231 tumors from mice treated with 20 mg/kg SR-3029 vs. vehicle daily for 7 days (n=4; *, p=0.05; **, p=0.01; ***, p=0.001). (C) Effects of SR-3029 on tumor Cyclin D1 protein expression at day 7. Right panel shows quantification (n=3; **, p=0.01). (D) Frequency of CSNK1D copy number amplifications in renal papillary cell carcinoma (n=172) and bladder cancer tumors (n=220; TCGA). (E) Correlation of CSNK1D DNA copy number and CK1δ expression in renal papillary cell carcinoma (n=172) and bladder cancer (n=220). (F) −log10 p values showing significant overlap between Wnt/β-catenin pathway genes and CK1δ signature lists (p<0.05, fold change >1.5) for indicated cancer types (red line is threshold of significance, p=0.05).
Collectively, these findings established a link between activation of a CK1δ-to-β-catenin pathway and sensitivity to SR-3029, and suggested that features of this pathway would define tumors that will respond to this targeted therapy. Analyses of additional TCGA cancer datasets revealed CSNK1D copy number amplifications (high and low) in over 70% of patients with papillary renal cell carcinoma and in nearly 50% of patients with bladder cancer, and gene amplification in these tumors also correlated with increased CK1δ expression (Fig. 6D and E and tables S8–11). There was a significant overlap between the CK1δ gene signature list and Wnt pathway genes in both cancer types (CK1δ high vs. CK1δ low, p < 0.05, fold change 1.5), (Fig. 6F, fig. S13, and tables S12 and 13). Thus, the CK1δ-to-β-catenin signaling circuit may be an unexploited vulnerability across a spectrum of human malignancies.
DISCUSSION
Identification of specific drivers of human breast cancer has instructed the development of targeted therapies such as trastuzumab for the treatment for HER2 amplified breast cancers and hormonal therapies for the treatment of ER+ breast cancers, and these targeted agents have improved the survival and clinical management of these diseases (32). In contrast, patients with relapsed disease and those with TNBC lack targeted therapies and represent an urgent unmet clinical need. The data presented herein implicate CK1δ as an attractive therapeutic target with potential benefit for HER2+ and TN breast cancer patients aberrantly expressing CK1δ.
Heretofore, the roles of CK1δ in human cancer have been poorly understood, and prior small molecule modulators of CK1δ have lacked the potency and/or selectivity required to validate CK1δ as an anticancer target (9, 13–15, 33). For example, the probe molecule IC261 used in several studies has subsequently been shown to act not by inhibition of CK1δ/CK1ε, but rather by blocking tubulin function (13). Moreover, studies with the CK1δ/CK1ε dual inhibitor PF670462 have shown that it lacks anti-cancer activity (13, 14), and that this is likely due to important off-target activity against multiple kinases, including several having pro-apoptotic activity (16). In contrast, our small molecule inhibitor (SR-3029) is highly selective, potent (16), and efficacious in multiple preclinical models of human breast cancer. Further, our findings demonstrate that overexpression of CK1δ predicts sensitivity to SR-3029 in cell-based models of breast cancer, suggesting that dependence on CK1δ is cell type and context specific. Overexpression of CK1δ, which is widespread across each of the four major breast cancer subtypes, may thus identify tumors that will respond to this targeted treatment strategy. Further study across a broader spectrum of patient-derived tumor samples is needed to fully investigate this hypothesis.
Gain- and loss-of-function mutations in positive (β-catenin) and negative (APC, AXIN1, etc.) regulatory components of the Wnt pathway are prevalent at a high frequency in human cancers (reviewed by (34)). In contrast, although aberrant activation of the Wnt pathway is frequent in breast cancer (21, 35, 36), mutations in Wnt pathway components are rare in these malignancies (20, 21, 37). Here we have shown that CK1δ-to-β-catenin signaling is activated in a subset of human breast cancers, where silencing or pharmacological inhibition of CK1δ is sufficient to disable β-catenin activity and provoke breast cancer cell apoptosis. Our findings thus implicate CK1δ as a key target kinase that can be exploited (for instance by SR-3029) to disable aberrant Wnt/β-catenin signaling that is manifest in several breast cancer subtypes. Previous reports have described several pathways where CK1δ plays an important role (23, 33). Thus, although the anti-breast cancer activity of SR-3029 clearly targets Wnt/β-catenin signaling, additional effectors could contribute to its substantial anti-tumor activity in vivo.
