Abstract
Objective
Our objective was to identify perinatal risk factors that are available within 1 hour of birth that are associated with severe brain injury after hypothermia treatment for suspected hypoxic-ischemic encephalopathy.
Study Design
One hundred nine neonates at ≥35 weeks' gestation who were admitted from January 2007 to September 2012 with suspected hypoxic-ischemic encephalopathy were treated with whole-body hypothermia; 98 of them (90%) underwent brain magnetic resonance imaging (MRI) at 7-10 days of life. Eight neonates died before brain imaging. Neonates who had severe brain injury, which was defined as death or abnormal MRI results (cases), were compared with surviving neonates with normal MRI (control subjects). Logistic regression models were used to identify risk factors that were predictive of severe injury.
Results
Cases and control subjects did not differ with regard to gestational age, birthweight, mode of delivery, or diagnosis of non-reassuring fetal heart rate before delivery. Cases were significantly (P ≤ .05) more likely to have had an abruption, a cord and neonatal arterial gas level that showed metabolic acidosis, lower platelet counts, lower glucose level, longer time to spontaneous respirations, intubation, chest compressions in the delivery room, and seizures. In multivariable logistic regression, lower initial neonatal arterial pH (P = .004), spontaneous respiration at >30 minutes of life (P = .002), and absence of exposure to oxytocin (P = .033) were associated independently with severe injury with 74.3% sensitivity and 74.4% specificity.
Conclusion
Worsening metabolic acidosis at birth, longer time to spontaneous respirations, and lack of exposure to oxytocin correlated with severe brain injury in neonates who were treated with whole-body hypothermia. These risk factors may help quickly identify neonatal candidates for time-sensitive investigational therapies for brain neuroprotection.
Keywords: brain injury, hypoxic-ischemic encephalopathy, magnetic resonance imaging, neonate, whole-body hypothermia
Despite advances in perinatal medicine during the past several decades, the incidence of cerebral palsy attributed to intrapartum asphyxia has remained unchanged.1 The incidence of hypoxic-ischemic encephalopathy (HIE) has remained steady at 1-2 cases per 1000 term births, despite the increasing use of antepartum fetal testing in high-risk pregnancies and intrapartum electronic fetal heart rate monitoring.2,3 Although antepartum testing and electronic fetal monitoring have been unable to decrease the incidence of HIE, some studies have found an association between antepartum and intrapartum risk factors and pediatric neurologic morbidity.4-8 A population-based study of all term infants with newborn encephalopathy in Western Australia found that 69% had only antepartum risk factors, that 24% had antepartum and intrapartum risk factors, and that 5% had only intrapartum risk factors.5 The causes of newborn encephalopathy were heterogeneous, but only 2% of the infants had no recognized antepartum or intrapartum risk factors. Although antenatal risk factors may be present, a prospective study of 351 term neonates with encephalopathy that used magnetic resonance imaging (MRI) or postmortem examination concluded that events in the immediate perinatal period are most important in neonatal brain injury, which has led some investigators to conclude that brain injury in most newborn infants with encephalopathy occurs at or near the time of birth and may be amenable to postnatal interventions.9,10
Although many therapeutic interventions are being studied to help mitigate long-term morbidity and death in neonates with HIE, hypothermia is the only widely accepted therapy at this time.11 More than 1500 neonates have been included in a metaanalysis of the 6 major hypothermia studies, and many more thousands around the world have been treated because hypothermia has become the standard of care in HIE treatment.12 Although a standard 72-hour course of hypothermia appears safe and effective, nearly one-half the neonates who are treated will have death or significant morbidity by childhood.13 Because HIE is not a single disease with a single cause but rather is characterized by great diversity in the timing and magnitude of brain injury, it is unreasonable to expect any single intervention to provide uniformly favorable outcomes.14 With hypothermia, intervention has proved effective only when implemented within 6 hours of birth, and it is possible that there is a gradation of therapeutic effect with time of implementation of hypothermia during this 6-hour period.15
A recent systematic review and meta-analysis found that newborn infants with severe HIE show less benefit from therapeutic hypothermia than those with moderate HIE (relative risk reduction: 17% severe vs 33% moderate).16 Immediate/early identification of those neonates with the most severe injury is of paramount importance to improve outcomes. HIE in term neonates has been associated with injury affecting predominately the cerebral cortex and subcortical white matter due to “prolonged partial” asphyxia, and injury affecting predominately the deep gray nuclei, thalamus, and basal ganglia to “acute total” perinatal asphyxia.10,17
MRI is a sensitive, specific, and well-accepted imaging modality for the evaluation of brain injury after the first week of life. Serial neuroimaging has shown that brain injury evolves over days, if not weeks, which means that early conventional MRI is not the ideal modality for the prediction of encephalopathy in the critical first few hours after birth, because lesions do not become visible until 7-10 days of life.10,18-20 Diffusion tensor imaging and magnetic resonance spectroscopic imaging may be able to identify brain lesions within the first 3 days of life,21 but affected neonates are usually too sick to take out of the neonatal intensive care unit (NICU) at this time, and many are born in hospitals where even conventional MRI is not available.
