Abstract
Background
Obese and overweight women are more likely to deliver a large infant or an infant with increased adiposity, however the underlying mechanisms are not well established. We tested the hypothesis that placental capacity to transport fatty acid is increased in overweight/obese women.
Methods
Fifty-seven pregnant women with body mass index (BMI) ranging from 18.4 to 54.3 kg/m2 and with uncomplicated term pregnancies were recruited for collection of blood samples and placental tissue. Maternal and fetal levels of non-esterified fatty acids (NEFAs) were measured in plasma. The expression and localization of CD36/fatty acid translocase (FAT), fatty acid transport protein (FATP)2, and FATP4 was determined in fixed placental tissue and in isolated syncytiotrophoblast plasma membranes from normal and high BMI mothers.
Results
Maternal and fetal plasma NEFA levels did not correlate (n=42). FATP2 and FATP4 expressions were approximately four-fold higher in the basal plasma membrane (BPM) compared to the microvillous membrane (P<0.001; n=7) per unit membrane protein. BPM expression of FATP2 correlated with maternal BMI (P<0.01; n=30); there was no association between CD36/FAT or FATP4 expression and maternal BMI.
Conclusion
The polarization of FATPs to the BPM will facilitate fatty acid transfer across the placenta. In overweight/obese pregnancies, the increased FATP2 expression could contribute to increased fatty acid delivery to the fetus and while we have no direct data we speculate that this could lead accelerated fetal growth or increased fat deposition.
Keywords: Placenta, Pregnancy, FATP, CD36/FAT, fatty acid transport
Introduction
Large numbers of women in developed countries like the USA are overweight or obese when they become pregnant [1, 2], and therefore they are more likely to deliver a large for gestational age infant [3, 4] or an infant with increased adiposity [5]. Large size at birth is associated with perinatal complications [6]. Furthermore, children of obese mothers are 2–3 times more likely to become obese themselves, an effect largely independent of birth weight [7]. Although the adverse short- and long-term consequences of maternal obesity on her offspring are well established [6], the underlying mechanisms are largely unknown.
Increased fetal lipid availability may contribute to fetal overgrowth and/or increased fat accumulation in fetuses of obese mothers. First, circulating levels of lipids [8, 9] are elevated in obese pregnant women as compared to normal weight pregnant women, which could enhance fatty acid transfer across the placenta. Second, some indirect evidence suggests that maternal obesity may increase placental capacity to transport lipids. In animal models of maternal obesity, the expression of several placental fatty acid transporters is increased [10, 11], consistent with an elevated capacity to transfer fatty acids. In human placental homogenates, maternal obesity has been shown to decrease the expression of fatty acid transporter protein (FATP)4, while increasing the expression of CD36/fatty acid translocase (FAT) [12]. However, the expression of these membrane-bound proteins in placental homogenates may not be representative of their expression in the syncytiotrophoblasts plasma membranes, the cell-layer considered the primary barrier for placental nutrient transport [13].
Numerous proteins involved in fatty acid transfer are expressed in the placenta, including cytosolic fatty acid binding proteins and membrane-bound FATPs [13]. The FATPs are important for cellular uptake of long-chain fatty acids [14]. The mammalian placenta expresses mRNA for five of the six known FATP isoforms: FATP1-4 and FATP6 [15]. FATP1 and FATP4, as well as CD36/FAT, have been demonstrated in both the basal plasma membrane (BPM) and the microvillous membrane (MVM) of the syncytiotrophoblast of human placenta [12, 16, 17].
Regulation of placental expression of FATPs has been studied in cultured human primary trophoblasts. These studies have identified the peroxisome proliferator-activated receptor (PPAR)γ /RXR signaling pathway as a differential regulator of FATP2 and FATP4 mRNA expression [18]. Other factors affecting trophoblast fatty acid transporter expression are interleukin (IL)-6 which reduces FATP4 mRNA expression [19] and leptin which is a positive regulator of CD36/FAT [20]. We have recently shown maternal circulating levels of leptin, but not IL-6, are increased in a population of high BMI predominantly Hispanic women [21].
In this study we tested the hypothesis that the protein expression of placental fatty acid transporters is up-regulated in response to maternal overweight and obesity. To this effect, we determined the protein expression of FATP2, FATP4, and CD36/FAT in isolated syncytiotrophoblast plasma membranes from term placentas of women with varying BMI.
