Abstract
Background
Rabies is an acute fatal encephalitis caused by all members of the Lyssavirus genus. The first human rabies survivor without benefit of prior vaccination was reported from Milwaukee in 2005. We report a second unvaccinated patient who showed early recovery from rabies and then died accidentally during convalescence, providing an unparalleled opportunity to examine the histopathology as well as immune and virological correlates of early recovery from human rabies.
Methods
Case report, rapid fluorescent focus inhibition test, enzyme-linked immunosorbent assay, indirect and direct fluorescent antibody assays, reverse-transcriptase polymerase chain reaction, phylogenetic reconstruction, isolation in tissue culture, pathology and immunohistochemistry.
Results
The 9 year old died 76 days after presenting with rabies of vampire bat phylogeny transmitted by cat bite. Antibody response in serum and cerebrospinal fluid was robust and associated with severe cerebral edema. No rabies virus was cultured at autopsy. Rabies virus antigen was atypical in size and distribution. Rabies virus genome was present in neocortex but absent in brainstem.
Conclusions
Clinical recovery was associated with detection of neutralizing antibody and clearance of infectious rabies virus in the central nervous system by 76 days but not clearance of detectable viral subcomponents such as nucleoprotein antigen or RNA in brain.
Keywords: rabies, therapy, pathology, immunology, recovery of function, cerebral edema
Rabies is an acute fatal encephalitis caused by all members of the Lyssavirus genus, including rabies virus (RABV). While vaccine preventable for over a century, RABVs remains the leading global zoonosis, killing more than 55,000 persons annually.1 The first human rabies survivor without benefit of prior vaccination was reported from Milwaukee in 2005.2 We report a second unvaccinated patient who showed early recovery from rabies and then died accidentally during convalescence, providing an unparalleled opportunity to examine the histopathology as well as immune and virological correlates of early recovery from human rabies. These findings add to a small body of laboratory studies of human rabies that provide insights into the chronology of RABV–host interactions.3–7
MATERIALS AND METHODS
Detection of RABV-neutralizing Antibodies by the Rapid Fluorescent Focus Inhibition Test (RFFIT)
This cell culture–based microneutralization test assessed virus inhibition by serially diluted serum and cerebrospinal fluid (CSF) against a standardized amount of reference RABV (CVS-11, laboratory strain). The test was performed on serial (5-fold) dilutions of heat-inactivated serum and CSF samples in 8-well LabTek chamber slides (Thermo Scientific, Waltham, MA) using mouse neuroblastoma (MNA) cell culture as described.8 The World Health Organization standard serum (2 IU/mL) was used for calibration.
Detection of RABV Antibodies by an Enzyme-linked Immunosorbent Assay (ELISA)
The Platelia Rabies II (Bio-Rad Laboratories, Hercules, CA) is a kit that detects glycoprotein G–binding antibodies in serum and CSF samples. ELISA plates were coated with purified RABV glycoprotein, and serial dilutions of serum and CSF were assayed. Titers of glycoprotein-binding antibodies were estimated based on a standard curve using reference serum according to the manufacturer’s instructions.9
Detection of Class-specific RABV-binding Antibodies by an Indirect Fluorescent Antibody (IFA) Assay
This assay detected CSF and serum antibodies binding to RABV structural proteins. A monolayer of MNA cells, infected with the CVS-11 RABV strain and fixed with acetone, was used as antigen. Serial dilutions of serum and CSF were placed on the fixed 4-well antigen-coated slides for antibody quantification. A secondary goat or rabbit anti-human IgG or IgM fluorescein isothiocyanate-labeled conjugates identified the presence of antibodies as described elsewhere.10
Detection of RABV Antigens by Direct Fluorescent Antibody (DFA) in a Skin Biopsy
Horizontal and vertical planes of a skin biopsy, mounted in Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC), were frozen and cut in 8-μm sections and DFA stained as described.11,12
Detection of RABV Nucleic Acid by a Heminested Reverse-transcription Polymerase Chain Reaction (RT-PCR)
This technique targeted the RABV nucleoprotein (N) gene in saliva, nuchal skin and brain tissues. Total RNA was extracted with the use of TRIZol reagent (Invitrogen, San Diego, CA) according to the manufacturer’s instructions. For maximum sensitivity, we did a 2-step primary RT-PCR with primer sets 001-550B, 550F-304 and 1066Fdeg-304 followed by a second round of heminested PCR reactions as follows: 001-550B product was reamplified with primers N7deg-550B; the 550F-304 PCR product was amplified with primers 550F-1066deg reverse and 1066Fdeg-304 in separate reactions and the 1066Fdeg-304 PCR product was amplified with primers 1087Fdeg-304 and 504S-304 in separate reactions (Table 1).11,13,14 RT was performed for 90 minutes at 42°C followed by a 4°C hold. PCR conditions were as follows: 1 minute at 94°C followed by 40 cycles of 94°C for 30 seconds, 37°C for 30 seconds and 72°C for 90 seconds, followed by 7-minute extension at 72°C. Amplicons were purified and subjected to direct sequencing according to the manufacturer’s instructions (ABI Prism Big dye terminator v1.1 ready reaction mix Cycle sequencing kit, and sequence analyzer 3023; Applied Biosystems, Foster City, CA).13,15
TABLE 1.
Primers Detection of Rabies Virus by Polymerase Chain Reaction
| Designation ID | Broadly Reactive or Degenerate Primers
|
|||
|---|---|---|---|---|
| Orientation | Genome Position* | Sequence | Reference | |
| 001 | Forward | 1–16 | ACGCTTAACGAMAAA | 13 |
| 550 F† | Forward | 647–666 | ATGTGYGCTAAYTGGAGYAC | 13 † |
| 550B | Reverse | 647–666 | GTRCTCCARTTAGCRCACAT | 13 |
| 1087Sdeg | Forward | 1157–1173 | GAGAARGAACTTCARGA | 11 |
| 1066 deg F | Forward | 1136–1155 | GARAGAAGATTCTTCAGRGA | 11 |
| 1066deg B† | Reverse | 1136–1155 | TCYCTGAAGAATCTTCTYTC | 11† |
| 304‡ | Reverse | 1514–1533 | TTGACGAAGATCTTGCTCAT | 14 |
| 504S‡ | Forward | 1296–1312 | TCATGATGAATGGAGGT | 11 |
According to the full genome sequence of the fixed rabies virus strain, SAD B19 (Conzelmann et al. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175:485–499.
Nondegenerate primers.
Reverse complement of a published primer.
RABV Typing Using Sequence Analysis and Phylogenetic Reconstruction
Sequences newly generated during this study were submitted to GenBank for assignation of consecutive accession numbers JF693449–JF693484.