Newer investigational treatments such as longer and deeper hypothermia regimens and other therapies including erythropoietin, xenon, antiepileptic drugs, and neural stem cells may benefit the most severely injured neonates if they can be identified accurately soon after birth and treated as the injury evolves.22 Animal models of HIE have demonstrated a neuroprotective effect of erythropoietin, as have small human studies without hypothermia.23-25 Recently, a multicenter phase I trial was conducted that involved 24 neonates with moderate-to-severe encephalopathy to test the safety of erythropoietin in conjunction with hypothermia.26 Further studies with other pharmacologic interventions in conjunction with hypothermia are ongoing, and the armamentarium of neuroprotective strategies should increase in the coming years.11 We hypothesize that there are risk factors present within 1 hour of birth that may help to identify neonates with severe brain injury that result from HIE that qualify for treatment with whole-body hypothermia or other experimental therapies. Our objective was to identify those perinatal risk factors that are present within the first hour of life that identify the most severely injured neonates as evidenced by abnormal MRI at 7-10 days of life or death.
Materials and Methods
Subjects
We conducted a case-control study of all neonates with suspected HIE who were admitted to our NICU for treatment with whole-body hypothermia from January 2007 to September 2012. This study was approved by our medical center Institutional Review Board. To qualify for whole-body hypothermia neonates had to be admitted and begin cooling within 6 hours of birth. Neonates were eligible for treatment with whole-body hypothermia if moderate-severe encephalopathy27 was present at birth that manifested as lethargy, stupor, coma, decreased or no activity, distal flexion, complete extension, decerebrate posture, hypotonia or flaccidity, abnormal primitive reflexes, bradycardia, periodic breathing, apnea, or seizures. Infants were eligible for whole-body hypothermia if they had a cord gas or early neonatal gas at < 1 hour with pH ≤7.0, a base deficit >16 mM, cord/early neonatal gas at <1 hour with pH 7.01–7.15 and base deficit 10–15.9 mM (if moderate-severe encephalopathy was present with evidence of an acute sentinel event and a 10-minute Apgar score <5), or a need for assisted ventilation that was initiated at birth with continuation for at least 10 minutes. A sentinel event was defined as a signal event that occurred immediately before or during labor that could lead to fetal hypoxia such as ruptured uterus, abruption, massive fetomaternal hemorrhage, umbilical cord prolapse, ruptured vasa previa, amniotic fluid embolism, shoulder dystocia, or maternal cardiopulmonary arrest.28 Exclusion criteria included >6 hours of life, gestational age <35 weeks, severe growth restriction (birthweight, <1800 g), major congenital anomaly, severe persistent pulmonary hypertension with an anticipated need for extracorporeal membrane oxygenation, coagulopathy with active bleeding, and suspected sepsis with severe hemodynamic compromise that required large doses of pressors. Those neonates who were transported from outside institutions were started on passive cooling immediately on recognition of need for therapeutic hypothermia with instructions for temperature goal. All neonates were cooled to a rectal temperature of 33.5°C for 72 hours. After 72 hours of whole-body hypothermia, warming was initiated by increasing the set point of the automatic control on the servomechanism of the hypothermia system by 0.5°C per hour, which warmed the cooled blanket by 0.5°C per hour. All children who were enrolled in the hypothermia protocol were evaluated by a pediatric neurologist within 18 hours.
Infant and maternal medical records were reviewed to identify relevant clinical data. Intrauterine growth restriction was defined as an estimated fetal weight <10th percentile.29 Oligohydramnios was defined as an amniotic fluid index <5.0 cm with intact membranes at the time of the admission in which the delivery occurred. Preeclampsia was defined as proteinuria, edema, and the presence of new onset hypertension. The clinical diagnosis of chorioamnionitis was made in the presence of maternal fever, with at least 1 other finding of fetal tachycardia, uterine tenderness, or purulent vaginal discharge. Patients who were diagnosed with clinical chorioamnionitis were started immediately on intravenous antibiotics. Sepsis was considered present only for neonates with positive blood and/or cerebrospinal fluid cultures. The diagnosis of nonreassuring fetal heart rate tracing was made by the physician who attended the delivery before performing a vacuum, forceps, or cesarean delivery.