Patients and Methods
Study subjects
Human placental tissue and blood samples were collected with informed written consent. The protocol was approved by the Institutional Review Board at University of Texas Health Science Center, San Antonio (HSC20100262H), and blood and placental samples obtained at delivery were added to a tissue repository. From this tissue repository, 62 women who delivered at term (≥37 weeks gestation) with no major pregnancy complications were selected and the repository provided coded plasma and placental tissue samples and de-identified relevant medical information. The women were grouped into either “normal BMI” (<25.0 kg/m2, n=27) or “high BMI” (>25.0 kg/m2, n=35) based on their pre-pregnancy or early pregnancy BMI. Only deliveries by Caesarean section prior to the onset of labor were included in this study. Additional samples from normal BMI women were used for localization studies.
Plasma NEFA analysis
Fasting maternal blood samples (n=42) were collected prior to Caesarean section and fetal venous cord blood was obtained immediately after delivery. Plasma concentrations of non-esterified fatty acids (NEFAs) were determined using an enzymatic colorimetric assay (Wako Diagnostics, Richmond, VA).
Immunofluorescence assay
Placenta (n=5) were obtained immediately after delivery and several small villous tissue pieces were rinsed in cold physiological saline before placed in formalin fixation solution. The fixed tissue was embedded in paraffin and was cut into (3 μm) sections. Paraffin was removed and antigen retrieval performed by submerging tissue sections in sodium citrate buffer (10mM sodium citrate, 0.025% Tween 80, pH 6) at 95C for 10 minutes. Sections were blocked in 5% BSA for 30 min before incubation with primary antibodies, polyclonal rabbit anti-human CD36/FAT (Abcam, ab78054), polyclonal rabbit anti-human FATP2 (Abcam, ab85801), polyclonal rabbit anti-human FATP4 (Sigma-Aldrich, HPA007293), and polyclonal sheep anti-human Anti-Placental Alkaline Phosphatase (Abcam, ab64671) for 60 min at RT. In negative controls, the primary antibody was omitted. Sections were then incubated with a mixture of secondary antibodies Alexa Fluor 555 donkey anti-rabbit (Molecular Probes, A31572) and Alexa Fluor 488 donkey anti-sheep (Molecular Probes, A11015) for 60 min RT in a dark chamber. All slides were mounted with ProLong Gold anti-fade reagent with DAPI (Molecular Probes, P36935). Images were viewed at 63x magnification with oil immersion using Zeiss LSM 780 confocal microscope with Zen Black 2.1 software.
Preparation of placental homogenates
Placentas (n=37) were obtained immediately after delivery. After removing decidua basalis and chorionic plate, approximately 100 grams of villous tissue was collected and rinsed in cold physiological saline. The villous tissue was transferred to ice-cold buffer D (250 mM sucrose, 10 mM hepes, pH 7.4) containing protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO) and homogenized on ice. Placental homogenates were frozen in liquid nitrogen and stored at −80 °C until further processing for isolation of plasma membranes.
Isolation of microvillous and basal plasma membranes
Syncytiotrophoblast BPM and MVM were isolated according to a previously described protocol [23, 24] with some modifications. Thawed villous tissue was re-homogenized and centrifuged for 15 min (10,000g, 4°C). The supernatant was collected, the pellet was re-suspended in buffer D, homogenized, and centrifuged (10 min at 10,000g, 4°C). The supernatants from both spins were combined and centrifuged for 30 min (125,000g, 4°C). The resulting pellet was re-suspended in buffer D, MgCl2 was added to a final concentration of 12 mM, and the mixture was stirred on ice for 20 min and subsequently centrifuged for 15 min (2,500g, 4°C). The MVM containing supernatant was centrifuged for 30 min (125,000g, 4°C), while the BPM containing pellet was re-suspended, homogenized, and layered on a sucrose gradient. The BPM was separated by centrifugation for 60 min (144,000g, 4°C). The collected MVM and BPM were centrifuged for 30 min (125,000g, 4°C), resulting pellets re-suspended in buffer D containing protease and phosphatase inhibitors, frozen in liquid nitrogen, and stored at −80 °C.