Viral gene sequences obtained from patient’s antemortem or postmortem samples were analyzed together with recent and historic RABV-positive samples obtained in Colombia and other countries in the United States (Table 2). Phylogenetic reconstructions were run with MEGA software v 5.0, using maximum-likelihood and neighbor-joining methods.16
TABLE 2.
Sample List Used as an Epidemiological Context for Reconstruction of Phylogenetic Tree
| Sample Code | Host | Year | Geographical Location in Colombia | Antigenic Variant | GeneBank Accession Number |
|---|---|---|---|---|---|
| H01/08 | Human | 2008 | Quilichao-Cauca | 3 | JF693456 |
| H02/08 | Human | 2008 | Quilichao-Cauca | 3 | JF693457 |
| CT1/06 | Cat | 2006 | Sasaima-Cundinamarca | 3 | JF693458 |
| CT1/07 | Cat | 2007 | Linares-Nariño | 3 | JF693459 |
| H04/08 | Human | 2008 | Bolívar-Cauca | 3 | JF693460 |
| H01/04 | Human | 2004 | Bajo Baudó-Chocó | 3 | JF693461 |
| C07/96 | Dog | 1996 | Armenia-Quindío | 3 | JF693462 |
| BV6/97 | Bovine | 1997 | Neira-Caldas | Atipical. | JF693463 |
| CT1/97 | Cat | 1997 | Zipaquirá-Cundinamarca | 3 | JF693464 |
| BV8/97 | Bovine | 1997 | Leticia-Amazonas | 3 | JF693465 |
| BV1/06 | Bovine | 2006 | Samaniego-Nariño | 5 | JF693466 |
| BV7/97 | Bovine | 1997 | Curumaní-Cesar | 3 | JF693467 |
| BV1/03 | Bovine | 2003 | Sardinata-Nte Santander | 3 | JF693468 |
| HR1/02 | Horse | 2002 | Pto Carreño-Vichada | 3 | JF693469 |
| H02/07 | Human | 2007 | San Luis-Casanare | 3 | JF693470 |
| CT1/94 | Cat | 1994 | Neiva-Huila | 8 | JF693471 |
| CT3/96 | Cat | 1996 | La Mesa-Cundinamarca | Atipical | JF693472 |
| H03/08 | Human | 2008 | Floridablanca-Santander | 3 | JF693478 |
| H01/03 | Human | 2003 | Quipile-Cundinamarca. | 8 | JF693477 |
| H02/94 | Human | 1994 | Sasaima-Cundinamarca | Atipical | JF693476 |
| H04/95 | Human | 1995 | María la Baja-Bolívar | 3 | JF693475 |
| CT4/96 | Cat | 1996 | Viotá-Cundinamarca | Atipical | JF693473 |
| CT2/06 | Cat | 2006 | Sasaima-Cundinamarca | 3 | JF693474 |
| Bat | 1988 | Florida, United States | 9 | AF394876 | |
| Bat | 2008 | Uruguay, South America | 4 | EU981920 | |
| B01/04 | Bat | 2004 | Pereira-Risaralda | 4 | JF693481 |
| B01/08 | Bat | 2008 | Cartago-Valle | 4 | JF693479 |
| B02/08 | Bat | 2008 | Cartago-Valle | 4 | JF693480 |
| Bat | 2002 | Sao Paulo, Brazil | Atipical | AB201814 | |
| Bat | 2001 | Sao Paulo, Brazil | Atipical | AB201813 | |
| F01/07 | Fox | 2007 | Honda-Tolima | Atipical | JF693482 |
| F21/03 | Fox | 2003 | Sardinata-Nte Santander | Atipical | JF693483 |
| Bat | 1992 | British Columbia, Canada | Atipical | AF351839 | |
| Bat | 1993 | Ontario, Canada | Atipical | AF351827 | |
| Bat | 1985 | Colorado, United States | Atipical | AY039228 | |
| B02/02 | Bat | 2002 | Cartago-Valle | Atipical | JF693484 |
| Bat | 1998 | Pennsylvania, United States | Atipical | AY705373 | |
| Bat | 1991 | Ontario, Canada | 6 | AF351856 | |
| Bat | 1993 | Ontario, Canada | 6 | AF351846 | |
| Skunk | 2001 | Arizona, United States | 8 | AF483524 | |
| Raccoon | 1989 | Pennsylvania, United States | Atipical | RVU27221 | |
| C14/95 | Dog | 1995 | Tunja-Boyacá | 1 | JF693453 |
| C20/95 | Dog | 1995 | Samacá-Boyaca | 1 | JF693454 |
| H01/00 | Human | 2000 | Orito-Putumayo | 1 | JF693455 |
| C01/06 | Dog | 2006 | Ariguaní-Magdalena | 1 | JF693451 |
| F02/07 | Fox | 2007 | Plato-Magdalena | 1 | JF693450 |
| C02/07 | Dog | 2007 | Bosconia-Cesar | 1 | JF693449 |
| H01/07 | Human | 2007 | Sta Marta-Magdalena | 1 | JF693452 |
RABV Isolation From Brain Tissues and Saliva in Cell Culture
Approximately 100–500 μL of saliva was used to prepare suspensions in 1000 μL of MEM-10% fetal calf serum. Additional washings of brain tissue sediment through resuspension and centrifugation at 500g were introduced. Aliquots of this inoculum (100–400 μL) were incubated with a suspension of MNA cells (4–5 × 106). RABV presence was monitored in the LabTek slides at 24, 48, 72 and 96 hours using the DFA test as described.11,17 Supernatants were passaged serially to maximize detection of wildtype RABV.
Detection of RABV Antigens by the DFA Test
Brain touch impressions were examined for the presence of RABV antigens using the national standard protocol for rabies diagnosis in the United States, as described.12
Detection of RABV Antigens by the Direct Rapid Immunohistochemistry (IHC) Test
The direct rapid IHC test was performed on fresh brain, brainstem, cerebellum and hippocampus, as described.18
Detection of RABV Antigens by IHC
Routine hematoxylin and eosin stains and IHC were performed on representative brain tissues as well as skin and salivary glands. Hematoxylin and eosin slides were evaluated for histopathology. IHC was performed using a colorimetric polymer-based indirect immunoalkaline phosphatase procedure as described.19
Case Report
On July 10, 2008, a 9-year-old girl was bitten by her unvaccinated cat in a southwestern rural area of Colombia, South America. The cat was then killed without laboratory testing for rabies. She was taken to the clinic 3 hours away, where the wounds were cleaned, tetanus vaccine given and antibiotics dispensed. Rabies vaccine and immune globulin were not available. On August 8, the girl developed fevers, headache, myalgia, vomiting and trouble swallowing. The next day, she showed alternating agitation and somnolence. On August 10, she was taken to the clinic and diagnosed with either meningitis or encephalitis. She was sent to a regional hospital and then to Hospital Universitario del Valle, in Cali, Colombia, by August 12.