Imaging
A brain MRI with diffusion tensor images was performed between day of life 7 and 10. Cases had severe brain injury, defined as an abnormal brain MRI or death. Control subjects were surviving neonates with normal MRI. These MRIs were reviewed by an experienced pediatric neuroradiologist at our institution (T.A.G.M.H.). The images were reviewed for focal or diffuse lesions related to hypoxic-ischemic injury. Neuroimaging abnormalities of an acute perinatal insult were defined as brain swelling; cortical highlighting; focal or global loss of grey-white matter differentiation; abnormal signal intensity in the basal ganglia and thalami; loss of normal signal intensity in the posterior limb of the internal capsule; acute and subacute parenchymal, intraventricular, or extracerebral hemorrhage; and acutely evolving focal infarction in an arterial territory or in a parasagittal or watershed distribution.9
Statistics
Univariate analysis was performed with the Student t test to evaluate continuous variables, and χ2 or Fisher exact test for categoric variables. A probability value of < .05 was considered statistically significant. Logistic regression models were created with forward stepwise selection of risk variables with a probability value of < .1 and biologic plausibility after elimination of similar confounding variables. Models were used to determine the predictive value of factors that were hypothesized to impact severe brain injury. Receiver operating characteristic curves were generated, and sensitivity and specificity were calculated. Stata software was used for statistical analysis (version 10; StataCorp LP, College Station, TX). Cross-validation analysis was performed by dividing the participants evenly into 5 groups, combining 80% of the participants, or 4 groups at a time, and creating a model based on those 4 groups. Logistic regression was then run on the aggregate of 4 groups and used to predict the outcome for the fifth group. This process was repeated until each group had been the test group.
Results
During this 5-year 8-month period, there were 109 neonates admitted to our NICU with suspected HIE for treatment with whole-body hypothermia; 39% were born at 1 of 2 hospitals within our system, and 61% were transferred from outside institutions within our state. Of these 109 neonates, 98 (90%) had an MRI performed at 7-10 days of life; 8 died without having imaging; 1 had a computed tomography scan, and 2 survivors were discharged before having an MRI performed. Fifteen neonates were missing data on the commencement of spontaneous respirations, and 2 neonates were missing Apgar scores. The 2 patients for whom we are missing Apgar scores were both born unintentionally at home. Cord pH at birth was available for 84.7% of cases and 85.2% of control subjects; cord base deficit was available for 71.7% of cases and 82.0% of control subjects. Cord gases were not always done routinely at birth in referring institutions. Of the 8 neonates who died without imaging, 6 were emergent cesarean deliveries for nonreassuring fetal heart rate tracing; 3 had sentinel events that included abruption, uterine rupture, and maternal cardiac arrest, and 5 had seizures. The 8 neonates who died before having an MRI were diagnosed with Sarnat stage III encephalopathy before starting cooling within 6 hours of life. The 46 cases with an abnormal brain MRI or death were compared with 60 survivors with a normal MRI. In the case group, cerebral white matter injury occurred in 29 of 46 neonates (63%), and basal ganglia/thalamic injury occurred in 21 neonates (45.7%). Areas of infarcted white matter were noted in 6 neonates (13%). Extracerebral hemorrhage occurred in 10 neonates (21.7%): 9 had subdural hematoma, and 1 had cephalohematoma, all of which were associated with either cerebral white matter and/or basal ganglia injury. Acute and subacute parenchymal and intraventricular hemorrhage occurred in 7 neonates (15.2%), always in conjunction with other abnormalities.