As a marker for MVM enrichment, alkaline phosphatase activity was measured in isolated MVM-vesicles and placental homogenates. Alkaline phosphatase activity was 15.3±0.8 fold higher in MVM-vesicles compared to placental homogenates (n=30), and did not significantly differ between the groups (normal BMI, 14.9±1.5; high BMI, 15.6±1.1). Enrichment of BPM was verified using protein expression of the iron transporter Ferroportin-1 (Thermo Scientific cat no. PA5-22993). This transporter is almost exclusively expressed in the BPM and mediates unidirectional transport of iron from the mother to the fetus [25–27]. The mean enrichment of Ferroportin-1 expression in BPM compared to placental homogenates was 36 ± 8 (n=35), and did not significantly differ between the groups.
Western blotting
Western blots were performed on 10% SDS-PAGE. After electrophoresis the proteins were transferred to PVDF membranes (overnight, 4°C) and subsequently blocked in 5% milk/TBS-tween (1 hour, room temperature). Primary antibodies were purchased from Abcam (Cambridge, MA): CD36/FAT (ab78054), FATP2 (ab85801), or Santa Cruz Biotechnology (Santa Cruz, CA): FATP4 (sc-101271). After washing the membranes, they were incubated (1 hour, room temperature) with the appropriate peroxidase labeled secondary antibody diluted in 5% milk/TBS-tween. Immunolabelling was made visible with enhanced chemiluminescence detection solution (Thermo Scientific, Rockford, IL). Membranes were stripped (2% SDS, 62.5 mM Tris-HCl, 100 mM β-mercaptoethanol; 35 minutes at 60°C) and re-probed with an antibody targeting β-actin (A2228; Sigma-Aldrich). Densitometry analysis was performed using ImageJ software (1.44p; National Institutes of Health, USA) and target protein expression was adjusted for amount of β-actin.
Data presentation and statistical analysis
Data are presented as mean ± SEM. Statistical analysis was carried out using GraphPad Prism 5 (version 5.04; GraphPad Software, La Jolla, CA), and differences evaluated by t-test or Pearson’s correlation test. P<0.05 was considered significant.
Results
Clinical characteristics
We evaluated samples either serum or placentas from a total of 62 mother/infant pairs with some overlap between the measurements. The majority of women (77%) were of Hispanic descent, reflecting the population in San Antonio, TX. The women with a normal BMI (≤ 25 kg/m2) delivered newborns who were significantly leaner (as estimated by ponderal index; Table 1) than the women with a high BMI (>25 kg/m2). Birth weight, birth length, and placental weight did not significantly differ between the two groups (Table 1).
Table 1.
Clinical Characteristics of Study Participants
| Normal BMI (≤ 25.0 kg/m2) | High BMI (> 25.0 kg/m2) | P-value (t-test) | ||
|---|---|---|---|---|
| Mother | N | 27 | 35 | - |
| BMI (kg/m2) | 22.3 ± 0.4 | 32.7 ± 1.0 | <0.0001 | |
| Age (years) | 29 ± 1.2 | 27 ± 0.8 | 0.06 | |
| Ethnicity (% Hispanic) | 73% | 80% | - | |
| Gestational Age | 39.3±0.2 | 39.2±0.1 | 0.6 | |
| Weight gain | 29.6 ± 2.3 | 24.5 ± 2.1 | ||
| % mothers under/within/above IOM weight gain guideline | 33/33/33 | 12/29/59 | ||
| Newborn | Fetal gender (female/male) | 10/12 | 24/11 | - |
| Birth weight (g) | 3390 ± 61 | 3430 ± 80 | 0.7 | |
| Length (cm) | 51.3 ± 0.3 | 50.3 ± 0.4 | 0.06 | |
| Ponderal Index (gx100/cm3) | 2.5 ± 0.04 | 2.7 ± 0.05 | <0.01 | |
| Placental weight (g) | 679 ± 22 | 729 ± 26 | 0.2 |
Data are presented as mean ± sem.
Circulating plasma NEFAs
There was no significant correlation between maternal and fetal plasma NEFA concentrations (Figure 1; n=42). We have previously reported that maternal plasma NEFAs correlate positively with pre-pregnancy/early pregnancy BMI in a subset of these women [9], a weak correlation was also observed for this larger cohort (r = 0.286, p<0.05). The mean NEFA levels for normal BMI (546 + 37.5 μM) and obese (615 + 35 μM) women were not significantly different.