Collectively, the findings presented herein identify CK1δ as an efficacious therapeutic target with potential for clinical relevance in a subset of breast cancers, where CK1δ is: (i) activated via amplification and/or overexpression; (ii) a necessary driver of β-catenin activity in these tumor subtypes; and (iii) necessary for the growth and survival of cell and patient-derived preclinical models of human breast cancer. Further, given the discovery of additional cancers with an activated CK1δ-β-catenin circuit, CK1δ may be an attractive target for a broad spectrum of human malignancies. Moving forward, further medicinal chemistry efforts are required for the development of a SR-3029 analog suitable as a clinical candidate for testing in humans.
MATERIALS AND METHODS
Study Design
This study was designed to assess the involvement of CK1δ and CK1ε in human breast cancer and to investigate the efficacy of a highly specific dual CK1δ/CK1ε inhibitor in pre-clinical models of human breast cancer. Five human orthotopic breast xenograft models, as well as pharmacological and genetic studies were used to validate targeting CK1δ in subsets of breast cancer that overexpress this kinase. Power analyses suggested that based on the difference in tumor volume between groups and the standard deviation of tumor volumes within each group an n of at least 7 or greater is required for confidence of 90%,. Our experiments therefore included 7–12 tumor-bearing mice per experimental or control (vehicle) cohort, with mice randomized before treatment to determine random sampling such that the median tumor size between cohorts was the same. All tumor sizes were measured throughout the duration of the experiment and graphed in figures without excluding any samples. For survival analyses, mice were euthanized when moribund and/or when tumors became ulcerated or exceeded a volume of 1.2 cm3. All cell-based assays were performed in triplicate and repeated at least 3 times.
Xenograft Tumor Models and Bioluminescent Imaging of Mice
All animal studies were approved by the Scripps Florida IACUC. Stable pools of MDA-MB-231-Luc, MDA-MB-231, MDA-MB-468, SKBR3, or BT474 cells were established by injection of 2 × 106 cancer cells into the mammary fat pads of 6-week-old female athymic nude mice (Charles River Laboratories). Establishment of BCM-4013 patient-derived xenografts was performed as described (19). Briefly, fresh xenograft tumor fragments (~1 mm3) were transplanted into the cleared mammary fat pad of recipient SCID/Bg mice (Charles River Laboratories). Mice were treated with SR-3029 or vehicle (10:10:80, DMSO:Tween-80:Water) at 20 mg/kg daily by i.p. injection. Tumor volumes were measured as the indicated intervals using calipers or by luminescence imaging with the IVIS 100 imager (exposure time, 1–60 sec; binning 8; field of view 15-cm; f/stop 1; open filter) after subcutaneous injection of luciferin (15 mg/ml, Goldbio Technology). Average radiance (p/s/cm2/sr) was determined from tumor region-of-interest (ROI) using Living-Image (Xenogen) analysis software.
Cell Proliferation and Clonogenicity Assays
Cell proliferation was measured 72 hours after SR-3029 or vehicle treatment using Cell-Titer Glo (Promega) according to the manufacturer’s instructions. EC50 values were determined by non-linear regression and a four-parameter algorithm (GraphPad Prism5). For clonogenic assays, cells were plated in 6-well dishes in triplicate at a density of 500–1000 cells per well. After overnight incubation, SR-3029 or vehicle (DMSO) was added to the medium for 72 hours, and cells were allowed to grow out for 7–10 days, during which medium was changed every 2–3 days without adding compound. Colonies were fixed in 4% paraformaldehyde/PBS, stained with 0.5% methylene blue in 50% ethanol for 1 hour at room temperature, and de-stained with water. Colonies with more than 50 cells were counted using a low magnification light microscope.