In univariate analysis, cases and control subjects did not differ on gestational age, sex, race, birthweight, mode of delivery, sentinel events, positive neonatal blood cultures, or diagnosis of nonreassuring fetal heart rate tracing before delivery (Tables 1 and 2). There were 33 cesarean deliveries performed in the case group, of which 29 deliveries were done for nonreassuring fetal heart rate tracing, 1 emergency cesarean delivery was done after a motor vehicle accident, 1 emergency cesarean delivery was done after a maternal seizure, 1 cesarean delivery was done after a failed vacuum delivery of a 4140-g infant, and 1 cesarean delivery was done for a previous myomectomy at which time there was difficulty extracting the infant for 10 minutes, which required vacuum assistance and the extension of the uterine incision. There were 35 cesarean deliveries in the control group, of which 30 were done for nonreassuring fetal heart rate tracing, 1 was an elective repeat cesarean during which the placenta was incised during delivery, 1 for arrest with chorioamnionitis and fetal tachycardia, and 3 for arrest with unremarkable fetal heart rate tracings. There was 1 forceps delivery in the case group and 3 in the control subjects, all with nonreassuring fetal heart rate tracing noted before delivery. There were 2 vacuum deliveries in the case group, both with nonreassuring fetal heart rate tracing; there were 6 vacuum deliveries in the control subjects of which 3 infants had nonreassuring fetal heart rate tracing. Of those infants with meconium-stained fluid noted at delivery, 4 of 17 cases (24%) and 9 of 25 control subjects (36%) were noted to have thick meconium (P = .39). Cases were significantly more likely to have had an abruption (P = .03), 5-minute Apgar score <5 (P = .049), higher initial white blood cell count (P = .025), lower initial neonatal platelet counts (P = .03), require chest compressions (P = .02) and intubation (P = .02) in the delivery room, first spontaneous respiration at >30 minutes of life (P = .001), and seizures (P = .03; Tables 1 and 2). Cases were significantly more likely to have metabolic acidosis with pH <7.0 and a base deficit of > 12 mM on the cord gas at birth or the initial neonatal arterial gas. The incidence of the initial neonatal arterial pH being <7.0 was 20 of 46 for the cases (43.5%) and 8 of 60 for the control subjects (13.3%; P = .0005).
Table 1. Univariate analysis: comparison of maternal variables.
| Variable | Cases (n = 46) | Control subjects (n = 60) | P value |
|---|---|---|---|
| Maternal age, y | 29.1 ± 8.2 | 26.5 ± 6.8 | .08 |
| Nulliparous, n (%) | 27 (59) | 34 (57) | .80 |
| Race, n (%) | .80 | ||
| White | 19 (41) | 25 (42) | |
| Black | 20 (43) | 29 (48) | |
| Hispanic | 3 (7) | 3 (5) | |
| Other | 4 (9) | 3 (5) | |
| Cesarean delivery, n (%) | 33 (72) | 35 (58) | .45 |
| Multiple birth, n (%) | 0 | 1 (2) | 1.00 |
| Preterm premature rupture of membranes, n (%) | 0 | 1 (2) | 1.00 |
| Preeclampsia, n (%) | 3 (7) | 7 (12) | .51 |
| Antenatal magnesium, n (%) | 2 (4) | 2 (3) | 1.00 |
| Tobacco use, n (%) | 2 (4) | 1 (2) | .58 |
| Cocaine use, n (%) | 2 (4) | 4 (7) | .70 |
| Intrauterine growth restriction, n (%) | 3 (7) | 5 (8) | 1.00 |
| Oligohydramnios, n (%) | 2 (4) | 3 (5) | 1.00 |
| Abruption, n (%) | 12 (26) | 6 (10) | .03a |
| Meconium, n (%) | 17 (37) | 25 (42) | .69 |
| Oxytocin for labor induction/augmentation, n (%) | 8 (17) | 21 (35) | .05 |
| Nonreassuring fetal heart rate tracing, n (%) | 32 (70) | 37/58 (64) | .40 |
| Sentinel event, n (%) | 20 (43) | 23 (38) | .59 |
| Clinical chorioamnionitis, n (%) | 5 (11) | 9 (15) | .53 |
Among all neonates with suspected hypoxic-ischemic encephalopathy who were treated with whole-body hypothermia, univariate analysis compared maternal variables between cases with abnormal brain imaging or death and control subjects who survived with normal brain imaging.
P < .05.
Table 2. Univariate analysis: comparison of neonatal variables.