Figure 1. Circulating maternal and fetal plasma levels of non-esterified fatty acids.
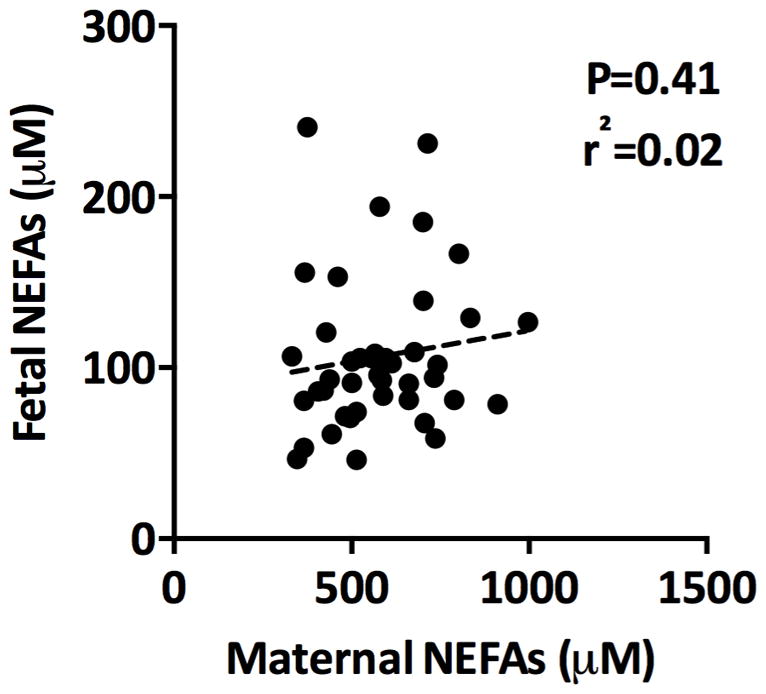
Fasting maternal blood was collected prior to Caesarean section and fetal venous cord blood immediately after delivery. There was no association between maternal and fetal circulating plasma NEFA levels, n=42.
Cellular localization
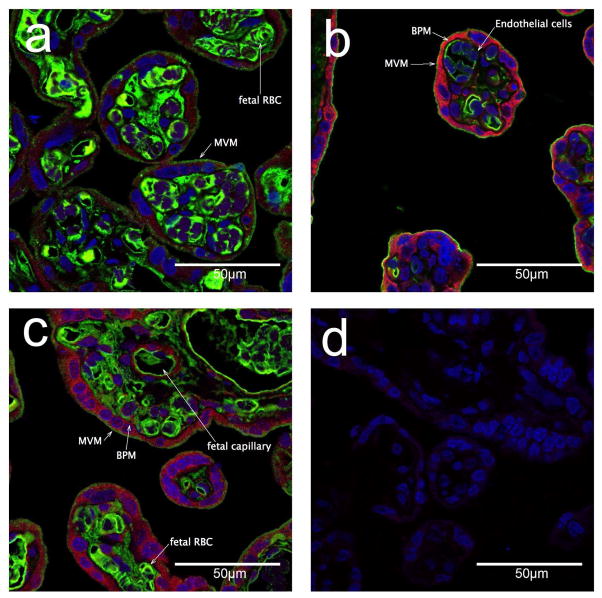
Cellular localization of fatty acid transporters was investigated by immunofluorescence in sections of term placenta form women with normal BMI. CD36/FAT, FATP2, and FATP4 were predominantly expressed in the syncytiotrophoblast layer and the endothelial cells. We found stronger staining in the BPM (Figure 2) for both FATP4 and FATP2 with very little staining of these transporters in the MVM, as indicated by the co-localization with alkaline phosphatase, a strong MVM marker. The localization of CD36 appeared to be punctate in the MVM and co-localized with alkaline phosphatase and generally very low expression. Diffuse staining for lipid transporters FATP2, 4 and CD36/FAT was found in the cytoplasm of the syncytium as well as in the endothelial cells of the fetal capillaries.
Figure 2. Immunofluoresence imaging of fatty acid transporters in human term placenta.