At time of transfer, on the fourth day of illness [hospital day (HD) 3, based on admission to the first hospital], the patient was somnolent and salivating. Her temperature was 38°C, pulse 98/min, respirations 24/min and blood pressure of 97/66 mm Hg. She did not respond to commands, with eye opening and extensor posturing to pain. She had left hemiparesis and left gaze preference. Samples of saliva, serum, CSF and corneal impressions were sent to the National Institute of Health in Bogota, Colombia. White blood cells (WBCs) were 4600/mm3 (60% neutrophils and 24% lymphocytes), hemoglobin 12.5 g/dL, platelets 441,000/mm3 and C-reactive protein 200 μg/L. CSF showed 1 red blood cell, 233 WBC (4% neutrophils), glucose 71 mg/dL (serum 91 mg/dL) and protein 48 mg/dL. Chest radiograph (CXR) and computed tomography (CT) of the head were normal.
A meeting with hospital leadership, pediatricians, intensivists, infectious disease consultants, psychologists and the legal department was held. The decision was made to attempt treatment according to the Milwaukee Protocol adapted to local resources.20 After laboratory confirmation of rabies on HD6, therapy was initiated with ventilation, ketamine, midazolam, phenobarbital, thiopental, magnesium sulfate, amantadine, oral ribavirin, sublingual interferon alpha and medroxyprogesterone. The case was discussed with experts at the Centers for Disease Control and Prevention, Atlanta, GA, and the Medical College of Wisconsin; ribavirin and interferon were stopped and barbiturates tapered.
The patient became unstable on HD7, with anisocoria, hypertension, bradycardia with extrasystoles and metabolic acidosis. Serum sodium was 149 mEq/L. Lidocaine, mannitol and hypertonic saline were administered and the patient improved. Repeat CT was normal. The patient became hypotensive and bradycardic, requiring plasma, plasma expanders and 2 boluses of epinephrine. Bispectral index monitoring [a simplified form of electroencephalogram (EEG) used in anesthesiology] showed a decline in scores of 58 to 21 over the next 4 days. A urinary tract infection with resistant Escherichia coli was treated. CSF on HD10 showed 154 WBC, 70 red blood cells, protein 146 mg/dl and glucose 48 mg/dl. Serum prolactin, used to monitor central nervous system (CNS) dopamine production, was 5.61 ng/mL (normal: 3.25–29.1). Sapropterin and vitamin C were initiated.21 EEG on HD11 showed reduced activity on the right, with alpha rhythm predominance. CSF on HD12 showed 133 WBC, 5 red blood cells, protein 203, glucose 130 and lactate dehydrogenase 272 IU/L. On HD13, the bispectral index score abruptly decreased to 7, and sedation was tapered; bispectral index score increased to 25 with increased blood pressure. Serum sodium was 136. Pupils remained symmetric and reactive.
On HD14, signs of recovery were first noted. She showed fluttering of eyelids and return of corneal, cough and ankle reflexes. In contrast, CT showed diffuse cerebral edema without midline shift and hypodensity in the left basal ganglia. Mannitol and hypertonic saline were administered. Neurosurgeons advised against placing an intraventricular drain because of a concurrent outbreak of bacterially infected ventricular shunts. On HD16, the patient breathed spontaneously. Sedation was increased to control agitation. Patellar and ankle reflexes were brisk. EEG showed response to stimuli, and recovery of right-sided activity but diminution in the fronto-central-parietal areas bilaterally.
HD17 to HD32 were characterized by 3 episodes of anisocoria and hypertension, treated with hypertonic saline, mannitol and thiopental. Serum sodium concentrations were extremely labile, ranging from 129 to 156, and confounded by runs of hypertonic saline and use of diuretics. CT on HD17 showed worsening of left cortical edema with obliteration of the lateral ventricle and marked edema of the basal ganglia. CT angiography on HD22 showed effacement of the cortical gyri, transtentorial herniation with compression of the posterior cerebral artery and possible left occipital lobe infarction. There was no venous thrombosis. The patient was markedly acidotic (pH 7.06 with a pCO2 of 105) on return from CT. On HD31, CT showed improved edema, with a left occipital hematoma. On HD32, a tracheostomy was performed. During HD33 to HD51, serum sodium concentrations were stabilized with enteral sodium supplementation. She had frequent episodes of hypothermia associated with bradycardia.
On HD 39, CT showed less edema, with irregular hypodensities in frontal and occipital lobes and basal ganglia. Sedation was discontinued. The next day, the patient breathed independently, had equal and reactive pupils and moved her head to avoid light in her eyes. She spontaneously moved her legs and had increased reflexes in arms and legs. By HD44, she opened her mouth, moved her tongue and sucked on swabs. On HD45, she turned her head to voice and showed subtle arm movements. She was treated for a urinary tract infection.
She was weaned off the ventilator on HD47. By HD48, she appeared more awake, sucked on a candy and moved 2 fingers on her left hand to command. There was eye deviation to the left. On HD50, based on low CSF tetrahydrobiopterin and monoamine neurotransmitter concentrations, sapropterin was restarted with gradual increase to 20 mg/kg/d. On HD51, she clearly preferred the voices of her mother and her regular nurse to others, also becoming emotional when she heard them. When presented with her mother’s face, she increased the frequency of her mouth movements.
On HD55, neurology consultation documented an awake patient with spontaneous movements of head, legs and left hand, strength 3/5. She did not follow commands consistently. She did not blink to a loud noise. Right eye was deviated outward; left eye had mesial and lateral paresis. In a seated position, she did not support her head and did not follow commands. The following day, she moved her right hand and both shoulders. Sodium supplementation was discontinued. On HD58, speech therapy certified adequate swallowing and protection of the airway. Sapropterin dosing reached therapeutic range. EEG showed greater activity on the left, reacting to stimuli, with theta rhythm. Over several days, she was somnolent correlating with serum sodium of 134, with improvements in alertness with serum sodium concentrations in the 140s, so oral supplementation at 9 mg/kg/d was restarted.
On HD61, the patient was removed from isolation. Visual-evoked responses were negative. Auditory-evoked responses showed decreased amplitudes, with normal brainstem and spiral ganglion cell responses. Magnetic resonance imaging on HD62 showed increased T2 signal in parieto-occipital regions, caudate and putamen, T1 changes in caudate nuclei and left temporo-occipital infarct, with mildly enlarged ventricles and cerebellar atrophy. Cervical cord was normal. On HD63, she extended her arms on stimulation of the arms and face and controlled her head position. On HD66, she was discharged from the intensive care unit.