| Variable | Cases (n = 46) | Control subjects (n = 60) | P value |
|---|---|---|---|
| Gestational age, wk | 38.6 ± 1.5 | 39.0 ± 1.7 | .17 |
| Birthweight, g | 3224 ± 659 | 3256 ± 597 | .79 |
| Female sex, n (%) | 18 (39) | 22 (37) | .80 |
| 5-minute Apgar score <5, n (%) | 34 (74) | 32 (55; n = 58) | .049a |
| Cord pH | 6.85 ± 0.15 (n = 39) | 6.98 ± 0.14 (n = 52) | < .0001a |
| Cord base deficit, mM | 19.6 ± 6.6 (n = 33) | 13.5 ± 7.5 (n = 50) | .0003a |
| Cord pH <7.0 and base deficit >12 mM, n (%) | 36 (88; n = 41) | 37 (70; n = 53) | .038a |
| First arterial pH | 7.05 ± 0.20 | 7.17 ± 0.15 | .0009a |
| First arterial base deficit, mM | 20.5 ± 9.5 (n = 45) | 15.4 ± 7.9 | .0035a |
| Neonatal length of stay, d | 21.3 ± 15.8 | 15.9 ± 9.2 | .03a |
| Respiratory distress, n (%) | 13 (28) | 24 (40) | .21 |
| Intraventricular hemorrhage, n (%) | 2 (4) | 1 (2) | .58 |
| Hyperbilirubinemia, n (%) | 4 (9) | 12 (20) | .17 |
| Seizures, n (%) | 25 (54) | 20 (33) | .03a |
| Initial neonatal white blood cell count, n | 20,694 ± 8493 | 17,190 ± 7301 | .025a |
| Initial neonatal platelets, K/cu mm | 158 ± 49 | 181 ±58 | .03a |
| Initial neonatal hematocrit, % | 47.2 ± 8.1 | 44.4 ± 8.1 | .08 |
| Glucose level, mg/dL | 124 ± 130 | 108 ± 63 | .43 |
| Days to oral feeding | 29.1 ± 61.6 (n = 29) | 12.9 ± 20.1 (n = 59) | .07 |
| Chest compressions in delivery room, n (%) | 23 (50) | 17 (28) | .02a |
| First respiration at >30 minutes, n (%) | 29 (74; n = 39) | 21 (40; n = 52) | .001 |
| Intubation in delivery room, n (%) | 40 (87) | 40 (67) | .02a |
| Positive blood culture, n (%) | 3 (6) | 1 (2) | .31 |
Among all neonates with suspected hypoxic-ischemic encephalopathy who were treated with whole-body hypothermia, univariate analysis compared neonatal variables between cases with abnormal brain imaging or death and control subjects who survived with normal brain imaging.
P < .05.
Additional variables with a probability value of < .1 after univariate analysis included maternal age (P = .08), absence of intrapartum exposure to oxytocin (P = .05), and neonatal length of stay (P = .03). Cord pH, cord base deficit, and initial neonatal arterial base deficit were not included in the multivariable regression model because of confounding with initial neonatal arterial pH. Initial neonatal arterial blood gas was chosen over cord blood gas because more umbilical cord data were missing. Intubation in the delivery room was not included because it is performed routinely when chest compressions are administered and may impact spontaneous respirations. Given that our goal was to examine early predictors of neurologic injury (within 1 hour of life), neonatal length of stay was not included in the model. Therefore, the following variables were included in the multivariable analysis: maternal age; oxytocin exposure in labor; abruption; 5-minute Apgar score <5; chest compressions in delivery room; initial neonatal arterial pH; initial neonatal white blood cell count, platelets, and hematocrit level; neonatal seizures, and spontaneous respirations at >30 minutes.
In multivariable logistic regression, initial neonatal arterial pH (P = .004), spontaneous respiration at >30 minutes of life (P = .002), and absence of intrapartum exposure to oxytocin (P = .033) predicted a significantly increased risk of abnormal brain MRI at 7-10 days or death (Tables 3 and 4). All patients had contractions, and the risk of abruption was significantly lower for patients who received intrapartum oxytocin (1/30 women; 3.3%), compared with those without oxytocin exposure (17/61 women; 27.9%; P = .02). For those women without intrapartum exposure to oxytocin, the cesarean delivery rate was 50 of 77 women (64.9%); for those women who were exposed to oxytocin, the cesarean delivery rate was 18 of 29 women (62.1%), which was not significantly different (P = .78) and showed that the threshold for performing a cesarean delivery seems to be roughly equivalent in both groups.
Table 3. Multivariable logistic regression analysis.
| Variable | Coefficient | 95% CI | P value |
|---|---|---|---|
| Maternal age | 0.01 | −0.003 to 0.023 | .13 |
| Intrapartum oxytocin exposure | −0.23 | −0.44 to −0.01 | .04a |
| Abruption | 0.07 | −0.014 to 0.157 | .16 |
| 5-minute Apgar score <5 | −0.0001 | −0.24 to 0.24 | 1.00 |
| Chest compressions in delivery room | −0.06 | −0.31 to 0.19 | .65 |
| Initial neonatal arterial pH | −0.73 | −1.28 to −0.18 | .01a |
| Initial neonatal white blood cells | 8.78e–06 | −3.61e–06, 2.12e–05 | .16 |
| Initial neonatal hematocrit level | 0.007 | −0.005 to 0.019 | .24 |
| Initial neonatal platelets | −0.002 | −0.0033 to 0.0002 | .08 |
| Neonatal seizures | 0.08 | −0.12 to 0.28 | .43 |
| Spontaneous respirations at >30 min | 0.27 | 0.05–0.50 | .02a |
Multivariable logistic regression analysis to determine whether variables with a probability value of < .10 on univariate analysis are associated with abnormal brain imaging or death in neonates with suspected hypoxic-ischemic encephalopathy who were treated with whole-body hypothermia.
