Abstract
The stability of frontal electroencephalogram (EEG) asymmetry, temperamental activity level and fear, as well as bidirectional relations between asymmetry and temperament across the first four years of life were examined in a sample of 183 children. Children participated in annual lab visits through 48 months, providing EEG and maternal report of temperament. EEG asymmetry showed moderate stability between 10 and 24 months. Analyses revealed that more left asymmetry predicted later activity level across the first three years. Conversely, asymmetry did not predict fear. Rather, fear at 36 months predicted more right asymmetry at 48 months. Results highlight the need for additional longitudinal research of infants and children to increase understanding of bidirectional relations between EEG and temperament in typically developing populations.
Keywords: EEG asymmetry, temperament, activity level, fear, infancy, early childhood
Conceptualizations of temperament often focus on affective-motivational systems and maintain that temperament is biologically based, relatively stable over time, and sensitive to experiential influence (Rothbart & Bates, 2006). Temperament is broadly defined as an inherited disposition that predisposes individuals toward certain patterns of emotional and behavioral reactivity. Although the relative stability of temperament is well established (Neppl et al., 2010; Rothbart & Bates, 2006), the underlying biological mechanisms that support this stability have received little attention in longitudinal research frameworks. Furthermore, the bidirectional relations between temperament and its biological underpinnings throughout development have yet to be explored. In this study, we investigate the relation between two important dimensions of temperament—activity level and fear—and EEG asymmetry at four time points across the first four years of life.
EEG Asymmetry: Relations to Temperament
Brain electrical activity (i.e., electroencephalogram, EEG) recorded from frontal scalp locations is correlated with temperament. In general, patterns of resting EEG asymmetry have been linked to temperamental differences in reactivity and regulation whereas patterns of stimulus-related EEG asymmetry have been linked to emotional state (Diaz & Bell, 2012; Fox, 1991, 1994; Fox, Calkins, & Bell, 1994). In particular, children with greater resting left frontal activity tend to be approach-oriented and display more positive affect, and children with greater resting right frontal activity tend to be withdrawal-oriented and display more negative affect (Coan & Allen, 2004; Davidson & Rickman, 1999; Fox, 1991, 1994; Fox et al., 1994). Similarly, in response to emotional events or stimuli, greater left activation is typically evident during positive emotional states and greater right activation is typically evident during negative emotional states (Dawson, 1994; Fox, 1991).
The relation of EEG asymmetry to emotional state is relatively clear, yet the relation of EEG asymmetry to temperament appears to be more complex. Existing work has provided evidence that certain temperament profiles are consistently associated with particular patterns of brain asymmetry. To date, however, there has been no work to test whether there are bidirectional relations between temperament and asymmetry over time. Research on the longitudinal reciprocal relations of EEG asymmetry to temperament will provide insight as to when and how neurophysiology and temperament may predict one another.
In accordance with a developmental view of temperament, the self-organization framework provides a natural platform to support the notion of longitudinal bidirectional relations between individual neurophysiology and temperament (Derryberry & Rothbart, 1997). Specifically, bidirectional relations between frontal EEG asymmetry and temperament may evolve as a function of neural plasticity. At birth, neural connections are initially extremely diverse. Upon exposure to the environment, however, the more active synapses tend to be strengthened and stabilize. This process is largely influenced by an individual’s active participation in the environment. Thus, a child’s response tendencies, be it activity- or fear-related behaviors, may result in the child’s exposure to specific types of information across development, which may strengthen particular patterns of synaptic connections, which may in turn influence later behavior, and so on. Thus, understanding bidirectional relations between patterns of asymmetry and temperament over time will allow researchers to further investigate whether biology drives observed differences in behavior or vice versa. Extant research on the relations between EEG asymmetry and temperament offers empirical support for extreme temperament profiles using selected samples. However, when samples have not been selected based on child temperament, these relations are less clear.