Reagents, Cell Lines and Transfection
Unless otherwise stated, all chemicals were purchased from Sigma Aldrich. MDA-MB-231, MDA-MB-436, MDA-MB-468, HS578T, BT474, SKBR3, MDA-MB-453, MCF7, and T47D breast cancer cells, as well as immortal MCF-10A breast epithelial cells, were from the American Type Culture Collection (ATCC). For CK1δ and CK1ε knockdown, siRNA duplexes were prepared according to the manufacturer’s instructions (Qiagen), and specific knockdown conditions were optimized using the HiPerfect transfection reagent (Qiagen). A final concentration of 20 nM total siRNA was used to achieve knockdown. FuGene6 (Roche) was used for DNA transfections as per manufacturer’s instructions. SiRNA, shRNA, and oligonucleotide sequences used for PCR are listed as supplementary tables (tables S14–16).
Lentiviral Transduction
Lentiviral vectors expressing CK1δ (Y3989-Lv105-0200, GeneCopoeia), GFP (EGFP-Lv105-0200, GeneCopoeia), luciferase, or the TCF reporter 7TFP (Addgene) were co-transfected with pPACKH1 packaging plasmids into HEK293T cells to produce lentiviral particles per the manufacturer’s recommendations (System Biosciences). To stably express specific shRNAs (table S16) shRNA oligonucleotides were cloned into the Tet-pLKO-Puro vector using the recommended protocol (38), and lentiviruses were generated using the Mission Packaging System (Sigma). MDA-MB-231 cells were transduced with optimized titers of lentiviruses, and infected cells were selected in puromycin (1 μg/ml) or blasticidin (5 μg/ml for luciferase lentivirus) containing medium to expand stably infected pools.
Immunoblotting
SDS-PAGE gel electrophoresis was performed using NuPAGE 4–12% Bis-Tris gels (Invitrogen), and proteins were transferred to PVDF membranes by semi-dry transfer using trans-blot transfer medium (Biorad). Membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences) and incubated overnight at 4°C with primary antibodies. After repeated washes with TBST (20 mM Tris, pH 7.6, 140 mM NaCl, and 0.1% Tween-20), blots were incubated with the appropriate IRDye-conjugated secondary antibody (LI-COR Biosciences) and imaged using the LI-COR Odyssey. Bands were quantified using the Odyssey software (LI-COR Biosciences). The following antibodies were used in this study: CK1δ and Histone H4 (Abcam), CK1ε, c-Myc (9E10), Cyclin-D1, and β-actin (Santa Cruz), β-catenin (Cell Signaling), GAPDH (Millipore), and CD44 (R&D Systems).
Quantitative Real-Time PCR
Total RNA was obtained with the RNeasy Plus Mini Kit (Qiagen), and 1–2 μg of RNA was reverse transcribed with Superscript III First Strand Synthesis System (Life Technologies). Quantitative PCR was performed with the Power SYBR Green PCR Master Mix (Life Technologies) on the ABI7900HT Fast Real-Time PCR System. Intron-spanning gene-specific primer pairs were designed using the Primer3 algorithm, and relative expression values for each gene of interest were obtained by normalizing to GAPDH mRNA expression using the ΔΔCt method.
Immunocytochemistry and H&E Staining
To detect apoptosis in cryosections, tumors and small intestines were fixed in 10% buffered formalin for 2 hours, incubated in 20% sucrose overnight, and embedded in OCT. Frozen sections (5 μM) were mounted and stained using the ApopTag Red In Situ Kit according to the manufacturer’s instructions (CHEMICON). For H&E staining, tissues were fixed in 10% buffered formalin for 48 hours, transferred to 70% ethanol/PBS, and embedded in paraffin. Staining was performed on 5 μM sections after deparaffinization (AML Laboratories).
Flow Cytometry
2 × 105 cells seeded in triplicate in 6-well dishes were cultured with SR-3029 or vehicle for 72 hours. Cells were then harvested and stained with Annexin V-FITC and PI using the Annexin V-FITC Apoptosis Detection Kit (BioVision) per the manufacturer’s instructions and analyzed with the LSRI II flow cytometer (Becton Dickinson). Staurosporine (Cell Signaling) treated cells (1 μM) were used as positive controls.
TCF Reporter Assays
MDA-MB-231 or HEK293 cells stably expressing the TCF luciferase reporter 7TFP (39) were transfected with β-catenin-S33Y or empty vector (pcDNA). After 18 hours, cells were seeded onto a 96-well plate at a density of 6000 cells/well. After 24 hours, cells were treated with SR-3029 or vehicle and incubated for 6 hours before addition of 1 μg/ml recombinant human Wnt3a (R&D Systems) or PBS. After 3 hours, we performed reporter assays with BriteLite Plus (Perkin Elmer), which was added in equal volume directly to the medium, and luminescence was detected with a Spectramax plate reader (Molecular Devices).