CI, confidence interval.
P< .05.
Table 4. Stepwise multivariable logistic regression analysis.
| Variable | Odds ratio | 95% CI | P valuea |
|---|---|---|---|
| Spontaneous respirations at >30 min | 5.24 | 1.88–14.6 | .002 |
| Initial neonatal arterial pH | 0.02 | 0.0009–0.26 | .004 |
| Pitocin exposure intrapartum | 0.26 | 0.075–0.90 | .033 |
Stepwise multivariable logistic regression analysis to determine whether variables with a probability value of < .10 on univariate analysis are associated with abnormal brain imaging or death in neonates with suspected hypoxic-ischemic encephalopathy who were treated with whole-body hypothermia.
CI, confidence interval.
P < .05.
The receiver operating characteristic curve for the model that included these 3 variables revealed an area under the curve of 0.797 (Figure) and had a maximal ability to predict severe brain injury with a sensitivity of 74.3% and specificity of 74.4%. Results of the internal validation confirmed our findings with the same sensitivity and specificity.
Figure. Receiver operating characteristic curve.
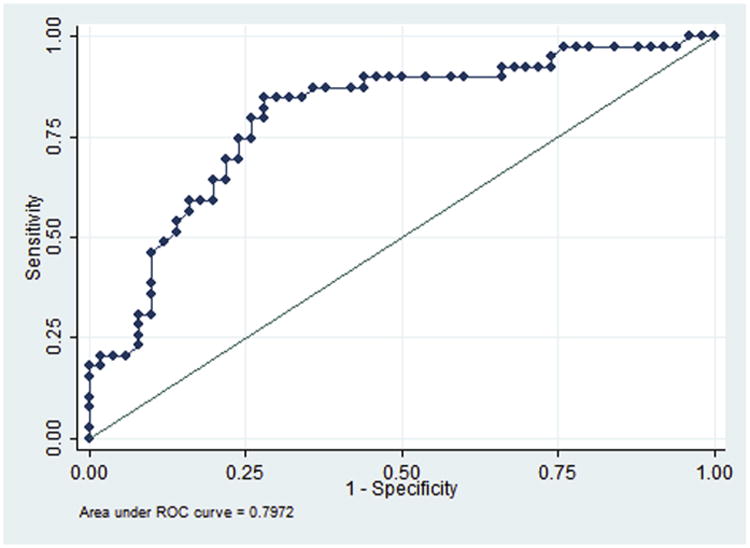
Receiver operating characteristic (ROC) curve for multivariable logistic regression that used initial neonatal arterial pH, spontaneous respiration at >30 minutes of life, and absence of exposure to oxytocin to identify abnormal brain magnetic resonance imaging at 7-10 days or death.
Comment
The early perinatal factors of initial neonatal arterial pH, time to spontaneous respirations, and absence of intrapartum exposure to oxytocin were associated with 70% of neonates who were admitted for hypothermia treatment and who had severe brain injury, as evidenced by an abnormal brain MRI result at 7-10 days of life or death. The identification of these severely injured neonates could be complete within 1 hour of life, and they could be triaged to more intensive neuroprotective therapy beyond the current standard 72 hours of hypothermia. Our study demonstrates the importance of the magnitude of fetal and neonatal acidosis in the risk of brain injury related to HIE. Although an umbilical arterial pH at birth frequently is used to measure perinatal asphyxia, existing reports of the association between umbilical arterial pH and adverse outcome are conflicting. However, a recent systematic review and meta-analysis found that low umbilical arterial pH showed strong, consistent, and temporal associations with clinically important neonatal outcomes that are biologically plausible.30 They found the most substantial association at a pH threshold of 7.00; the odds ratio for an association between umbilical arterial pH and HIE was 13.8 (95% confidence interval [CI], 6.6–28.9), for seizures was 8.1 (95% CI, 3.0–21.9), for intraventricular hemorrhage or periventricular leukomalacia was 2.9 (95% CI, 2.1–4.1), and for cerebral palsy was 2.3 (95% CI, 1.3–4.2). Shah et al31 and Ambalavanan et al32 have also demonstrated the importance of metabolic acidosis in predicting neonatal outcomes, looking specifically at the magnitude of base deficit. The most severely injured babies in our study not only had a higher degree of metabolic acidosis in cord blood at birth but also continued to be severely acidotic at the time of the initial neonatal arterial blood gas sample. We found that the time to spontaneous respirations was predictive of severe brain injury, as was shown in the model developed by Shah et al.31 In both our model and that of Shah et al,31 the most severely injured neonates did not have spontaneous respirations until >30 minutes of life.