On HD70, she developed fever, difficulty breathing, desaturations and rhonchi and produced purulent sputum. She was pale and listless. WBC was 8300 with 80% neutrophils. C-reactive protein was 224 μg/L. CXR showed right basilar alveolar infiltrates. The patient was moved to the intensive care unit and given diuretics and antimicrobials. On HD72, she became hypertensive, tachycardic, with serum sodium greater than 200 mEq/L. Vasopressin test excluded central diabetes insipidus. The patient required 70% inspired oxygen concentration. CXR showed worsening infiltrates. She was intubated. She was given free water, and her serum sodium decreased by 28 mEq/L over 24 hours. On HD74, she became hypotensive. Attempts to place central lines resulted in bilateral pneumothoraces with hypotension and oxygen desaturations of 50% for over 2 hours. The patient was placed on dopamine and nor-epinephrine drips and transfused. On HD75, cardiac echo showed diastolic dysfunction with an ejection fraction of 40%. The patient developed refractory hypotension and died.
RESULTS
Antemortem Results
Humoral Immune Response
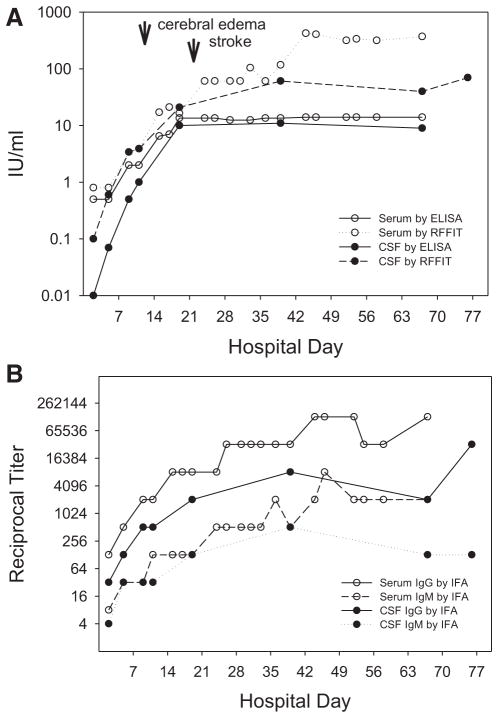
At the National Institute of Health in Bogota, Colombia, RABV antiglycoprotein (G) antibodies were evaluated using a commercial ELISA kit (Platelia Rabies II). A titer of 0.5 IU/mL was detected in serum 4 days after onset of symptoms (HD3), while no antibody was detected in CSF (Fig. 1A, B). In a symptomatic, unvaccinated patient, detection of serum antibodies is diagnostic of rabies. RFFIT (detecting RABV-neutralizing antibody) and IFA tests (detecting antibodies that bind to various RABV proteins, predominantly to the abundant nucleoprotein), performed at Centers for Disease Control and Prevention a week later using the same serum and CSF aliquots, detected neutralizing activity and RABV-binding IgM and IgG in both serum and CSF (Fig. 1A, B). Titers in serum and CSF then rapidly rose, with the maximum humoral immune response in CSF and serum at days 41 (HD40) and 46 (HD45) after disease onset, respectively. While showing comparable sensitivity at lower concentrations of anti-G antibodies, the ELISA became asymptotic around 14 IU/mL, while RFFIT titers were at least 10-fold higher. IFA titers of RABV-binding antibody paralleled RFFIT titers in this individual. The IgG titer in brain tissue was about 10-fold higher than the last CSF sample (Fig. 1B).
FIGURE 1.
A) Anti-rabies virus antibody kinetics in serum and CSF, monitored by ELISA and RFFIT. Increasing humoral immune response was elicited in both CSF and serum. RFFIT detected earlier and higher antibody titers in CSF and serum compared with ELISA. Timing of first detection of cerebral edema and stroke are illustrated. B) Anti-rabies virus class-specific antibody kinetics in serum and CSF, monitored by IFA. IgG class antibodies were detected in increasing titers in both sera and CSF, occurring in higher titers than IgM antibodies. Humoral immune responses tend to be higher in serum than in CSF.
Detection of RABV Antigens by DFA Test
Corneal impressions taken at time of transfer to the tertiary hospital (HD3) and in a biopsy of nuchal skin taken 13 days (HD12) after the onset of symptoms were negative for RABV antigens.
Detection of RABV Nucleic Acids
By RT-PCR, RABV RNA (N gene) was detected in saliva only on HD14 and HD18. RABV RNA was absent in the only skin biopsy sample available at HD14 (Table 3).
TABLE 3.
Detection of Rabies-specific Antibody in Serum and CSF by ELISA, RFFIT and IFA Assays and Rabies Virus Amplicons in Saliva and Skin by RT-PCR
| Date | Days After Onset of Symptoms | Hospital Day | Serum
|
CSF
|
Saliva
|
Skin
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISA (IU/mL) | RFFIT (IU/mL) | IFA Titer† (IgG) | IFA Titer† (IgM) | ELISA (IU/mL) | RFFIT (IU/mL) | IFA Titer† (IgG) | IFA Titer† (IgM) | PCR | PCR | |||
| August 8, 2012 | 4 | 3 | 0.5 | 0.8 | 128 | 8 | 0.0 | 0.1 | 32 | 4 | nd | nd |
| August 15, 2008 | 7 | 6 | 0.5 | 0.8 | 512 | 32 | 0.07 | 0.6 | 128 | 32 | − | nd |
| August 19, 2008 | 11 | 10 | 2.0 | 3.4 | 2048 | 32 | 0.5 | 3.4 | 512 | 32 | − | nd |
| August 21, 2008 | 13 | 12 | 2.0 | 3.9 | 2048 | 128 | 1.0 | 3.9 | 512 | 32 | + | − |
| August 25, 2008 | 17 | 16 | 6.5 | 17.1 | 8192 | 128 | + | nd | ||||
| August 27, 2008 | 19 | 18 | 7.0 | 21.1 | 8192 | 128 | − | nd | ||||
| August 29, 2008 | 21 | 20 | 13.5 | 16.5 | 8192 | 128 | 10.0 | 20.9 | 2048 | 128 | − | nd |
| September 3, 2008 | 26 | 25 | 13.5 | 60.9 | 8192 | 512 | nd | nd | ||||
| September 5, 2008 | 28 | 27 | 13.5 | 60.9 | 32,768 | 512 | − | nd | ||||
| September 8, 2008 | 31 | 30 | 12.5 | 60.9 | 32,768 | 512 | − | nd | ||||
| September 10, 2008 | 33 | 32 | ND | 60.9 | 32,768 | 512 | − | nd | ||||
| September 12, 2008 | 35 | 34 | 12.5 | 104.4 | 32,768 | 512 | − | nd | ||||
| September 15, 2008 | 38 | 37 | 13.5 | 60.9 | 32,768 | 2048 | − | nd | ||||
| September 18, 2008 | 41 | 40 | 13.5 | 117.4 | 32,768 | 512 | 11.0 | 60.9 | 8192 | 512 | − | nd |
| September 23, 2008 | 46 | 45 | 14.0 | 424.5 | 131,072 | 2048 | − | nd | ||||
| September 25, 2008 | 48 | 47 | 14.0 | 407.1 | 131,072 | 8192 | − | nd | ||||
| October 1, 2008 | 54 | 53 | 14.0 | 317.5 | 131,072 | 2048 | − | nd | ||||
| October 3, 2008 | 56 | 55 | 14.0 | 336.8 | 32,768 | 2048 | − | nd | ||||
| October 7, 2008 | 60 | 59 | 14.0 | 317.5 | 32,768 | 2048 | nd | nd | ||||
| October 16, 2008 | 69 | 68 | 14.0 | 372.1 | 131,072 | 2048 | 9.0 | 40.0 | 2048 | 128 | nd | nd |
| October 25, 2008* | 78 | 77 | 70.0 | 32,768 | 128 | − | − | |||||
Postmortem brain suspension.