Bioinformatics Analyses
TCGA data retrieval: breast cancer (BRCA), kidney renal papillary cell carcinoma (KIRC), and bladder cancer (BLCA) gene expression and copy number datasets were downloaded from TCGA portal (http://tcga-data.nci.nih.gov/). For expression profiling, we downloaded level 3 expression data of 20,475 genes from the RNASeqV2 platform.
For gene expression profiling analysis, RNA-Sequencing (RNA-Seq) by Expectation-Maximization (RSEM) normalized count was used to analyze gene expression estimates for the RNASeqV2 data from TCGA breast cancer dataset. Log2 normalized counts were imported into GeneSpring GX V12.1 (Agilent Technologies). Baseline transformation was set as the median for all samples for each dataset (919 breast, 228 kidney, and 260 bladder cancer samples).
To identify the CK1δ gene signature list, the upper 100 and lower 100 tumor breast tumor samples (CK1δ-high and CK1δ-low groups) or upper and lower quartiles for smaller datasets (kidney and bladder cancer) were defined on the basis of CK1δ (CSNK1D) expression. Out of 20,501 genes, only genes with higher than median expression in at least one sample were filtered for downstream analysis. GeneSpring Volcano Plot function was used to identify differentially expressed genes between the CK1δ-high and CK1δ-low groups. Statistical test parameters were set as follows: selected test, unpaired t-test; p value computation, asymptotic, multiple testing correction, Benjamini-Hochberg. Corrected p value cut-off was set to 0.05, and fold change cutoff was as indicated in the text.
To generate heatmaps, we used the GeneSpring hierarchical clustering algorithm. The similarity measure was set to Pearson centered, and the linkage rule was set to average. Ingenuity Pathway Analysis software (Qiagen) was used to identify canonical pathways that overlap with the CK1δ gene signature in the breast cancer dataset. Significance was calculated by Fisher’s exact test (p value < 0.05). For analysis of additional cancer datasets, we imported the list of IPA Wnt/β-catenin signaling genes (172 genes) into GeneSpring and calculated the p value of overlap with the CK1δ signature lists using GeneSpring software (probability of overlap formula).
Copy number analysis, TCGA RNA-seq, and GISTIC2 thresholded copy number data were ordered on the basis of CSNK1D RNA-Seq expression. To confirm the correlation, we generated a scatter plot in GraphPad Prism 6 on the basis of log2 mRNA expression and log2 copy number values (40). Pearson r and p value were calculated using GraphPad Prism 6. The data shown in all scatter plots and correlation analyses are provided in supplementary tables S1–4 and S7–11.
Statistical Analysis
All values in figures are presented as means ± SE unless otherwise stated. Survival curves were calculated by using the Kaplan-Meier method, and differences between the curves were determined by log rank test. Correlation coefficients were calculated using the Pearson test. All other experiments were analyzed using Student’s two-tailed t test in Excel or Prism, and p values ≤ 0.05 were considered significant.
Supplementary Material
Fig. S1. CK1δ, but not CK1ε, is overexpressed in human breast cancer and associated with CSNK1D gene amplification.
Fig. S2. CK1δ is overexpressed in human breast cancer specimens.
Fig. S3. Silencing of CK1δ and CK1ε, but not CK1ε alone, impairs growth and survival of MDA-MB-231 breast cancer cells.
Fig. S4. Selective inhibition of FLT3 has no effect on proliferation of MDA-MB-231 breast cancer cells.
Fig. S5. Treatment with SR-3029 impairs growth of triple negative and HER2+ breast tumor models.
Fig. S6. Long-term daily dosing with SR-3029 has no adverse effects on major organ histology.
Fig. S7. Selective silencing of CK1δ is sufficient to impair growth of MDA-MB-231 breast cancer cells.
Fig. S8. Wnt/β-catenin pathway genes are a hallmark of CK1δ-expressing breast cancers.
Fig. S9. Constitutively active β-catenin N90 rescues the growth inhibitory effects of CK1δ inhibition and silencing.