Another variable that was found to be significantly predictive of injury in our model was the absence of intrapartum exposure to oxytocin. Intrapartum exposure to oxytocin was protective of adverse neurologic outcome as defined by abnormal brain imaging at 7-10 days or death (odds ratio, 0.28; 95% CI, 0.08–0.95). This finding differs significantly from the finding of increased risk of birth asphyxia as defined by a 5-minute Apgar score <7 in those neonates who had been exposed to oxytocin augmentation (odds ratio, 2.9; 95% CI, 1.4–6.3) in a retrospective study by Milsom et al.33 Compared with the study by Milsom et al, our study used more stringent criteria for neurologic injury by evaluating objective neuroimaging as opposed to a 5-minute Apgar score <7. Neonates who were exposed to intrapartum oxytocin in our study fared better than those without exposure. This finding agrees with an Irish case-control study of 237 cases of HIE compared with 489 control subjects that found that the induction of labor by any method did not increase the odds of encephalopathy.34 Although the finding that oxytocin exposure is protective from neurologic injury may seem counterintuitive, this may be related to the significantly lower risk of abruption in patients who received oxytocin in this population. The Irish study found a number of factors that were associated with HIE that included meconium-stained fluid, intrauterine growth restriction, oligohydramnios, male sex, fetal bradycardia, and maternal pyrexia.34 These variables were not associated with severe neurologic injury in our study. Current obstetric practice places a great reliance on electronic fetal heart rate monitoring, despite poor specificity.35 Our study also found that nonreassuring fetal heart rate tracings, as defined by the obstetrician who managed the labor, as an indication for operative delivery with forceps, vacuum, or emergent cesarean delivery, which occurred in 70% of cases and 62% of control subjects, was not associated with abnormal brain MRI or death.
Our study has several limitations. It is important to understand that predictive modeling in any study population may not be as accurate in a different population. The diversity of our patient population from both the university and surrounding private hospitals is both a strength and weakness of our study in that the applicability of the results are improved, but some data were not recorded uniformly in all the medical records at all institutions. Data were not complete for our entire cohort because we were missing data on the time of spontaneous respirations for 15 outborn neonates, on Apgar scores for 2 control babies who were born at home, on days to oral feeding for 8 babies, and complete umbilical cord gas values. The use of neonatal blood gas values, although important, is not as ideal as umbilical artery blood gas to present the clearest picture of fetal status at the time of birth. Most of our patients were resuscitated partially to varying degrees before having neonatal arterial blood gas drawn. A strength of our study is that it is a relatively large single institution population of term and near-term neonates with suspected HIE, which is fortunately a rare perinatal outcome in a resource-rich setting, which ensures consistent clinical treatment of the infants once they reached the referral center.
Newer therapies that target these at-risk neonates are being developed and are undergoing clinical trials at the time of this study. The identification of at-risk neonates soon after birth will allow for the implementation of such strategies that may improve the neurologic outcome for this vulnerable patient population. These risk factors may help to identify an at-risk neonate within 1 hour of birth that may benefit from the initiation of individualized cooling therapy in the delivery room and additional therapies that could prevent life long disability.
Footnotes
The authors report no conflict of interest.
Presented in poster format at the 33rd annual meeting of the Society for Maternal-Fetal Medicine, San Francisco, CA, Feb. 11-16, 2013.
Reprints not available from the authors.