Titer expressed as the inverse of the dilution factor.
IU/mL indicates international units per milliliter; nd, not done.
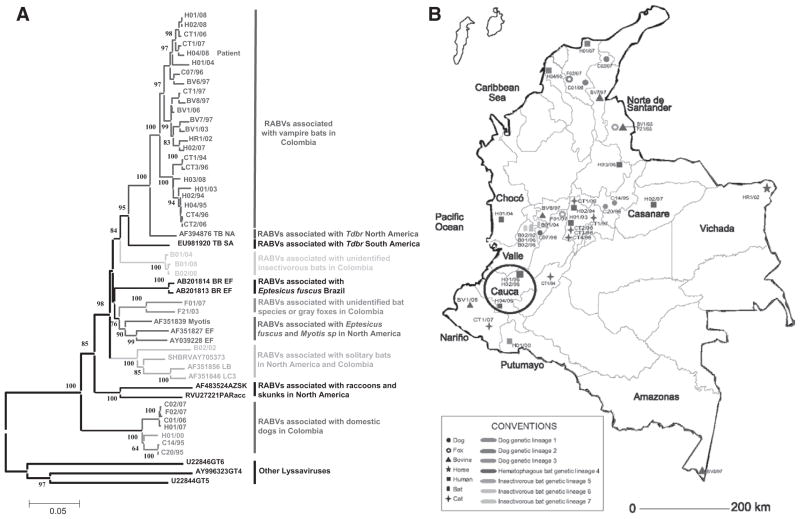
The sequence of viral N gene obtained from the patient’s saliva presented the highest nucleotide similarity (99.2%) with sequences of a RABV variant circulating in vampire bats near the patient’s hometown (Fig. 2 and Table 2).
FIGURE 2.
A) Phylogenetic relationships among 51 selected RABV isolates from Colombia and the United States 1985–2008. Neighbor joining with a maximum composite likelihood nucleotide substitution model was used for the phylogenetic reconstruction. The significance of each clade was estimated by a bootstrap algorithm applying 1000 iterations. Numbers at nodes indicate bootstrap values greater than 60%. The Lyssavirus species Duvenhage virus (DUVV) and European Bat Lyssavirus 2 (EBL-2) were included as outgroup. B) Geographical location of selected rabies isolates in Colombia 1994–2008. Circle A shows the location of patient case (H04/08). Conventions show host and genetic lineage for each isolate color coded matching their respective branches in the phylogenetic reconstruction.
Postmortem Results
RABV Isolation Attempts
No RABV was isolated from brain samples including brain stem, cerebellum, hippocampus, temporal and occipital lobes as well as from saliva after 3 passages of 15 days in MNA cells. Intracerebral isolation in suckling mice was not attempted because susceptibility of MNA cells to RABV is equal or greater than susceptibility of suckling mice at the contributing rabies reference center.
Detection of RABV Antigens
Samples including brain stem, cerebellum, hippocampus, temporal and occipital lobes, kidney, liver, salivary glands, skin and saliva were available at autopsy. RABV antigens were detected by DFA in the hippocampus. Scant and atypically distributed intracytoplasmic inclusions with sizes ranging from 0.1 to 1 μm were observed in some sections of the brain stem, temporal and occipital lobes but not in other sections. No RABV antigens were detected in an autopsy skin specimen. Other tissues were not tested by DFA.
Direct rapid IHC test was performed on the same material subjected to DFA. Results by this technique were inconclusive because only a limited number of scattered inclusions, different in distribution and intensity from the positive control samples, were observed. Human brain without rabies showed no inclusions.
Histopathology
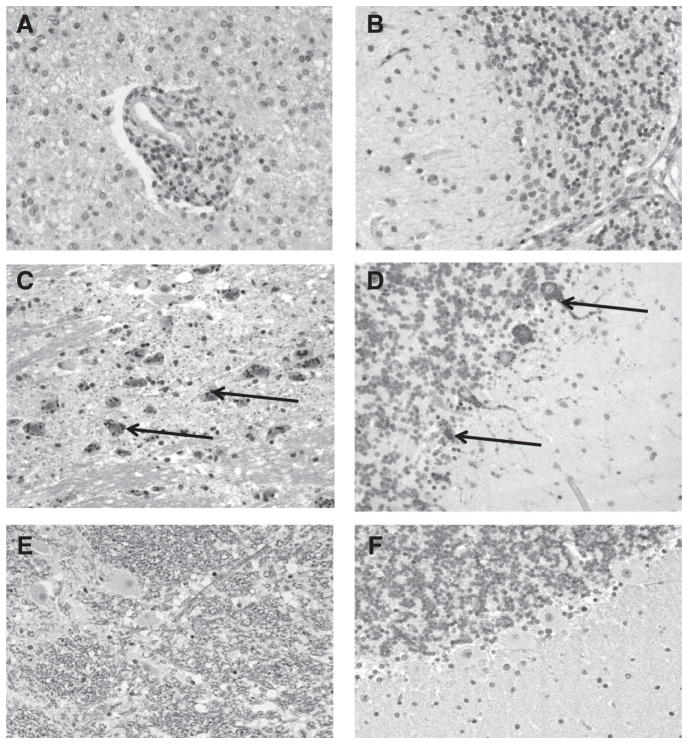
Microscopic pathological examination of CNS specimens showed necrosis, gliosis and inflammatory infiltrates in parenchyma and perivascular space. The inflammatory infiltrates were mainly composed of lymphocytes and macrophages. No significant pathology was observed in skin and salivary gland. No RABV antigens were detected in any specimens tested by IHC (Fig. 3).
FIGURE 3.