Cellular localization of CD36/FAT, FATP4, and FATP2 (stained in Alexa Fluor 555, red) in term human placenta at 63x magnification a) CD36/FAT (red) and alkaline phosphotase (AP) stained in Alexa Fluor 488 (green) b) FATP4 (red), AP (green) c) FATP2 (red), AP (green). d) control, DAPI nuclear staining (blue) only, primary antibodies omitted. MVM = microvillous membrane, apical surface of the syncytiotrophblast, stained with alkaline phosphatase. BPM = basal plasma membrane of the syncytiotrophoblast , not stained by alkaline phosphatase.
Syncytiotrophoblast plasma membrane expression
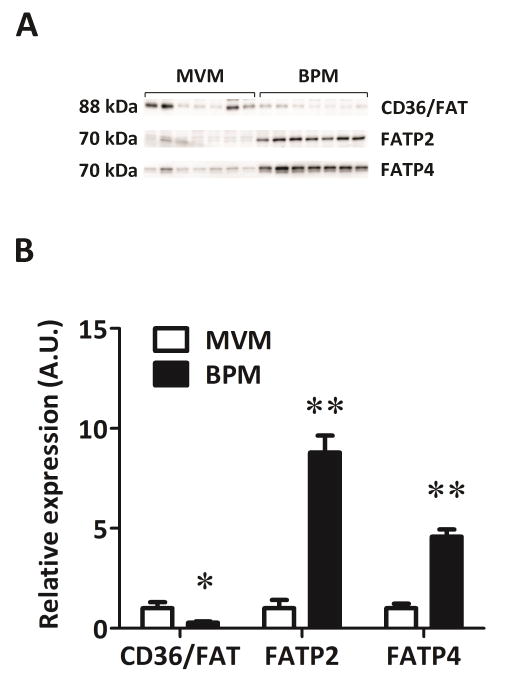
In a subset of syncytiotrophoblast plasma membranes (n=7) isolated from placentas of women with normal BMI (<25 kg/m3), the expression level in MVM compared to BPM was tested. We found CD36/FAT was higher in MVM compared to BPM (P<0.05; Figure 3). The expression of both FATP2 and FATP4 were strongly polarized and markedly higher in the BPM compared to the MVM. FATP2 was nearly 10-fold and FATP4 5-fold higher in the BPM compared to MVM per unit membrane protein (P<0.001; Figure 3).
Figure 3. Expression of fatty acid transporters in isolated syncytiotrophoblast plasma membranes from placenta of normal BMI women.
Western blots for CD36/FAT, FATP2, and FATP4 (A). Densitometric analysis of expression levels (B). The MVM expression was assigned an arbitrary value of 1; *P < 0.05, **P<0.001, paired t-test, n=7.
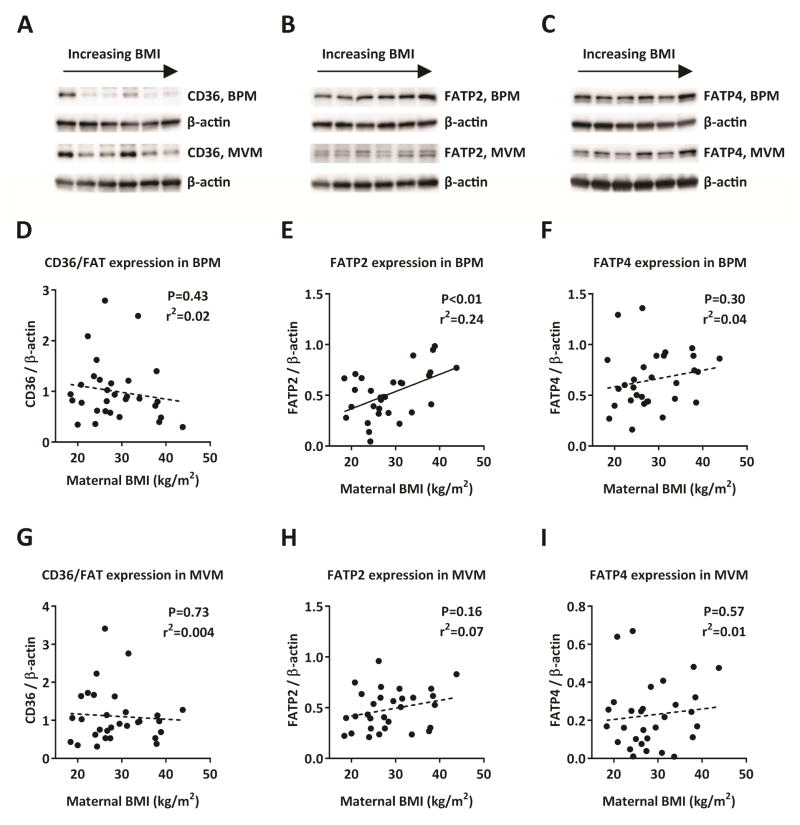
In the BPM, expression of FATP2 was significantly positively correlated with maternal BMI (P<0.01, n=30; Figure 4E) while the BPM expressions of CD36/FAT and FATP4 were not associated with maternal BMI (n=30; Figure 4D, F). In the MVM, expression levels of CD36/FAT, FATP2, or FATP4 were not correlated with maternal BMI (n=30; Figure 4G–I). Due to a slight imbalance in the distribution of fetal sex in the BMI categories, we evaluated the expression of FATP2, 4 and CD36/FAT by fetal sex in both normal and high BMI categories and found no significant difference in expression in male vs female placentas for any of the transporters in either BPM or MVM (data not shown).
