Abstract
Donor age has become the dominant donor factor used to predict graft failure (GF) after liver transplantation (LT) in HCV recipients.
AIM
To develop and validate a model of Corrected Donor Age (CDA) for HCV LT recipients that transforms the risk of other donor factors into the scale of donor age.
METHODS
We analyzed all first LT recipients with HCV in the UNOS registry from 1/1998–12/2007 (development cohort, n=14,538) and 1/2008–12/2011 (validation cohort, n=7,502) using Cox regression, excluding early GF (<90 days from LT). Accuracy in predicting 1yr GF (death or Re-LT) was assessed with the net reclassification index (NRI).
RESULTS
In the development cohort, controlling for pre-LT recipient factors and geo-temporal trends (UNOS region, LT year), the following donor factors were independent predictors of GF (Hazard Ratio); all p<0.05; donor age (1.02/yr), circulatory death (DCD) (1.31), diabetes (1.23), height<160cm (1.13), AST>120 U/L (1.10), female (0.94), cold ischemia time (CIT) (1.02/hr), donor non-AA : recipient AA (1.65). Transforming these risk factors into the donor age scale yielded the following: DCD=+16yrs, diabetes=+12yrs, height<160cm=+7yrs, AST >120 U/L=+5yrs, female=−4yrs, CIT=+1yr/hr>8hrs and −1yr/hr<8 hrs. There was a large effect of donor-recipient race combinations; +29yrs for donor non-AA : recipient AA but only +5yrs for donor AA : recipient AA, and −2yrs for donor AA : recipient non-AA. In a validation cohort, CDA better classified risk of 1yr GF versus actual age (NRI 4.9%, p=0.009) and versus the donor risk index (9.0%, p<0.001).
CONCLUSIONS
The CDA, compared to actual donor age, provides an intuitive and superior estimation of graft quality for HCV-positive LT recipients since it incorporates additional factors that impact LT GF rates.
Keywords: donor age, liver transplantation, risk score, donor quality
Introduction
Liver transplantation (LT) can be a lifesaving intervention for patients with acute or chronic liver disease. Organ shortage is perhaps the greatest challenge facing the field of organ transplantation today1, prompting a push for aggressive graft utilization practices by the transplant community yet this effort could adversely affect outcome without appropriate donor selection2. Several analyses have identified specific donor characteristics that affect the risk of graft failure (GF), not always reaching consensus3–5. However, there is no controversy about the impact of donor age, considered to be the most important factor related to patient and graft survival. The strong negative impact of older donors on LT outcomes has long been recognized6, 7, with an increasing relative risk of GF associated with each decade of increasing donor age, beginning at 40 years.
When considering hepatitis C (HCV) patients, still the most common indication for LT in United States and worldwide, the evidence regarding the negative impact of donor age in patient and graft survival is overwhelming6–8. Lake et al analyzed the impact of several risk factors on survival outcomes of adult LT recipients, and found that donor age surpassed all other risk factors for poor graft and patient survival in patients with HCV6 prompting restrictive modifications in donor selection based on age9. Nevertheless, during the last decade some investigators have shown favorable early- and middle-term results with elderly donors 10, 11, even in HCV-positive recipients, highlighting that other donor and recipient factors contribute to graft loss risk. Therefore, estimating the risk that other risk factors add to actual donor age in a fast and simple way could facilitate successful donor selection. The aim of this study was to develop and validate a model of Corrected Donor Age (CDA) for HCV-infected LT recipients that transforms the risk of other donor factors into the scale of donor age.
Methods
Study Population
We obtained data on LT recipients, their respective donors and transplant factors from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research files. The development cohort included adults (>=18 years of age) with a primary, secondary or other diagnosis of HCV receiving a primary, single-organ, deceased donor LT between January 1998 and December 2007 with at least 90 days of post-transplant follow-up. Patients receiving a partial or split liver, infected with HIV or having fulminant status were excluded from the analysis.
Statistical Analysis
Donor, recipient and transplant characteristics were described with means (standard deviations [SD]), and medians (interquartile ranges [IQR]) for continuous variables and frequency distributions for categorical variables. Variables missing greater than 20% of responses were excluded from further evaluation. Donor height was evaluated by 10 cm increments and in the final model dichotomized at 160 cm due to a lack of statistical difference in outcomes between 10 cm groupings. Similarly, AST was dichotomized at 120 U/L after evaluating the relationship between outcomes and AST by 40 unit increments.
Cox proportional hazards regression was used to estimate the impact of donor factors on liver GF. Time-to-event was defined as the number of days from LT to the date of retransplant, death or censoring at last follow-up, whichever occurred first. Donor factors evaluated in univariable analysis included anti-CMV serology, HBV core antibody, anti-HCV serology, cause of death (head trauma [reference category], anoxia, cerebrovascular/stroke and other causes including CNS tumor), ethnicity, sex, non-heart beating donor/donation after circulatory death (DCD), antihypertensives and vasodilators within 24 hours of pre-cross clamp, synthetic antidiuretic hormone, cardiac arrest post brain death, allocation type, age, height, weight, body mass index, serum creatinine, total bilirubin, AST, ALT, cold ischemic time, warm ischemic time and history of diabetes, cigarette use, hypertension and cancer. Donor factors with p<0.1 in the univariable analysis were eligible for entry into the multivariable model. The donor model was constructed using stepwise selection with a significance level of 0.05 for model entry and removal.