Postmortem RABV-specific immunohistochemistry test in brain stem (left column) and cerebellum (right column). A), B) Tissues of the patient addressed in this study where absence of rabies components is observed. Some histopathological findings included parenchymal necrosis, gliosis and inflammatory infiltrates in parenchyma and perivascular space. No prominent intracytoplasmic inclusion bodies were seen. C), D) Positive controls from a rabies-positive human brain obtained from the archive. Arrows indicate specific intracytoplasmic rabies virus antigen stained in red. All cell nuclei are stained in navy blue. E), F) Negative controls from a human brain confirmed negative for rabies.
Detection of RABV Nucleic Acid
RABV RNA was detected by both primary and heminested RT-PCR in cerebellum, hippocampus and brain cortex (temporal and occipital regions), but not in brain stem. RABV RNA was not found in the kidneys, lungs, skin biopsy, salivary glands or saliva of the patient. RT-PCR amplicons from brain tissues were sequenced and compared among each other and with sequences obtained initially from the saliva samples at days HD12 and HD16. All were 100% identical to each other.
DISCUSSION
This patient was the second known human to show recovery from rabies without benefit of prior vaccination, thereby establishing that Homo sapiens can recover from wildtype rabies. Many other species also produce occasional rabies survivors.22 After the first report of survival after human rabies without prior immunization, operational definitions of survival, therapeutic futility and lack of transmissibility (for infection control) were developed.2,23 Survival was defined in 2005 by 4 a priori criteria: (1) rabies diagnosis confirmed by a rabies reference laboratory, (2) absence of detectable RABV in saliva and/or skin, (3) concurrent development of neutralizing antibodies to RABV and (4) discharge of the convalescent patient from the intensive care unit. This patient met all 4 criteria for survival from rabies. A threshold duration of survival after onset of rabies was not considered as an ad hoc criterion for survival in 2005 because the 90th percentile for survival with rabies in the United States was only 18 days, and there were fewer than 10 patients over a 40-year span with rabies (all vaccine recipients) who had survived beyond 30 days. Our definition of survival is in retrospect problematic because our patient died 76 days after hospitalization, but not as a clear consequence of RABV infection. Whether to call our patient a survivor is debatable. Given the rarity of any survival from rabies, alternative statistical approaches quantifying duration of survival may be preferred.24 Conversely to having met ad hoc criteria for survival, our patient never met ad hoc criteria for therapeutic futility, which were defined in 2007.6,25 This patient had CSF protein levels greater than 200 mg/dL, which is 1 of 5 ad hoc criteria for therapeutic futility. The other 4 criteria, such as, fixed pupils, isoelectric EEG, diabetes insipidus and CSF lactate greater than 4.0 mM, were not present concurrently. This patient also met ad hoc criteria for discontinuation of isolation beyond standard precautions.2 Droplet isolation was discontinued when 3 saliva samples after HD16 were negative for RABV nucleic acid by heminested RT-PCR, and the patient had demonstrated the presence of RABV-neutralizing antibodies indicative of adequate humoral response.
This patient showed initial clinical improvement on HD14, at a time when both systemic and CSF concentrations of neutralizing antibody likely exceeded 1.0 IU/mL. Neurological recovery in this patient was faster than in the first vaccine-naive survivor.2 Her course was then complicated by hyponatremia and refractory cerebral edema, causing an occipital stroke. It is almost certain that she would have had significant neurological sequelae, including blindness, had she survived. After partial resolution of her cerebral edema, she followed commands inconstantly and showed preference for familiar voices and a passion for sweets. She breathed independently, swallowed well, had head control and was regaining use of her 4 extremities, but was not verbal at the time of her premature demise. From over 140 case reports of rabies in the peer-reviewed English literature, we are aware of only one other report of human rabies describing recovery of function during medical care of a nonsurvivor, and that patient was treated similarly to our patient but died of renal failure.3 The referenced patient did not meet our a priori definition of survival and was not reported as a survivor. For a nonsurvivor to regain any neurological function is otherwise unprecedented in the medical literature.
Four rabies vaccine-naive patients have now putatively survived rabies.2,26,27 Confirmation of the diagnosis of the 2011 India survivor is uncertain while the diagnosis of the California and Texas survivors relied on immunofluorescence assays that have been challenged.28 The 2011 California survivor did not mount a RABV-neutralizing antibody response.26 The putative rabies survivor in Texas showed mild symptoms, like the India survivor, and did not show consistent RABV-neutralizing antibody in serum or CSF, like the California survivor.29 There is considerable debate about the California and Texas cases, but there is no acceptable alternative hypothesis. Testing of the California patient for autoimmune disease was extensive. High-volume reference laboratories associated with the California Encephalitis Project and the Centers for Disease Control and Prevention do not encounter positive IFA results for RABV in other encephalitis cases referred for diagnosis, and there are no other known Lyssaviruses in the United States that cross-react by IFA with RABV. However, a new vesiculovirus has been reported in North American bats that might theoretically cross-react.1 The novel vesiculovirus has not been cultivated. Our report provides more robust laboratory proof of concept that rabies vaccine-naive humans may survive clinically severe rabies. Individual case reports are unlikely to elucidate mechanisms associated with survival but may generate testable hypotheses for animal models or therapeutic trials.
The dynamics of RABV clearance in humans are becoming clearer. Partial to complete loss of cultivable virus has been reported in brain 11–22 days after detection of serum neutralizing antibodies >1.0 IU/mL, while RABV antigen was still detectable in 3 of 4 instances.4,7,30,31 In this patient, 67 days after developing a CSF antibody response >1.0 IU/mL, RABV RNA and antigen were still detectable, but isolation of infectious RABV failed. (The failure to detect RABV antigen by IHC while fluorescent antibodies delineated intracytoplasmic inclusions likely relates to the logarithmically higher signal provided by fluorescent probes over conventional tissue stains.) Clearance of RABV antigen (and cultivable virus) was complete in the brain of a patient 127 days after exposure to high titers of neutralizing antibody.5 In mice surviving wildtype rabies, RABV antigen was present for up to 30 days; in a ferret that survived wildtype rabies, RABV antigen was undetectable after 76 days.32,33 RABV antigen was present 5 days longer than infectious virus and viral RNA in murine models of fixed (laboratory adapted) RABV infection.34 These aggregate observations in mice and man support the concept that clinical recovery occurs commensurate with RABV-specific antibody production and loss of infectious virus, while clearance of RABV antigen and RNA from brain takes several weeks after first detection of RABV-neutralizing antibodies.
In typical human rabies cases, intracytoplasmic inclusions are homogeneously distributed throughout brain stem, hippocampus, cerebellum and several other regions of the brain, ranging from 0.25 to 27 μm in size. In this patient, intracytoplasmic inclusions were smaller (0.1–1 μm) and similar to those found in previously vaccinated rabid animals. Changes in intracytoplasmic inclusion size may therefore provide an additional immunological marker of RABV clearance.