Fig. S10. CK1δ expression in MCF7 breast cancer cells sensitizes them to SR-3029.
Fig. S11. Cyclin-D1 expression is suppressed in TNBC MDA-MB-231-derived tumors following knockdown of CK1δ.
Fig. S12. Inhibition of CK1δ/CK1ε has no effects on intestinal homeostasis.
Fig. S13. Canonical Wnt signaling genes are correlated with CK1δ expression in human renal and bladder cancer.
Table S1. TCGA RNA-seq and GISTIC2 copy number data and correlation analysis for breast cancer (BRCA) tumors.
Table S2. TCGA RNA-seq and GISTIC2 copy number data for Basal-like Pam50 breast tumors.
Table S3. TCGA RNA-seq and GISTIC2 copy number data for HER2 Pam50 positive breast tumors.
Table S4. TCGA RNA-seq and GISTIC2 copy number data for Luminal A Pam50 breast tumors.
Table S5. TCGA RNA-seq and GISTIC2 copy number data for Luminal B Pam50 breast tumors.
Table S6. Potency of the CK1δ/CK1ε inhibitor SR-3029 for human breast cancer subtypes.
Table S7. Non-toxic long term daily treatment with SR-3029.
Table S8. TCGA RNA-seq and GISTIC2 copy number data for renal papillary (KIRP) tumors.
Table S9. TCGA RNA-seq and GISTIC2 copy number data for bladder cancer (BLCA) tumors.
Table S10. Renal papillary cell carcinoma correlation analysis.
Table S11. Bladder cancer correlation analysis.
Table S12. Wnt signaling genes significantly associated with CK1δ expression in renal papillary cell carcinoma.
Table S13. Wnt signaling genes significantly associated with CK1δ expression in bladder cancer.
Table S14. Oligonucleotides used for qPCR.
Table S15. SiRNA sequences.
Table S16. ShRNA sequences.
Acknowledgments
We sincerely thank Weimin Li for technical support, Ms. Pamela Clark-Spruill for secretarial assistance and members of the Duckett, Cleveland and Roush laboratories for input and editing of the manuscript.
Funding: This work was supported in part by NIH Grant CA175094 (W.R.R, J.L.C. and D.R.D.), the NIH Molecular Library Screening Center Network grant U54MH074404 (Chemistry portion, W.R.R.), NCI Comprehensive Cancer Center Support Grant P30-CA076292 awarded to the H. Lee Moffitt Cancer Center & Research Institute, The Rendina Family Foundation (D.R.D.), The Shear Family Foundation (D.R.D and W.R.R), ThinkPink Kids Foundation (J.L.C.), and by funds from the State of Florida to Scripps Florida and the Moffitt Cancer Center & Research Institute.
Footnotes
Author contributions: L.H.R. was the primary author of the manuscript and designed and executed experiments; collected, analyzed, and interpreted data; and wrote the manuscript. M.L. designed and executed experiments; collected data and reviewed the manuscript with contributions from V.Q. C.Y., collected and contributed data. M.F. performed bioinformatics analyses and reviewed the manuscript. M.B., Y.N., W.R.R., J.L.C. W.G., W.C. contributed materials critical to this work. J.C. contributed patient derived models critical to this work. D.R.D. oversaw the study, contributed to experimental design, Interpreted data and co-wrote the manuscript with substantial contribution from J.L.C. and W.R.R.
Competing interests: The authors declare that a provisional patent for CK1δ inhibitors and their use in breast cancer has been filed.