Recipient and transplant factors evaluated for association with liver graft loss by univariable Cox proportional hazards regression included hepatocellular carcinoma, life support pre-transplant, sex, ethnicity, age, diabetes, previous upper abdominal surgery, dialysis prior week to transplant, height, serum creatinine, total bilirubin, albumin, INR, MELD, region, transplant year and ABO compatibility. Complete data for INR at transplant and MELD were only available after March 1, 2002 and were therefore evaluated from this date forward. While MELD was statistically significant, creatinine was the only MELD component significantly associated with graft loss. Therefore, creatinine was evaluated in the multivariable analysis (and available for all 10 years of the study). To create a donor model adjusted for recipient and transplant factors, the likelihood ratio test was used to compare the full (model with the recipient variable) and reduced (model without the recipient variable) models for factors with p<0.1 in the univariable analysis. Recipient and transplant factors with p<0.05 from the likelihood ratio test were included in the adjusted model. The final model was adjusted for seven recipient factors (age, sex, hepatocellular carcinoma, life support, diabetes, serum creatinine and albumin) and two transplant factors (region and transplant year). The proportional hazards assumption was tested using Schoenfeld residuals. We evaluated potential interactions between donor age and donor characteristics (sex, non-heart beating/DCD, diabetes, height, AST), recipient age and cold ischemia time, between donor and recipient sex and between donor and recipient ethnicity. Interactions with p<0.05 were included in the final model. Significant donor factors were transformed into the scale of donor age12 (using the regression coefficients from the multivariable model) to develop a novel donor risk model of CDA. For example, the regression coefficient for the significant donor factor was divided by the regression coefficient for a 1 year increase in donor age and rounded to the nearest integer. The integer value for each donor component was then summed to calculate the CDA for a given donor.
Model Validation
To validate and compare the CDA model to actual donor age and DRI, we identified an independent validation cohort of HCV patients receiving a liver transplant between 2008 and 2011 from the UNOS/OPTN database. The original selection criteria were applied to the validation dataset. Probability of liver graft failure within one year of transplant was predicted by CDA and compared to actual donor age and DRI13. Overall c-index and net reclassification index (NRI) were calculated to evaluate model discrimination (the ability of the model to correctly classify recipients into events and non-events14) and improvement in model performance (quantify the proportion correctly reclassified regarding risk of graft loss15), respectively. We used a priori 1-year graft loss risk groups (<7.5%, 7.5% to <10%, 10% to <12.5%, 12.5% to <15%, and >=15%) to quantify NRI. Thus, predicted probabilities from the new CDA model reclassifying recipients with events into higher risk groups and recipients without events into lower risk groups indicates correct reclassification compared to the predicted probabilities from actual donor age and DRI. Significance of reclassification was evaluated with a two-sided z test. Statistical analyses were conducted using SAS v9.3 (Cary, NC).
Results
Eligible LT Recipients
Between 1998 and 2007, 21,938 anti-HCV positive persons received a liver transplant and were followed for at least 90 days. LT recipients under 18 years of age (n=141, 0.6%), HIV positive (n=24, 0.1%), with fulminant status (n=66, 0.3%) or receiving a repeat LT (n=1,791, 8.2%), multiple organ transplant (n=856, 3.9%), partial or split liver (n=928, 4.2%), or a liver from a live donor (n=33, 0.2%) were excluded from the analysis. The final development cohort for creating the CDA model included 18,099 recipients. Of these, 14,538 patients in the development cohort had complete data for the variables included in the final adjusted model. Additionally, we identified an independent validation cohort with the same inclusion/exclusion criteria of 7,502 patients transplanted from 2008 and 2011 to assess model performance.
Recipient and donors characteristics
Demographic and clinical characteristics of LT recipients and liver donors are detailed in Table 1 within the development (separately for all eligible recipients and those with complete data for the characteristics included in the final model) and validation cohorts. Demographic characteristics that were statistically (p<0.05), but not necessarily clinically, different between the final development with complete data (n=14,538) and the validation data (n=7502) cohorts are noted. As expected there were some differences between the development and validation cohorts. LT recipients were primarily white and male. Nearly one-third of recipients were diagnosed with hepatocellular carcinoma and another 18% were diabetic. Liver donors were also primarily male with 85% of deaths due to trauma or stroke. African Americans (AA) represented 13% of donors. Few donors were anti-HCV positive (5%) or donated after circulatory death (3%). Recipient and donor characteristics were similar for both cohorts.
Table 1.