Figure 4. Placental expression of CD36/FAT, FATP2, and FATP4 in relationship to maternal BMI.
Representative western blots of CD36/FAT (A), FATP2 (B), and FATP4 (C) expression in BPM and MVM isolated from placentas of women with varying BMI. In the basal plasma membrane CD36/FAT (D) or FATP4 (F) expression levels were not associated with maternal BMI, but the expression of FATP2 was significantly higher in placentas from high BMI women (E; P < 0.01). In the microvillous membrane, CD36/FAT, FATP2, or FATP4 expression was not associated with maternal BMI (G–H). Pearson correlation, n=30.
Discussion
In this study we show for the first time that FATP2 is expressed in both plasma membranes of the syncytiotrophoblast of the human placenta, and that FATP2 expression in the BPM correlated with maternal BMI. The increased FATP2 expression likely facilitates an increased delivery of fatty acids to the fetus in pregnancies complicated by maternal obesity. Further we have shown that FATP2 and FATP4 expression is highly polarized to the BPM, while CD36/FAT expression is higher in the MVM. We also demonstrate that maternal BMI is not associated with the expression levels of CD36/FAT or FATP4 in the syncytiotrophoblast plasma membranes.
The mechanism by which fatty acids are transferred across the placenta from the maternal to the fetal circulation remains to be fully established. There are several proposed mechanisms, including diffusion across cell membranes down a concentration gradient (reviewed in [28]). We found no correlation between fetal and maternal serum total NEFA levels when obtained at the time of c-section delivery. This lack of association between maternal and fetal circulating NEFA levels suggests that simple diffusion may not be the primary mechanism for transfer of fatty acids across the placenta. In addition, a preferential uptake and transfer of LCPUFA such as archidonic acid and docosahexaenoic acid across the placenta have been previously reported [29, 30]. Together these observations emphasize the importance of mediated transport mechanisms likely including syncytial fatty acid transport proteins localized to the plasma membranes of the syncytiotrophoblast. FATP isoforms on the basal membrane are unlikely to result in uptake of fatty acids into the syncytium from the fetus. This is due to a large net NEFA gradient toward the fetus and fetal albumin acting as an acceptor molecule in the fetal capillary which is in close proximity to the basal membrane. These thermo-dynamic considerations indicate that the FATP isoforms in the BPM are likely to result in fatty acid transfer toward the fetus (9). Details of the forces contributing to net fatty acid transfer to the fetus remain unclear but the localization of fatty acid transport proteins as described in this study are an important first step. Further studies are required to better understand the functional significance of these results.