REFERENCES AND NOTES
- 1.Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Richardson AL, Schnitt SJ, Schmitt FC, Tan PH, Tse GM, Badve S, Ellis IO. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12:207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. published online EpubOct 4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. published online EpubJan–Feb . [DOI] [PubMed] [Google Scholar]
- 4.Andre F, Slimane K, Bachelot T, Dunant A, Namer M, Barrelier A, Kabbaj O, Spano JP, Marsiglia H, Rouzier R, Delaloge S, Spielmann M. Breast cancer with synchronous metastases: trends in survival during a 14-year period. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3302–3308. doi: 10.1200/JCO.2004.08.095. published online EpubAug 15 . [DOI] [PubMed] [Google Scholar]
- 5.Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, D’Amico R. Trastuzumab containing regimens for early breast cancer. The Cochrane database of systematic reviews. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–692. doi: 10.1038/nrclinonc.2010.154. published online EpubDec . [DOI] [PubMed] [Google Scholar]
- 7.Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2011;16(Suppl 1):61–70. doi: 10.1634/theoncologist.2011-S1-61. [DOI] [PubMed] [Google Scholar]
- 8.Kaucka M, Plevova K, Pavlova S, Janovska P, Mishra A, Verner J, Prochazkova J, Krejci P, Kotaskova J, Ovesna P, Tichy B, Brychtova Y, Doubek M, Kozubik A, Mayer J, Pospisilova S, Bryja V. The Planar Cell Polarity Pathway Drives Pathogenesis of Chronic Lymphocytic Leukemia by the Regulation of B-Lymphocyte Migration. Cancer Research. 2013;73:1491–1501. doi: 10.1158/0008-5472can-12-1752. published online EpubMar 1 . [DOI] [PubMed] [Google Scholar]
- 9.Brockschmidt C, Hirner H, Huber N, Eismann T, Hillenbrand A, Giamas G, Radunsky B, Ammerpohl O, Bohm B, Henne-Bruns D, Kalthoff H, Leithauser F, Trauzold A, Knippschild U. Anti-apoptotic and growth-stimulatory functions of CK1 delta and epsilon in ductal adenocarcinoma of the pancreas are inhibited by IC261 in vitro and in vivo. Gut. 2008;57:799–806. doi: 10.1136/gut.2007.123695. published online EpubJun . [DOI] [PubMed] [Google Scholar]
- 10.Tsai IC, Woolf M, Neklason DW, Branford WW, Yost HJ, Burt RW, Virshup DM. Disease-associated casein kinase I delta mutation may promote adenomatous polyps formation via a Wnt/beta-catenin independent mechanism. Int J Cancer. 2007;120:1005–1012. doi: 10.1002/ijc.22368. published online EpubMar 1 . [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez N, Yang JZ, Hasselblatt K, Liu SB, Zhou YL, Rauh-Hain JA, Ng SK, Choi PW, Fong WP, Agar NYR, Welch WR, Berkowitz RS, Ng SW. Casein kinase I epsilon interacts with mitochondrial proteins for the growth and survival of human ovarian cancer cells. Embo Molecular Medicine. 2012;4:952–963. doi: 10.1002/emmm.201101094. published online EpubSep . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirner H, Gunes C, Bischof J, Wolff S, Grothey A, Kuhl M, Oswald F, Wegwitz F, Bosl MR, Trauzold A, Henne-Bruns D, Peifer C, Leithauser F, Deppert W, Knippschild U. Impaired CK1 delta activity attenuates SV40-induced cellular transformation in vitro and mouse mammary carcinogenesis in vivo. PloS one. 2012;7:e29709. doi: 10.1371/journal.pone.0029709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheong JK, Nguyen TH, Wang H, Tan P, Voorhoeve PM, Lee SH, Virshup DM. IC261 induces cell cycle arrest and apoptosis of human cancer cells via CK1delta/epsilon and Wnt/beta-catenin independent inhibition of mitotic spindle formation. Oncogene. 2011;30:2558–2569. doi: 10.1038/onc.2010.627. published online EpubJun 2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SY, Dunn IF, Firestein R, Gupta P, Wardwell L, Repich K, Schinzel AC, Wittner B, Silver SJ, Root DE, Boehm JS, Ramaswamy S, Lander ES, Hahn WC. CK1epsilon is required for breast cancers dependent on beta-catenin activity. PloS one. 2010;5:e8979. doi: 10.1371/journal.pone.0008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foldynova-Trantirkova S, Sekyrova P, Tmejova K, Brumovska E, Bernatik O, Blankenfeldt W, Krejci P, Kozubik A, Dolezal T, Trantirek L, Bryja V. Breast cancer-specific mutations in CK1epsilon inhibit Wnt/beta-catenin and activate the Wnt/Rac1/JNK and NFAT pathways to decrease cell adhesion and promote cell migration. Breast Cancer Res. 2010;12:R30. doi: 10.1186/bcr2581. bcr2581 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bibian M, Rahaim RJ, Choi JY, Noguchi Y, Schurer S, Chen W, Nakanishi S, Licht K, Rosenberg LH, Li L, Feng Y, Cameron MD, Duckett DR, Cleveland JL, Roush WR. Development of highly selective casein kinase 1delta/1epsilon (CK1delta/epsilon) inhibitors with potent antiproliferative properties. Bioorganic & medicinal chemistry letters. 