Recipient and Donor Characteristics in the Development and Validation Cohorts
| Characteristic | Development | Validation | |
|---|---|---|---|
|
| |||
| n=18,099 | n=14,538 | n=7,502 | |
|
| |||
|
Recipient
| |||
| Age at LT* [mean (SD)] | 52 (8) | 52 (8) | 55 (6) |
| Male (%) | 75 | 75 | 76 |
| Race* (%) | |||
| Caucasian | 74 | 74 | 70* |
| African American (AA) | 9 | 9 | 12 |
| Other | 18 | 18 | 18 |
| HCC* (%) | 28 | 29 | 37* |
| Life support at LT* (%) | 3 | 3 | 4 |
| Diabetes* (%) | 18 | 18 | 21 |
| Lab MELD at LT* [median (IQR)] | 17 (12–23) | 17 (12–23) | 18 (12–26) |
| Serum creatinine at LT* [median (IQR)] | 1.0 (0.8–1.4) | 1.0 (0.8–1.3) | 1.0 (0.8–1.5) |
| Albumin at LT* [mean (SD)] | 2.8 (0.7) | 2.8 (0.7) | 3.0 (0.7) |
|
| |||
|
Donor
| |||
| Age at donation* [mean (SD)] | 40 (17) | 40 (17) | 41 (15) |
| Male* (%) | 62 | 62 | 60 |
| Race* (%) | |||
| Caucasian | 71 | 72 | 66 |
| African American (AA) | 13 | 13 | 18 |
| Other | 15 | 15 | 16 |
| Cause of death* (%) | |||
| Anoxia | 12 | 12 | 24 |
| Stroke | 43 | 43 | 40 |
| Trauma | 42 | 42 | 34 |
| Other | 3 | 3 | 3 |
| Anti-CMV positive (%) | 65 | 65 | 65 |
| Anti-HCV positive* (%) | 5 | 5 | 7 |
| Anti-HBc positive* (%) | 6 | 6 | 7 |
| DCD* (%) | 3 | 3 | 6 |
| Antihypertensives within 24 hrs pre-cross clamp* (%) | 18 | 18 | 23 |
| History cigarette smoking, @ >20 pack-yrs* (%) | 37 | 38 | 28 |
| Diabetes* (%) | 7 | 8 | 11 |
| History of hypertension* (%) | 28 | 29 | 35 |
| History of cancer (%) | 3 | 3 | 3 |
| Synthetic antidiuretic hormone* (%) | 31 | 31 | 16 |
| Cardiac arrest post brain death* (%) | 4 | 4 | 7 |
| Share allocation* (%) | |||
| Local | 75 | 74 | 77 |
| Regional | 19 | 20 | 19 |
| National | 6 | 6 | 4 |
| Donor (D) : Recipient (R) race combination* (%) | |||
| D non-AA : R non-AA | 80 | 80 | 73 |
| D AA : R AA | 2 | 2 | 3 |
| D AA : R non-AA | 12 | 12 | 15 |
| D non-AA : R AA | 7 | 7 | 9 |
| Cold ischemic time, hours* [mean (SD)] | 7.6 (2.8) | 7.6 (2.8) | 6.6 (2.5) |
| Height, cm* [mean (SD)] | 172 (11) | 172 (10) | 172 (10) |
| AST* [median (IQR)] | 44 (27–77) | 44 (27–77) | 45 (27–84) |
Recipient and donor characteristics in development cohort (without (n=18,099) and with (n=14,538) complete data on characteristics included in the final adjusted model) and validation cohort (n=7,502).
p<0.05 when testing for differences between demographic characteristic of development and validation cohorts.
Donor factors associated with graft survival
Univariable and multivariable factors associated with graft survival in the development cohort are shown in Table 2. After controlling for pre-LT recipient factors and geo-temporal trends (UNOS region, LT year), the following donor factors were independent predictors of GF (Hazard Ratio); donor age (1.02/yr), DCD (1.31), diabetes (1.23), height<160cm (1.13), AST>120 U/L (1.10), female (0.94), cold ischemia time (CIT) (1.02/hr), and donor non-AA : recipient AA (1.65) (Table 2).
Table 2.
Univariable and multivariable Cox proportional hazards ratios (HR) and 95% confidence intervals (95% CI) for risk of graft loss within the development cohort.