In retrospect, this patient was the first of 3 patients with bat-transmitted rabies who developed severe cerebral edema temporally associated with CSF RABV-neutralizing antibody titers at least 10-fold higher than those of the initial survivor (personal communications, G. Sanchez, Lima, Peru; C. Zahlouth, Brasilia, Brazil). A fourth patient, undergoing postexposure prophylaxis at time of onset, had a similar high range of CSF titers without developing severe cerebral edema (personal communication, G. Henriques, Recife, Brazil). In contrast to the disease caused by bat-derived RABV, severe cerebral edema was rarely encountered during the immune response to dog-transmitted RABV (unpublished data, RW). Peak CSF titers of RABV-neutralizing antibody in 18 rabies patients that acquired the infection from dogs were, with 1 exception, at least 100-fold lower than they were in this patient. One patient bitten by a dog, who completed postexposure prophylaxis before onset of rabies, developed similarly high titers in CSF and survived; we do not know whether cerebral edema occurred, but outcome was poor (personal communication, G. Al-Sulaiti, Doha, Qatar.) We hypothesize a possible dose relationship of CNS antibody response in causing cerebral edema, although the vaccinated Brazilian and Qatari survivors suggest that more complex mechanisms may be involved. Cerebral edema in rabies is almost never related to loss of integrity of the blood-brain barrier, so other mechanisms for cerebral edema must be considered.35–38 Antibody-mediated clearance may involve intracellular antibodies to the RABV, so cytoplasmic interaction of antibodies and virus subparticles may cause isotonic volume expansion.39 N-acetylaspartate, secreted by neurons to reduce cellular volume, is markedly elevated in CSF in rabies, supporting a possible disorder of volume regulation by neurons.40 Cerebral edema in our patient was complicated by what appeared to be hyponatremia from renal salt wasting, but salt wasting could not be conclusively diagnosed in this critically ill patient because of concomitant use of diuretics and sodium supplementation. Sympathetic innervation of the kidneys controls natriuresis, while RABV has been detected in proximal tubular cells or urinary sediment and associated with glycosuria, so renal salt wasting is biologically plausible by 2 mechanisms: dysautonomia and renal tubular dysfunction.4,19,41–43 Both cerebral edema and salt wasting lasted weeks.43 Management of hyponatremia in this patient was facilitated by use of enteral sodium supplementation. Based on further experience with other rabies patients, we recommend mineralocorticoids for prophylaxis and treatment of hyponatremia, salt wasting and cerebral vasospasm.23,44,45
This case identifies potential clinical differences in rabies of bat versus carnivore phylogeny that affect medical management. In this patient, early clinical recovery was associated with detection of neutralizing antibody and clearance of infectious (cultivable) RABV in the CNS by 76 days, but not clearance of detectable viral subcomponents such as nucleoprotein antigen or RNA in brain.
Acknowledgments
Supported, in part, by the Zach Jones Foundation and by the NIH 1RO1AI093369 (R.E.W.).
The authors thank the Colombian Ministry of Health, the Pan-American Health Organization-World Health Organization regional office, Martha Perez, MD, Jhon Montes, MD and Sean Turbeville, MD for their assistance during treatment of this patient. The authors thank Brigid C. Batten, MPH, Wun-Ju Shieh, MD, MPH, PhD, Sherif R. Zaki, MD, PhD, Keith Highland, PhD and Charles E. Rupprecht, DVM, PhD, for their assistance with laboratory investigation of this patient.
Footnotes
The authors have no other funding or conflicts of interest to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
References
- 1.Knobel DL, Cleaveland S, Coleman PG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 2.Willoughby RE, Jr, Tieves KS, Hoffman GM, et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352:2508–2514. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]
- 3.Dolman CL, Charlton KM. Massive necrosis of the brain in rabies. Can J Neurol Sci. 1987;14:162–165. doi: 10.1017/s0317167100026329. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DR, Hattwick MA, Gerdsen R, et al. Human rabies. Diagnosis, complications, and management. Am J Dis Child. 1974;127:862–869. doi: 10.1001/archpedi.1974.02110250088013. [DOI] [PubMed] [Google Scholar]
- 5.Emmons RW, Leonard LL, DeGenaro F, Jr, et al. A case of human rabies with prolonged survival. Intervirology. 1973;1:60–72. doi: 10.1159/000148833. [DOI] [PubMed] [Google Scholar]
- 6.McDermid RC, Saxinger L, Lee B, et al. Human rabies encephalitis following bat exposure: failure of therapeutic coma. CMAJ. 2008;178:557–561. doi: 10.1503/cmaj.071326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter M, Johnson N, Hedderwick S, et al. Immunovirological correlates in human rabies treated with therapeutic coma. J Med Virol. 2010;82:1255–1265. doi: 10.1002/jmv.21785. [DOI] [PubMed] [Google Scholar]
- 8.Smith JS, Yager PA, Baer GM. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibody. In: Meslin F-X, Kaplan MM, Koprowski H, editors. Laboratory Techniques in Rabies. 4. Geneva, Switzerland: World Health Organization; 1996. pp. 181–191. [Google Scholar]
- 9.Feyssaguet M, Dacheux L, Audry L, et al. Multicenter comparative study of a new ELISA, Platelia Rabies II, for the detection and titration of anti-rabies glycoprotein antibodies and comparison with the rapid fluorescent focus inhibition test (RFFIT) on human samples from vaccinated and non-vaccinated people. Vaccine. 2007;25:2244–2251. doi: 10.1016/j.vaccine.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Lee TK, Hutchinson HD, Ziegler DW. Comparison of rabies humoral antibody titers in rabbits and humans by indirect radioimmunoassay, rapid-fluorescent-focus-inhibition technique, and indirect fluorescent-antibody assay. J Clin Microbiol. 1977;5:320–325. doi: 10.1128/jcm.5.3.320-325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orciari LA, Rupprecht CE. Rabies Virus. Manual of Clinical Microbiology. Washington, D.C: ASM Press; 2011. pp. 1470–1480. [Google Scholar]
- 12. [Accessed January 6, 2015];Protocol for post-mortem diagnosis of rabies in animals by direct fluorescent antibody testing: minimum national standard. 2001 Available at: http://www.cdc.gov/rabies/pdf/rabiesdfaspv2.pdf.