2013;23:4374–4380. doi: 10.1016/j.bmcl.2013.05.075. published online EpubAug 1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finak G, Sadekova S, Pepin F, Hallett M, Meterissian S, Halwani F, Khetani K, Souleimanova M, Zabolotny B, Omeroglu A, Park M. Gene expression signatures of morphologically normal breast tissue identify basal-like tumors. Breast Cancer Res. 2006;8:R58. doi: 10.1186/bcr1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS. Supervised risk predictor of breast cancer based on intrinsic subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. published online EpubMar 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Claerhout S, Prat A, Dobrolecki LE, Petrovic I, Lai Q, Landis MD, Wiechmann L, Schiff R, Giuliano M, Wong H, Fuqua SW, Contreras A, Gutierrez C, Huang J, Mao S, Pavlick AC, Froehlich AM, Wu MF, Tsimelzon A, Hilsenbeck SG, Chen ES, Zuloaga P, Shaw CA, Rimawi MF, Perou CM, Mills GB, Chang JC, Lewis MT. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer research. 2013;73:4885–4897. doi: 10.1158/0008-5472.CAN-12-4081. published online EpubAug 1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. published online EpubApr . [DOI] [PubMed] [Google Scholar]
- 21.Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. published online EpubFeb . [DOI] [PubMed] [Google Scholar]
- 22.Bryja V, Schulte G, Arenas E. Wnt-3a utilizes a novel low dose and rapid pathway that does not require casein kinase 1-mediated phosphorylation of Dvl to activate beta-catenin. Cell Signal. 2007;19:610–616. doi: 10.1016/j.cellsig.2006.08.011. published online EpubMar . [DOI] [PubMed] [Google Scholar]
- 23.Knippschild U, Kruger M, Richter J, Xu P, Garcia-Reyes B, Peifer C, Halekotte J, Bakulev V, Bischof J. The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front Oncol. 2014;4:96. doi: 10.3389/fonc.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wend P, Runke S, Wend K, Anchondo B, Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, Lesniak MS, Wolsky R, Bentolila LA, Grant SG, Elashoff D, Lehr S, Latimer JJ, Bose S, Sattar H, Krum SA, Miranda-Carboni GA. WNT10B/beta-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO molecular medicine. 2013;5:264–279. doi: 10.1002/emmm.201201320. published online EpubFeb . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schade B, Lesurf R, Sanguin-Gendreau V, Bui T, Deblois G, O’Toole SA, Millar EKA, Zardawi SJ, Lopez-Knowles E, Sutherland RL, Giguere V, Kahn M, Hallett M, Muller WJ. beta-Catenin Signaling Is a Critical Event in ErbB2-Mediated Mammary Tumor Progression. Cancer Research. 2013;73:4474–4487. doi: 10.1158/0008-5472.Can-12-3925. published online EpubJul 15 . [DOI] [PubMed] [Google Scholar]
- 26.Moumen M, Chiche A, Decraene C, Petit V, Gandarillas A, Deugnier MA, Glukhova MA, Faraldo MM. Myc is required for beta-catenin-mediated mammary stem cell amplification and tumorigenesis. Mol Cancer. 2013;12:132. doi: 10.1186/1476-4598-12-132. published online EpubOct 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Hussaini H, Subramanyam D, Reedijk M, Sridhar SS. Notch signaling pathway as a therapeutic target in breast cancer. Molecular cancer therapeutics. 2011;10:9–15. doi: 10.1158/1535-7163.MCT-10-0677. published online EpubJan . [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. published online EpubMar 22. [DOI] [PubMed] [Google Scholar]
- 29.Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, Cha SY, Ryu JK, Choi YJ, Kim J, Fearon ER, Weiss SJ. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8:1398–1406. doi: 10.1038/ncb1508. published online EpubDec . [DOI] [PubMed] [Google Scholar]