| Donor characteristic | Univariable | Multivariable* | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | p | HR (95% CI) | p | |
| Age at donation | 1.02 (1.02–1.02) | <0.001 | 1.02 (1.02–1.02) | <0.001 |
| Female | 1.11 (1.05–1.17) | <0.001 | 0.94 (0.88–1.00) | 0.04 |
| Cause of death | ||||
| Anoxia | 1.19 (1.08–1.30) | <0.001 | -- | -- |
| Stroke | 1.48 (1.40–1.57) | <0.001 | -- | -- |
| Trauma | 1.00 | -- | -- | |
| Other | 1.36 (1.17–1.59) | <0.001 | -- | -- |
| Anti-CMV positive | 1.12 (1.06–1.18) | <0.001 | -- | -- |
| Anti-HCV positive | 1.09 (0.97–1.23) | 0.14 | -- | -- |
| Anti-HBc positive | 1.14 (1.02–1.26) | 0.02 | -- | -- |
| DCD | 1.36 (1.16–1.59) | <0.001 | 1.31 (1.10–1.56) | 0.002 |
| Antihypertensives within 24 hrs pre-cross clamp | 1.09 (1.02–1.16) | 0.02 | -- | -- |
| History cigarette smoking, @ >20 pack-yrs | 1.09 (1.04–1.15) | 0.001 | -- | -- |
| Diabetes | 1.62 (1.48–1.76) | <0.001 | 1.23 (1.11–1.36) | <0.001 |
| History of hypertension | 1.40 (1.33–1.48) | <0.001 | -- | -- |
| History of cancer | 1.41 (1.24–1.62) | <0.001 | -- | -- |
| Synthetic antidiuretic hormone | 0.89 (0.84–0.94) | <0.001 | -- | -- |
| Cardiac arrest post brain death | 1.04 (0.91–1.20) | 0.53 | -- | -- |
| Share allocation | ||||
| Local | 1 | -- | -- | |
| Regional | 1.14 (1.07–1.22) | <0.001 | -- | -- |
| National | 1.37 (1.23–1.52) | <0.001 | -- | -- |
| Donor : Recipient race combination | ||||
| D non-AA : R non-AA** | 1.00 | 1.00 | ||
| D AA : R AA | 1.13 (0.93–1.37) | 0.23 | 1.09 (0.88–1.36) | 0.41 |
| D AA : R non-AA | 0.97 (0.89–1.06) | 0.48 | 0.97 (0.88–1.07) | 0.56 |
| D non-AA : R AA | 1.76 (1.62–1.93) | <0.001 | 1.65 (1.50–1.83) | <0.001 |
| Cold ischemic time (per hour increase from 8 hrs) | 1.02 (1.01–1.03) | 0.001 | 1.02 (1.01–1.03) | 0.002 |
| Height >=160 cm (vs 100–159 cm) | 1.17 (1.08–1.26) | <0.001 | 1.13 (1.03–1.25) | 0.01 |
| AST >=120 (vs <120) | 0.99 (0.92–1.07) | 0.85 | 1.10 (1.01–1.20) | 0.04 |
Adjusted for recipient (age at LT, sex, HCC, life support, diabetes and laboratory values at LT (creatinine and albumin)) and transplant (region and transplant year) factors. D=donor R=Recipient.
Reference= donor non-AA; recipient non-AA
CDA model development
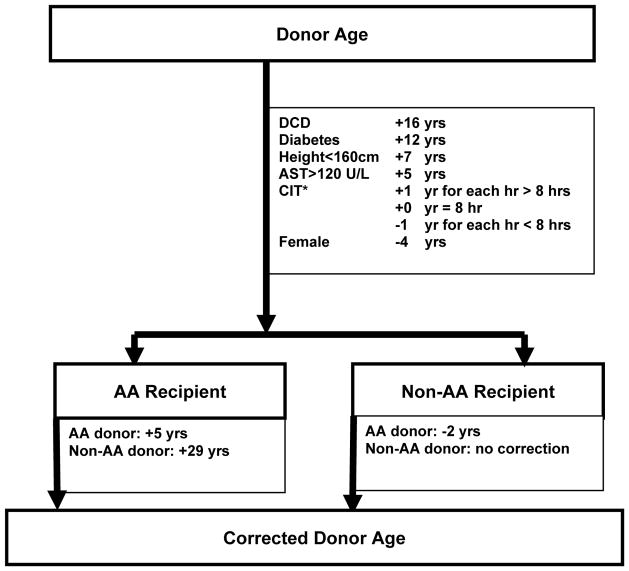
Transforming significant donor risk factors into the donor age scale yielded the following: DCD = +16yrs, diabetes = +12yrs, height <160 cm = +7yrs, AST >120 U/L = +5yrs, female = −4yrs, CIT = +1yr/hr >8hrs and -1yr/hr <8 hrs (Figure 1). The presence of each high risk feature would add the aforementioned amount of years to actual donor age, therefore allowing calculation of the CDA. Donor-recipient race combination mismatch had the largest effect; the highest risk association was represented by the combination of a non-AA donor with an AA recipient, adding 29 years to actual donor age. However, matching an AA donor with an AA recipient only added 5 years to actual donor age, and the AA donor : non-AA recipient combination subtracted 2 years to actual donor age. For the remaining donor characteristics analyzed, DCD, diabetes, height <160cm, AST>120 U/L and CIT >8 hours represented additional calculated donor years in decreasing order. CIT <8 hours and female sex subtracted 1 and 4 years respectively to actual donor age. No interactions between donor age and other donor factors were identified (p>0.05). Examples of combinations of actual donor age matched to donor and recipient race, and to the remaining analyzed donor characteristics are shown in Table 3. These examples reflect the strong negative influence of a non-AA donor matched to an AA recipient. For example, an AA recipient receiving a 40-year old graft from a non-AA donor would be predicted to have the same risk of graft loss as if receiving an 69 year old donor graft.