- 13.Markotter W, Kuzmin I, Rupprecht CE, et al. Isolation of Lagos bat virus from water mongoose. Emerg Infect Dis. 2006;12:1913–1918. doi: 10.3201/eid1212.060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JS. Rabies virus. In: Murray PR, Baron EJ, Pfaller MA, et al., editors. Manual of Clinical Microbiology. Washington, D.C: ASM Press; 1995. pp. 997–1003. [Google Scholar]
- 15.Smith JS. In: Molecular epidemiology. 1. Jackson AC, Wunner WH, editors. New York, NY: Academic Press; 2002. pp. 79–111. [Google Scholar]
- 16.Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trimarchi CV, Smith JS. Diagnostic evaluation. In: Jackson AC, Wunner WH, editors. Rabies. 1. San Diego, CA: Academic Press; 2002. pp. 307–350. [Google Scholar]
- 18.Lembo T, Niezgoda M, Velasco-Villa A, et al. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg Infect Dis. 2006;12:310–313. doi: 10.3201/eid1202.050812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan A, Burton EC, Kuehnert MJ, et al. Rabies in Transplant Recipients Investigation Team. Transmission of rabies virus from an organ donor to four transplant recipients. N Engl J Med. 2005;352:1103–1111. doi: 10.1056/NEJMoa043018. [DOI] [PubMed] [Google Scholar]
- 20.Willoughby RE., Jr [Accessed January 6, 2015];Rabies treatment protocol and registry. 2013 Available at: www.mcw.edu/rabies.
- 21.Willoughby RE, Opladen T, Maier T, et al. Tetrahydrobiopterin deficiency in human rabies. J Inherit Metab Dis. 2009;32:65–72. doi: 10.1007/s10545-008-0949-z. [DOI] [PubMed] [Google Scholar]
- 22.Feder HM, Jr, Petersen BW, Robertson KL, et al. Rabies: still a uniformly fatal disease? Historical occurrence, epidemiological trends, and paradigm shifts. Curr Infect Dis Rep. 2012;14:408–422. doi: 10.1007/s11908-012-0268-2. [DOI] [PubMed] [Google Scholar]
- 23.Willoughby RE., Jr [Accessed August 12, 2008];Rabies treatment protocol and registry. 2011 Available at: www.mcw.edu/rabies.
- 24.Diaz M, Neuhauser D. Pasteur and parachutes: when statistical process control is better than a randomized controlled trial. Qual Saf Health Care. 2005;14:140–143. doi: 10.1136/qshc.2005.013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aramburo A, Willoughby RE, Bollen AW, et al. Failure of the Milwaukee protocol in a child with rabies. Clin Infect Dis. 2011;53:572–574. doi: 10.1093/cid/cir483. [DOI] [PubMed] [Google Scholar]
- 26.Wiedeman J, Plant J, Glaser C, et al. Recovery of a patient from clinical rabies—California, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:61–65. [PubMed] [Google Scholar]
- 27.Rawat AK, Rao SK. Survival of a rabies patient. Indian Pediatr. 2011;48:574. [PubMed] [Google Scholar]
- 28.Rudd RJ, Appler KA, Wong SJ. Presence of cross-reactions with other viral encephalitides in the indirect fluorescent-antibody test for diagnosis of rabies. J Clin Microbiol. 2013;51:4079–4082. doi: 10.1128/JCM.01818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzmann-Pazgal G, Wanger A, Degaffe G, et al. Presumptive abortive human rabies—Texas, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:185–190. [PubMed] [Google Scholar]
- 30.Maton PN, Pollard JD, Davis JN. Human rabies encephalomyelitis. Br Med J. 1976;1:1038–1040. doi: 10.1136/bmj.1.6017.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin RH, Sullivan L, Summers R, et al. A case of human rabies in Kansas: epidemiologic, clinical, and laboratory considerations. J Infect Dis. 1970;122:318–322. doi: 10.1093/infdis/122.4.318. [DOI] [PubMed] [Google Scholar]
- 32.Lodmell DL, Bell JF, Moore GJ, et al. Comparative study of abortive and nonabortive rabies in mice. J Infect Dis. 1969;119:569–580. doi: 10.1093/infdis/119.6.569. [DOI] [PubMed] [Google Scholar]
- 33.Hamir AN, Niezgoda M, Rupprecht CE. Recovery from and clearance of rabies virus in a domestic ferret. J Am Assoc Lab Anim Sci. 2011;50:248–251. [PMC free article] [PubMed] [Google Scholar]
- 34.Hooper DC, Morimoto K, Bette M, et al. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72:3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabis MJ, Phares TW, Kean RB, et al. Blood-brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. Proc Natl Acad Sci U S A. 2008;105:15511–15516. doi: 10.1073/pnas.0807656105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A, Hooper DC. Immune evasion by rabies viruses through the maintenance of blood-brain barrier integrity. J Neurovirol. 2008;14:401–411. doi: 10.1080/13550280802235924. [DOI] [PubMed] [Google Scholar]
- 37.Laothamatas J, Wacharapluesadee S, Lumlertdacha B, et al. Furious and paralytic rabies of canine origin: neuroimaging with virological and cytokine studies. J Neurovirol. 2008;14:119–129. doi: 10.1080/13550280701883857. [DOI] [PubMed] [Google Scholar]
- 38.Laothamatas J, Hemachudha T, Mitrabhakdi E, et al. MR imaging in human rabies. Am J Neuroradiol. 2003;24:1102–1109. [PMC free article] [PubMed] [Google Scholar]
- 39.Dietzschold B, Kao M, Zheng YM, et al. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc Natl Acad Sci U S A. 1992;89:7252–7256. doi: 10.1073/pnas.89.15.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Sullivan A, Willoughby RE, Mishchuk D, et al. Metabolomics of cerebrospinal fluid from humans treated for rabies. J Proteome Res. 2013;12:481–490. doi: 10.1021/pr3009176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duenas A, Belsey MA, Escobar J, et al. Isolation of rabies virus outside the human central nervous system. J Infect Dis. 1973;127:702–704. doi: 10.1093/infdis/127.6.702. [DOI] [PubMed] [Google Scholar]
- 42.Warrell DA. The clinical picture of rabies in man. Trans R Soc Trop Med Hyg. 1976;70:188–195. doi: 10.1016/0035-9203(76)90037-7. [DOI] [PubMed] [Google Scholar]
- 43.Palmer BF. Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endocrinol Metab. 2003;14:182–187. doi: 10.1016/s1043-2760(03)00048-1. [DOI] [PubMed] [Google Scholar]
- 44.Moro N, Katayama Y, Kojima J, et al. Prophylactic management of excessive natriuresis with hydrocortisone for efficient hypervolemic therapy after subarachnoid hemorrhage. Stroke. 2003;34:2807–2811. doi: 10.1161/01.STR.0000103744.05430.99. [DOI] [PubMed] [Google Scholar]
- 45.Hasan D, Lindsay KW, Wijdicks EF, et al. Effect of fludrocortisone acetate in patients with subarachnoid hemorrhage. Stroke. 1989;20:1156–1161. doi: 10.1161/01.str.20.9.1156. [DOI] [PubMed] [Google Scholar]