- 30.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. published online EpubMar 25 . [DOI] [PubMed] [Google Scholar]
- 31.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. published online EpubNov . [DOI] [PubMed] [Google Scholar]
- 32.Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. published online EpubOct . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheong JK, Virshup DM. Casein kinase 1: Complexity in the family. The international journal of biochemistry & cell biology. 2011;43:465–469. doi: 10.1016/j.biocel.2010.12.004. published online EpubApr . [DOI] [PubMed] [Google Scholar]
- 34.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008052. published online EpubMay . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. published online EpubApr 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu WH, Liu ZB, Yang C, Qin W, Shao ZM. Expression of dickkopf-1 and beta-catenin related to the prognosis of breast cancer patients with triple negative phenotype. PLoS One. 2012;7:e37624. doi: 10.1371/journal.pone.0037624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown AM. Wnt signaling in breast cancer: have we come full circle? Breast Cancer Res. 2001;3:351–355. doi: 10.1186/bcr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiederschain D, Wee S, Chen L, Loo A, Yang G, Huang A, Chen Y, Caponigro G, Yao YM, Lengauer C, Sellers WR, Benson JD. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell cycle. 2009;8:498–504. doi: 10.4161/cc.8.3.7701. published online EpubFeb 1. [DOI] [PubMed] [Google Scholar]
- 39.Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLoS One. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12:R41. doi: 10.1186/gb-2011-12-4-r41. gb-2011-12-4-r41 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. CK1δ, but not CK1ε, is overexpressed in human breast cancer and associated with CSNK1D gene amplification.
Fig. S2. CK1δ is overexpressed in human breast cancer specimens.
Fig. S3. Silencing of CK1δ and CK1ε, but not CK1ε alone, impairs growth and survival of MDA-MB-231 breast cancer cells.
Fig. S4. Selective inhibition of FLT3 has no effect on proliferation of MDA-MB-231 breast cancer cells.
Fig. S5. Treatment with SR-3029 impairs growth of triple negative and HER2+ breast tumor models.
Fig. S6. Long-term daily dosing with SR-3029 has no adverse effects on major organ histology.
Fig. S7. Selective silencing of CK1δ is sufficient to impair growth of MDA-MB-231 breast cancer cells.
Fig. S8. Wnt/β-catenin pathway genes are a hallmark of CK1δ-expressing breast cancers.
Fig. S9. Constitutively active β-catenin N90 rescues the growth inhibitory effects of CK1δ inhibition and silencing.
Fig. S10. CK1δ expression in MCF7 breast cancer cells sensitizes them to SR-3029.
Fig. S11. Cyclin-D1 expression is suppressed in TNBC MDA-MB-231-derived tumors following knockdown of CK1δ.
Fig. S12. Inhibition of CK1δ/CK1ε has no effects on intestinal homeostasis.
Fig. S13. Canonical Wnt signaling genes are correlated with CK1δ expression in human renal and bladder cancer.
Table S1. TCGA RNA-seq and GISTIC2 copy number data and correlation analysis for breast cancer (BRCA) tumors.
Table S2. TCGA RNA-seq and GISTIC2 copy number data for Basal-like Pam50 breast tumors.
Table S3. TCGA RNA-seq and GISTIC2 copy number data for HER2 Pam50 positive breast tumors.
Table S4. TCGA RNA-seq and GISTIC2 copy number data for Luminal A Pam50 breast tumors.
Table S5. TCGA RNA-seq and GISTIC2 copy number data for Luminal B Pam50 breast tumors.
Table S6. Potency of the CK1δ/CK1ε inhibitor SR-3029 for human breast cancer subtypes.
Table S7. Non-toxic long term daily treatment with SR-3029.
Table S8. TCGA RNA-seq and GISTIC2 copy number data for renal papillary (KIRP) tumors.
Table S9. TCGA RNA-seq and GISTIC2 copy number data for bladder cancer (BLCA) tumors.
Table S10. Renal papillary cell carcinoma correlation analysis.
Table S11. Bladder cancer correlation analysis.
Table S12. Wnt signaling genes significantly associated with CK1δ expression in renal papillary cell carcinoma.
Table S13. Wnt signaling genes significantly associated with CK1δ expression in bladder cancer.
Table S14. Oligonucleotides used for qPCR.
Table S15. SiRNA sequences.
Table S16. ShRNA sequences.