Figure 1.
Age equivalent calculations
Note: *CIT reference time was 8 hours.
Table 3.
Case examples
| Donor Factor | Ref Non-AA Recip |
Case 1 AA Recip |
Case 2 Non-AA Recip |
Case 3 AA Recip |
Case 4 Non-AA Recip |
Case 5 AA Recip |
Case 6 Non-AA Recip |
|---|---|---|---|---|---|---|---|
| Actual Age (yrs) | 40 | 40 | |||||
| Race Donor | non-AA | non-AA | AA | non-AA | AA | non-AA | AA |
| Cardiac Death (DCD) | No | No | Yes | Yes | No | ||
| Diabetes | No | No | Yes | ||||
| Height <160 (cm) | No | No | |||||
| AST >120 (U/L) | No | No | |||||
| Sex | Male | Male | |||||
| CIT (hrs) | 8 | 8 | |||||
| Corrected Age (yrs) | 40 | 69 | 38 | 85 | 54 | 81 | 50 |
| 1yr Graft Survival (%) | 92 | 86 | 92 | 82 | 89 | 83 | 90 |
Note: AA: African-American.
CDA model validation
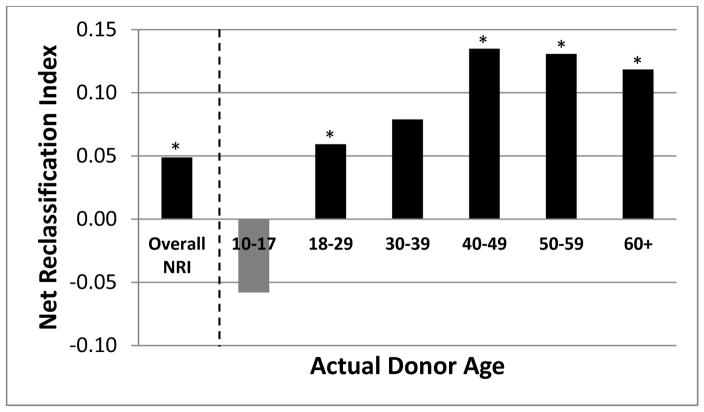
Within the validation cohort, the c-index for CDA was 0.60 and actual donor age was 0.59. Compared to actual donor age, CDA better predicted risk of 1-year GF, with 4.9% of patients correctly reclassified by CDA compared to donor age alone (NRI p=0.009). When actual donor age was stratified in decades (Figure 2), CDA risk reclassification further improved when considering donors >40 years old, achieving a correct reclassification of 12.0–13.5% of patients. Table 4 that displays the number of events, non-events and proportion of patient with events for each of the risk categories (<7.5%, 7.5 to <10%, 10 to <15%, >15%) for 1 year predicted graft failure by actual and corrected donor age. Next we examined the DRI in our validation cohort. The c-index for DRI was 0.58. When we compared 1-year predicted graft failure risk using CDA and DRI3, the NRI was 9.0% (p<0.001). That is, compared to DRI, CDA correctly classified the risk in 9.0% more of the patients.
Figure 2.
Improved risk reclassification with CDA by donor age category
Note: * p<0.05
Table 4.
One-year risks of graft failure as predicted by models with donor age versus corrected donor age (CDA).