The syncytiotrophoblast cell-layer is considered the primary barrier for transfer of nutrients [13], therefore transport capacity of the plasma membranes will impact nutrient transfer to fetus. In comparison to other macronutrients, there is a substantial gradient for lipids across the placenta, which therefore constitutes a barrier for NEFA transfer. Naturally the gradient is in part due to the fact that lipids are circulated in the form of lipoproteins in the maternal circulation, which requires additional transport processes to ensure delivery to the developing fetus. The positive vein-arterial concentration difference across the umbilical circulation is consistent with transfer of NEFAs across the placenta [31]. We observed on average 5-fold higher NEFA levels in the maternal circulation as compared to the fetal circulation, supporting a role of the syncytiotrophoblast as a major barrier for free fatty acid transfer. The large difference in maternal/fetal NEFA levels can be compared to the very small gradient for glucose across the placenta [32] and often higher amino acids levels in the fetal circulation compared to the maternal levels [13]. In this study we have evaluated the expression of FATP2 and FATP4 in both MVM and BPM and found that both are higher in the BPM compared to the MVM. The BPM was difficult to distinquish our immunofluorescence due to staining in the cytoplasm. But the lack of significant co-localization of FATP2 and FATP4 with alkaline phosphatase (Figure 2) is in good agreement with the western blot expression seen in the isolated plasma membranes (Figure 3). Whether uptake across MVM with a much greater surface area or transport over BPM (or both) are the rate limiting steps in transfer of fatty acids across the placenta remains to be determined. However, the high expression of these transporters on the BM indicates they may be critical for transfer of FA from the syncytial cytoplasm to the fetal capillary.
In this study we demonstrate that BPM FATP2 expression, but not FATP4 or CD36/FAT expression, is positively correlated to maternal pre-pregnancy BMI. The elevated FATP2 BPM expression in high BMI women, while not experimentally proven, could represent one mechanism contributing to accelerated fetal growth/adiposity commonly seen in obese mothers. Increased expression of FATP in the membrane directly apposed to the fetal capillary would potentially increase delivery of fatty acids to the fetal circulation. It has recently been shown that FATP4 expression is decreased, while CD36/FAT expression is increased in human placenta from obese women [12]. The discrepancy between our findings and the results by Dudé et al. [12] may be due to how the placental tissue was processed before analysis. Dudé and coworkers determined fatty acid transport expression in whole placental homogenates [12], which contain other cell types besides the syncytiotrophoblast. In contrast, we isolated the plasma membranes of the syncytiotrophoblast, which allows for a closer and more accurate characterization of transport features of the cell-layer composing the primary barrier for trans-placental nutrient transport [13]. Another recent study demonstrated effects of fetal sex and maternal BMI on CD36/FAT mRNA expression [33]. We did not measure mRNA levels, but in our study protein expression levels of CD36/FAT, FATP2, or FATP4 were not affected by fetal sex (data not shown).
This study indicates that the fatty acid transporters CD36/FAT, FATP2, and FATP4 are expressed in both plasma membranes of the syncytiotrophoblast in human placenta. The expression of FATP2 and FATP4 is substantially higher in the BPM than in the MVM, while CD36/FAT has greater expression in the MVM. The importance of such polarization remains to be established but suggests a clear mechanism to transfer NEFA to the fetal circulation where it is bound by fetal serum albumin and transported to the fetal liver. Increasing maternal BMI is positively associated with FATP2 BPM expression; which we speculate may represent one potential mechanism contributing to accelerated fetal growth and/or fetal adiposity in overweight/obese women.
Maternal and fetal circulating plasma NEFA levels do not correlate at term.
FATP2 and FATP4 expressions were approximately four-fold higher in the basal plasma membrane (BPM) compared to the microvillous membrane (MVM), a polarization that could facilitate fatty acid transfer across the placenta to the fetal circulation.
FATP2 expression on the BPM was positively correlated with maternal BMI.
Transplacental lipid transfer is not well understood and fatty acid transporters localized in the syncytial plasma membranes might be of importance.
Acknowledgments
We are grateful to patients and staff at the Labor & Delivery, University Hospital in San Antonio for making collection of blood and placental tissue possible. In addition, we are indebted to E. Miller (responsible for tissue collection), E. Dudley (excellent technical assistance) and Genelyn Dimasuay (confocal microscopy). This study was supported by grants from NIH (NIDDK RO1 DK89989; T.L.P.), the Swedish Research Council (S.L.), and the Swedish Society of Endocrinology (S.L.).