References
- 1.Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006;117(suppl):S28–33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- 2.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 3.Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;5:CD006066. doi: 10.1002/14651858.CD006066. [DOI] [PubMed] [Google Scholar]
- 4.Badawi N, Kurinczuk JJ, Keogh JM, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1549–53. doi: 10.1136/bmj.317.7172.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badawi N, Kurinczuk JJ, Keogh JM, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1554–8. doi: 10.1136/bmj.317.7172.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter BS, McNabb F, Merenstein GB. Prospective validation of a scoring system for predicting neonatal morbidity after acute perinatal asphyxia. J Pediatr. 1998;132:619–23. doi: 10.1016/s0022-3476(98)70349-x. [DOI] [PubMed] [Google Scholar]
- 7.Toh VC. Early predictors of adverse outcome in term infants with post-asphyxial hypoxic ischaemic encephalopathy. Acta Paediatr. 2000;89:343–7. [PubMed] [Google Scholar]
- 8.Talati AJ, Yang W, Yolton K, Korones SB, Bada HS. Combination of early perinatal factors to identify near-term and term neonates for neuroprotection. J Perinatol. 2005;25:245–50. doi: 10.1038/sj.jp.7211259. [DOI] [PubMed] [Google Scholar]
- 9.Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–42. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 10.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–60. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Buonocore G, Perrone S, Turrisi G, Kramer BW, Balduini W. New pharmacological approaches in infants with hypoxic-ischemic encephalopathy. Curr Pharm Des. 2012;18:3086–100. [PubMed] [Google Scholar]
- 12.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159:851–8.e1. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- 16.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 17.Roland EH, Poskitt K, Rodriguez E, Lupton BA, Hill A. Perinatal hypoxic-ischemic thalamic injury: clinical features and neuroimaging. Ann Neurol. 1998;44:161–6. doi: 10.1002/ana.410440205. [DOI] [PubMed] [Google Scholar]
- 18.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: Report of the quality standards subcommittee of the American Academy of Neurology and the practice committee of the child neurology society. Neurology. 2002;58:1726–38. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 19.Mercuri E, Rutherford M, Barnett A, et al. MRI lesions and infants with neonatal encephalopathy. Is the Apgar score predictive? Neuropediatrics. 2002;33:150–6. doi: 10.1055/s-2002-33412. [DOI] [PubMed] [Google Scholar]
- 20.McKinstry RC, Miller JH, Snyder AZ, et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology. 2002;59:824–33. doi: 10.1212/wnl.59.6.824. [DOI] [PubMed] [Google Scholar]
- 21.Gano D, Chau V, Poskitt KJ, et al. Evolution of pattern of injury and quantitative MRI on days 1 and 3 in term newborns with hypoxic-ischemic encephalopathy. Pediatr Res. 2013;74:82–7. doi: 10.1038/pr.2013.69. [DOI] [PubMed] [Google Scholar]
- 22.Ferriero DM. Timing is everything: delaying therapy for delayed cell death. Dev Neurosci. 2002;24:349–51. doi: 10.1159/000069048. [DOI] [PubMed] [Google Scholar]
- 23.Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–26. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 24.Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125:e1135–42. doi: 10.1542/peds.2009-2268. [DOI] [PubMed] [Google Scholar]
- 25.Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr Res. 2007;61:451–5. doi: 10.1203/pdr.0b013e3180332cec. [DOI] [PubMed] [Google Scholar]
- 26.Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130:683–91. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 28.American College of Obstetricians and Gynecologists. American Academy of Pediatrics. Neonatal encephalopathy and cerebral palsy: defining the pathogenesis and pathophysiology. Washington, DC: ACOG Distribution Center; 2003. [Google Scholar]
- 29.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 30.Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ. 2010;340:c1471. doi: 10.1136/bmj.c1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah PS, Beyene J, To T, Ohlsson A, Perlman M. Postasphyxial hypoxic-ischemic encephalopathy in neonates: outcome prediction rule within 4 hours of birth. Arch Pediatr Adolesc Med. 2006;160:729–36. doi: 10.1001/archpedi.160.7.729. [DOI] [PubMed] [Google Scholar]
- 32.Ambalavanan N, Carlo WA, Shankaran S, et al. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–93. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- 33.Milsom I, Ladfors L, Thiringer K, Niklasson A, Odeback A, Thornberg E. Influence of maternal, obstetric and fetal risk factors on the prevalence of birth asphyxia at term in a Swedish urban population. Acta Obstet Gynecol Scand. 2002;81:909–17. doi: 10.1034/j.1600-0412.2002.811003.x. [DOI] [PubMed] [Google Scholar]
- 34.Hayes BC, McGarvey C, Mulvany S, et al. A case-control study of hypoxic-ischemic encephalopathy in newborn infants at >36 weeks gestation. Am J Obstet Gynecol. 2013;209:29.e1–19. doi: 10.1016/j.ajog.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613–8. doi: 10.1056/NEJM199603073341001. [DOI] [PubMed] [Google Scholar]