| 1-Year Risk of Graft Failure with Actual Donor Age | 1-Year Risk of Graft Failure with CDA
|
Total | ||||
|---|---|---|---|---|---|---|
| <7.5% | 7.5% to <10% | 10% to <12.5% | 12.5% to <15% | ≥15% | ||
|
| ||||||
| <7.5% | ||||||
| Patients, n | 2536 | 361 | 94 | 13 | 2 | 3006 |
| Events, n | 353 | 71 | 17 | 2 | 0 | 443 |
| Nonevents, n | 2183 | 290 | 77 | 11 | 2 | 2563 |
| Proportion of events | 0.14 | 0.20 | 0.18 | 0.15 | 0.00 | 0.15 |
|
| ||||||
| 7.5% to <10% | ||||||
| Patients, n | 490 | 1448 | 262 | 110 | 40 | 2350 |
| Events, n | 76 | 263 | 68 | 30 | 13 | 450 |
| Nonevents, n | 414 | 1185 | 194 | 80 | 27 | 1900 |
| Proportion of events | 0.16 | 0.18 | 0.26 | 0.27 | 0.33 | 0.19 |
|
| ||||||
| 10% to <12.5% | ||||||
| Patients, n | 0 | 742 | 649 | 147 | 119 | 1657 |
| Events, n | 0 | 166 | 176 | 55 | 46 | 443 |
| Nonevents, n | 0 | 576 | 473 | 92 | 73 | 1214 |
| Proportion of events | 0.00 | 0.22 | 0.27 | 0.37 | 0.39 | 0.27 |
|
| ||||||
| 12.5% to <15% | ||||||
| Patients, n | 0 | 8 | 231 | 104 | 60 | 403 |
| Events, n | 0 | 2 | 71 | 35 | 28 | 136 |
| Nonevents, n | 0 | 6 | 160 | 69 | 32 | 267 |
| Proportion of events | 0.00 | 0.25 | 0.31 | 0.34 | 0.47 | 0.34 |
|
| ||||||
| ≥15% | ||||||
| Patients, n | 0 | 0 | 12 | 48 | 26 | 86 |
| Events, n | 0 | 0 | 6 | 15 | 11 | 32 |
| Nonevents, n | 0 | 0 | 6 | 33 | 15 | 54 |
| Proportion of events | 0.00 | 0.00 | 0.50 | 0.31 | 0.42 | 0.37 |
|
| ||||||
| Total | ||||||
| Patients, n | 3026 | 2559 | 1248 | 422 | 247 | 7502 |
| Events, n | 429 | 502 | 338 | 137 | 98 | 1504 |
| Nonevents, n | 2597 | 2057 | 910 | 285 | 149 | 5998 |
| Proportion of events | 0.14 | 0.20 | 0.27 | 0.32 | 0.40 | 0.20 |
Discussion
Donor age has been recognized as the most influential risk factor for both liver transplant graft and patient survival16. In the initial published paper by Lake et al, donor age surpassed all other risk factors for poor graft and patient survival in a particular subset of patients, HCV-positive recipients, and the effect was already evident in the first year post LT when analyzing donors over the age of 40 years6.
There is an ongoing disparity between the demand and supply of lifesaving liver grafts. Of the 15,116 candidates waiting for LT in the US in 2013, 19.7% died or were removed as too ill for transplant17. With an aging general population and high waitlist death rate, the use of older liver donors has risen17, 18. Yet, the use of older donors in HCV-positive patients has been associated with decreased graft and patient survival mainly due to universal viral infection recurrence, faster fibrosis progression and finally decompensated graft cirrhosis 6, 7, 19–23. However, favorable results with older donors have been reported, even with ≥80 year old donors, especially if careful donor evaluation and donor-to-recipient matching are undertaken11. These variable results reflect a simple fact; donors are heterogeneous. Among individuals of the same age, general health status, comorbidities and physiologic reserve vary markedly16. Recognizing this heterogeneity among donors and the singular importance of donor age, we developed and validated a simple metric – the CDA -- that captures the risk of GF.
In our analysis, eight donor characteristics were found to be significantly and independently associated with GF. Some of the included risk factors such as DCD, donor’s height, sex and CIT have also been described in other donor scoring systems such as the Donor Risk Index (DRI)3 and Liver Donor Risk Index (L-DRI)24. Our study confirms prior findings that donor history of diabetes is a strong independent risk factor for GF in HCV-positive recipients25. We show that donor history of diabetes added 12 years to CDA. Mismatched donor and recipient race has long been recognized as a factor influencing LT outcome in HCV-infected recipients. Recently, in another donor risk index (the AADRI-C) developed for AA recipients with HCV26, AA donor : AA recipient match attenuated the negative effect of increasing donor age on graft survival; therefore allowing the use of older donors. We analyzed the influence of donor-recipient race combination mismatch and found that the highest risk association was represented by a non-AA donor for an AA recipient, adding 29 years to actual donor age. Unlike previous findings reported in the AADRI-C, donor AA for an AA recipient was associated with higher risk of graft loss (represented by additional 5 years to actual donor age). Even though serum aminotransferase levels have not been found to be significant in several scoring models3–5, in a recent paper by Ghinolfi that analyzed the use of octogenarian donors for liver transplant the combination of donor age and AST were found to be predictive of GF11 and in our analysis, AST>120 U/L accounted for 5 additional years to actual donor age.
Although donor age is the dominant characteristic of liver graft quality in HCV-positive recipients, other characteristics clearly add to predictive models for graft survival. The advantage of the CDA over other models using graft quality characteristics, such as the DRI3 or AADRI-C26, is the simplicity of translating the risk associated with the additional donor characteristics into (more or less) years of donor age. The complexity of the DRI and AADRI-C can limit their use. Donor age then becomes the default for quick assessments of graft quality. Compared to age alone, our CDA model had superior classification of GF, correctly reclassifying 1-year risk of GF in 4.9% (p=0.009) of patients in the entire cohort. This improvement in risk classification was even more apparent when considering donors >40 years old, achieving correct reclassification of 12.0–13.5% (p<0.05) compared to donor age alone. In this HCV validation cohort, CDA also had improved risk classification for 1 year risk of GF when compared to the DRI with an NRI of 9.0% (p<0.001).