Grants: This study was supported by grants from NIDDK (R01DK089989; T.L.P.), the Swedish Research Council (S.L.), and the Swedish Society of Endocrinology (S.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004–2005. Matern Child Health J. 2009;13(5):614–20. doi: 10.1007/s10995-008-0388-3. [DOI] [PubMed] [Google Scholar]
- 2.Fisher SC, et al. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med. 2013;56(6):372–8. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91(3):436–40. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sebire NJ, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25(8):1175–82. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 5.Sewell MF, et al. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195(4):1100–3. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. Bjog. 2006;113(10):1126–33. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 8.Ramsay JE, et al. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87(9):4231–7. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 9.Lager S, et al. Oleic Acid Stimulates System A Amino Acid Transport in Primary Human Trophoblast Cells Mediated by Toll-Like Receptor 4. J Lipid Res. 2013;54(3):725–733. doi: 10.1194/jlr.M033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu MJ, et al. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1224–31. doi: 10.1152/ajpregu.00309.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strakovsky RS, Pan YX. A Decrease in DKK1, a WNT Inhibitor, Contributes to Placental Lipid Accumulation in an Obesity-Prone Rat Model. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dube E, et al. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod. 2012;87(1):14, 1–11. doi: 10.1095/biolreprod.111.098095. [DOI] [PubMed] [Google Scholar]
- 13.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012:article ID 179827. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta. 2012;1821(5):852–7. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishima T, et al. The expression and function of Fatty Acid transport protein-2 and -4 in the murine placenta. PLoS One. 2011;6(10):e25865. doi: 10.1371/journal.pone.0025865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell FM, et al. Detection and cellular localization of plasma membrane-associated and cytoplasmic fatty acid-binding proteins in human placenta. Placenta. 1998;19(5–6):409–15. doi: 10.1016/s0143-4004(98)90081-9. [DOI] [PubMed] [Google Scholar]
- 17.Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res. 2009;48(1):52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Schaiff WT, et al. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab. 2005;90(7):4267–75. doi: 10.1210/jc.2004-2265. [DOI] [PubMed] [Google Scholar]
- 19.Lager S, et al. Effect of IL-6 and TNF-alpha on fatty acid uptake in cultured human primary trophoblast cells. Placenta. 2011;32(2):121–127. doi: 10.1016/j.placenta.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Mousiolis AV, et al. Effects of leptin on the expression of fatty acid-binding proteins in human placental cell cultures. Mol Med Report. 2012;5(2):497–502. doi: 10.3892/mmr.2011.686. [DOI] [PubMed] [Google Scholar]
- 21.Aye ILMH, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biology of Reproduction. 2014;90(6):129. doi: 10.1095/biolreprod.113.116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ericsson A, et al. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod. 2005;20(2):521–30. doi: 10.1093/humrep/deh596. [DOI] [PubMed] [Google Scholar]
- 23.Illsley NP, et al. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta. 1990;1029(2):218–26. doi: 10.1016/0005-2736(90)90157-j. [DOI] [PubMed] [Google Scholar]
- 24.Johansson M, Jansson T, Powell TL. Na(+)-K(+)-ATPase is distributed to microvillous and basal membrane of the syncytiotrophoblast in human placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279(1):R287–94. doi: 10.1152/ajpregu.2000.279.1.R287. [DOI] [PubMed] [Google Scholar]
- 25.Donovan A, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776–81. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 26.Bastin J, et al. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol. 2006;134:532–543. doi: 10.1111/j.1365-2141.2006.06216.x. [DOI] [PubMed] [Google Scholar]
- 27.Bradley J, et al. Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am J Physiol Regul Integr Comp Physiol. 2004;287(4):R894–901. doi: 10.1152/ajpregu.00525.2003. [DOI] [PubMed] [Google Scholar]
- 28.Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr. 2010;30:237–55. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- 29.Gil-Sanchez A, et al. Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am J Clin Nutr. 2010;92(1):115–22. doi: 10.3945/ajcn.2010.29589. [DOI] [PubMed] [Google Scholar]
- 30.Larque E, et al. In vivo investigation of the placental transfer of (13)C-labeled fatty acids in humans. J Lipid Res. 2003;44(1):49–55. doi: 10.1194/jlr.m200067-jlr200. [DOI] [PubMed] [Google Scholar]
- 31.Lewis RM, Hanson MA, Burdge GC. Umbilical venous-arterial plasma composition differences suggest differential incorporation of fatty acids in NEFA and cholesteryl ester pools. Br J Nutr. 2011;106(4):463–7. doi: 10.1017/S0007114511000377. [DOI] [PubMed] [Google Scholar]
- 32.Acosta O, et al. Increased glucose and placental GLUT-1 in large babies of obese non-diabetic mothers. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Brass E, Hanson E, O'Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013;34(6):503–9. doi: 10.1016/j.placenta.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]