Several deceased donor scoring systems have been developed with the aim of helping clinicians assess the impact of different donor features on the outcome of LT. The development of the DRI and the L-DRI were a major advance since it permitted a formal assessment of the risks posed by a particular allograft and also allowed a standardized assessment of transplant practices3, 24. However, its use has not been widely adopted. In a survey conducted by Mataya et al that evaluated the role of the DRI in clinical practice, they found that even though the majority of responding physicians (62%) stated they were very familiar with the DRI, only a small minority (17%) discussed the concept DRI with wait list candidates, and only 7% disclosed a specific liver DRI number27. One of the concerns identified in this survey was that DRI was considered too complicated for patients to grasp. The CDA model has the benefit of being easy to calculate and simple to explain to potential recipients, making it a useful tool to promote shared decision making. Another asset of the CDA is its focus on HCV infected potential liver transplant recipients. In our HCV cohort, the HCV focused CDA model had improved risk classification compared to the more general DRI model. Additionally, the concept of the CDA could be extended to decision making when considering donor and recipient factors as has been done using the D-MELD model4, 28, 29 that uses the simple product of Donor Age and Recipient MELD score to identify high risk combinations.
Chronic HCV infection is the leading cause of end-stage liver disease and indication for liver transplantation in the Western world4, 30. Several studies have demonstrated worse survival outcomes in HCV-positive compared to HCV-negative LT recipients6, 25; therefore justifying the need for development of specific donor scoring models for this subset of patients such as our CDA. With the availability of novel HCV antiviral drugs, it is unclear whether HCV-infected patients undergoing treatment will have transplant outcomes similar to non-HCV-infected patients, rendering HCV-specific scores less important31. However, low and middle income countries, where more than 80% of chronically infected HCV patients reside, are still struggling for access to direct acting antivirals. Even though price reduction strategies for some of these new drugs are being announced in several low income countries, middle income countries are being excluded from these policies. No lower priced or generic medications will be available in the near future for millions of patients living in these areas, that is to say widespread affordability is still not guaranteed26, 32. For example, in South America where more than 2,500 liver transplantations are performed yearly, three of the top four countries with the highest liver transplant rates (Argentina with 10.4, Uruguay with 5.5 and Colombia with 5.1 transplants per million people per year)33, only first wave HCV protease inhibitors are currently available in combination with peg interferon and ribavirin. Without a clear strategy in place to obtain governmental coverage for DDA HCV medications 34, access will unlikely improve in the near future. In these resource-constrained regions, prioritization of transplant recipients for treatment is necessary, and prediction scores such as CDA help to identify patients at highest risk of graft loss for whom early HCV treatment should be considered.
In summary, we have developed and validated a Corrected Donor Age score that incorporates the key donor factors associated with graft survival and transformed them into a single scale of donor age, the most relevant feature associated with risk of graft loss, thereby yielding an intuitive and superior estimation of 1-year graft quality for HCV LT recipients.
Acknowledgments
Financial support: This work was funded in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK076565) and from Agency for Healthcare Research and Quality (DK076565) to SWB.
List of abbreviations in order of appearance
- GF
graft failure
- LT
liver transplantation
- HCV
Hepatitis C
- CDA
Corrected Donor Age
- UNOS
United Network for Organ Sharing
- Re-LT
repeat liver transplantation
- NRI
net reclassification index
- AA
African American
- non-AA
non-African American
- DCD
donation after circulatory death
- AST
aspartate aminotransferase
- CIT
cold ischemia time
- DRI
donor risk index
- L-DRI
liver donor risk index
Footnotes
Conflict of interest: The authors have no conflicts to disclose.
References
- 1.Saidi RF, Hejazii Kenari SK. Challenges of organ shortage for transplantation: solutions and opportunities. Int J Organ Transplant Med. 2014;5:87–96. [PMC free article] [PubMed] [Google Scholar]
- 2.Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015 doi: 10.1002/lt.24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 4.Halldorson JB, Bakthavatsalam R, Fix O, et al. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318–26. doi: 10.1111/j.1600-6143.2008.02491.x. [DOI] [PubMed] [Google Scholar]
- 5.Braat AE, Blok JJ, Putter H, et al. The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789–96. doi: 10.1111/j.1600-6143.2012.04195.x. [DOI] [PubMed] [Google Scholar]
- 6.Lake JR, Shorr JS, Steffen BJ, et al. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am J Transplant. 2005;5:549–57. doi: 10.1111/j.1600-6143.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 7.Machicao VI, Bonatti H, Krishna M, et al. Donor age affects fibrosis progression and graft survival after liver transplantation for hepatitis C. Transplantation. 2004;77:84–92. doi: 10.1097/01.TP.0000095896.07048.BB. [DOI] [PubMed] [Google Scholar]
- 8.Uemura T, Nikkel LE, Hollenbeak CS, et al. How can we utilize livers from advanced aged donors for liver transplantation for hepatitis C? Transpl Int. 2012;25:671–9. doi: 10.1111/j.1432-2277.2012.01474.x. [DOI] [PubMed] [Google Scholar]
- 9.Flemming JA, Vagefi PA, Freise CE, et al. Restricting liver transplant recipients to younger donors does not increase the wait-list time or the dropout rate: the hepatitis C experience. Liver Transpl. 2014;20:1202–10. doi: 10.1002/lt.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cescon M, Grazi GL, Cucchetti A, et al. Improving the outcome of liver transplantation with very old donors with updated selection and management criteria. Liver Transpl. 2008;14:672–9. doi: 10.1002/lt.21433. [DOI] [PubMed] [Google Scholar]
- 11.Ghinolfi D, Marti J, De Simone P, et al. Use of octogenarian donors for liver transplantation: a survival analysis. Am J Transplant. 2014;14:2062–71. doi: 10.1111/ajt.12843. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–60. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 13.Feng SS. New-concept chemotherapy by nanoparticles of biodegradable polymers: where are we now? Nanomedicine (Lond) 2006;1:297–309. doi: 10.2217/17435889.1.3.297. [DOI] [PubMed] [Google Scholar]
- 14.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 16.Freeman RB. Deceased donor risk factors influencing liver transplant outcome. Transpl Int. 2013;26:463–70. doi: 10.1111/tri.12071. [DOI] [PubMed] [Google Scholar]
- 17.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2013 Annual Data Report: Liver. Am J Transplant. 2015;15(Suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 18.Kim WR, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant. 2014;14(Suppl 1):69–96. doi: 10.1111/ajt.12581. [DOI] [PubMed] [Google Scholar]
- 19.Berenguer M, Prieto M, San Juan F, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;36:202–10. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 20.Wali M, Harrison RF, Gow PJ, et al. Advancing donor liver age and rapid fibrosis progression following transplantation for hepatitis C. Gut. 2002;51:248–52. doi: 10.1136/gut.51.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso O, Loinaz C, Moreno E, et al. Advanced donor age increases the risk of severe recurrent hepatitis C after liver transplantation. Transpl Int. 2005;18:902–7. doi: 10.1111/j.1432-2277.2005.00114.x. [DOI] [PubMed] [Google Scholar]
- 22.Rayhill SC, Wu YM, Katz DA, et al. Older donor livers show early severe histological activity, fibrosis, and graft failure after liver transplantation for hepatitis C. Transplantation. 2007;84:331–9. doi: 10.1097/01.tp.0000270313.31328.63. [DOI] [PubMed] [Google Scholar]
- 23.Mutimer DJ, Gunson B, Chen J, et al. Impact of donor age and year of transplantation on graft and patient survival following liver transplantation for hepatitis C virus. Transplantation. 2006;81:7–14. doi: 10.1097/01.tp.0000188619.30677.84. [DOI] [PubMed] [Google Scholar]
- 24.Akkina SK, Asrani SK, Peng Y, et al. Development of organ-specific donor risk indices. Liver Transpl. 2012;18:395–404. doi: 10.1002/lt.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Ahmed A, Kamal A. Donor diabetes mellitus is an independent risk factor for graft loss in HCV positive but not HCV negative liver transplant recipients. Dig Dis Sci. 2013;58:574–8. doi: 10.1007/s10620-012-2345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shores NJ, Dodge JL, Feng S, et al. Donor Risk Index for African American liver transplant recipients with hepatitis C virus. Hepatology. 2013;58:1263–9. doi: 10.1002/hep.26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mataya L, Aronsohn A, Thistlethwaite JR, Jr, et al. Decision making in liver transplantation--limited application of the liver donor risk index. Liver Transpl. 2014;20:831–7. doi: 10.1002/lt.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avolio AW, Cillo U, Salizzoni M, et al. Balancing donor and recipient risk factors in liver transplantation: the value of D-MELD with particular reference to HCV recipients. Am J Transplant. 2011;11:2724–36. doi: 10.1111/j.1600-6143.2011.03732.x. [DOI] [PubMed] [Google Scholar]
- 29.Halldorson JB, RL, Bhattacharya R, et al. D-MELD risk capping improves post-transplant and overall mortality under markov microsimulation. World J Transplant. 2014;4:206–15. doi: 10.5500/wjt.v4.i3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Hill A, Cooke G. Medicine. Hepatitis C can be cured globally, but at what cost? Science. 2014;345:141–2. doi: 10.1126/science.1257737. [DOI] [PubMed] [Google Scholar]
- 32.Graham CS, Swan T. A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Res. 2015 doi: 10.1016/j.antiviral.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Salvalaggio PR, Caicedo JC, de Albuquerque LC, et al. Liver transplantation in Latin America: the state-of-the-art and future trends. Transplantation. 2014;98:241–6. doi: 10.1097/TP.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 34.Botero RC, Tagle M. New therapies for hepatitis C Latin American perspectives. Clinical Liver Disease. 2015;5:8–10. doi: 10.1002/cld.438. [DOI] [PMC free article] [PubMed] [Google Scholar]