In research with asymmetry, behaviorally inhibited and uninhibited temperament profiles have been of particular interest. Children who are behaviorally inhibited react to novelty with fear and withdrawal, are likely to be characterized as shy, and are at risk for internalizing problems (Buss & Kiel, 2013; Fox, Henderson, Marshall, Nichols, & Ghera, & 2005). Children who are uninhibited react to novelty with approach-oriented behaviors, frequently show positive emotions, and tend to be active and social. Extremely uninhibited or exuberant children may also be impulsive, placing them at risk for externalizing problems (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Rothbart, Derrybery, & Hershey, 2000; Stifter, Putnam, & Jahromi, 2008). According to the Fox (1991, 1994) model of temperament and EEG asymmetry, inhibited children are expected to show patterns of right frontal asymmetry and uninhibited children are expected to show patterns of left frontal asymmetry.
The extant literature on the relation between EEG asymmetry and temperament has traditionally flowed from approaches whereby child characteristics are linked to observed behaviors; however, this research has not examined the bidirectional relations of biological underpinnings to temperament across development. In this study, we examine early asymmetry and later activity level and fear in two unique ways. First, we allow for the fact that there may be a bidirectional association where not only earlier asymmetries are related to later activity level and fear but also early activity level and fear relate to later asymmetries. Furthermore, we capitalize on multiple waves of data, recognizing that these relations may in fact change over time. In particular, we know of no work that examines the bidirectional nature of the associations between EEG asymmetry and temperament. This is particularly important because we know nothing about how these relations may change over time. In this paper, we examine whether biology appears to drive observed differences in behavior or if characteristics of temperament predict later biology.
EEG Asymmetry: Considerations of Stability
Temperamental reactivity and regulation have been shown to be relatively stable across time, and yet the stability of the associations with underlying EEG asymmetry patterns is rarely examined. It is thus difficult to precisely define the role of EEG asymmetry in maintaining these stable temperament traits over time. Examinations of the stability of EEG asymmetry patterns may be particularly likely to accurately represent trait- or temperament-linked patterns.
In one of the few studies of the stability of EEG asymmetry across early life, infants were selected for both high and low reactivity to novelty at four months, with just over one third of the sample selected for high negative reactivity (Fox et al., 1994). In this sample, approximately 26% of the infants who provided artifact-free EEG at 9 and 24 months showed stable patterns of greater right frontal activity and were more likely to have been highly negatively reactive at 4 months and more inhibited, more compliant, less impulsive, and more tolerant of frustration at 14 months compared to infants who showed stable patterns of greater left frontal activity. This behavioral profile is in line with what would be expected under the Fox model.
In a recent study from our lab that utilized a small subsample (n = 48) of the infants in the present study (selected because they also participated in a longitudinal study in another research lab at 30 months of age during which internalizing and externalizing data were collected), stable patterns of EEG asymmetry were examined. Stable left frontal activity from 10 to 24 months was related to higher levels of externalizing problem behaviors at 30 months, and stable right frontal activity was related to higher levels of internalizing problem behaviors (Smith & Bell, 2010). Again, this is line with what would be expected under the Fox model in that toddlers with greater left activation appeared to have less control of their approach motivation and toddlers with greater right activation appeared to have less control of their withdrawal motivation. In this sample, approximately half of the toddlers showed stable patterns of asymmetric activity (29% right, 19% left). Toddlers who did not show stable patterns of asymmetric activity had relatively low levels of internalizing and externalizing problem behaviors, suggesting that stability is an important consideration in research that examines the relation of EEG asymmetry to temperament and risk.
Taken together, stable patterns of resting frontal EEG asymmetry may reflect temperament-linked vulnerability to risk. Children with early stable patterns of frontal EEG asymmetry appear to be the most likely to have correspondingly stable and extreme temperaments that place them at risk for future emotional and behavioral problems. Importantly, understanding psychophysiological correlates of emotional and behavioral problems may allow researchers to identify children at risk and guide early intervention efforts to reduce the risk of later psychopathology (Bauer, Quas, & Boyce, 2002).
The Current Study
The pattern of findings from previous literature suggests that the relation between EEG asymmetry and temperament is dynamic across the early years of development and that this relation needs to be further explored to more fully understand the nature of the association. In this study, we examine the bidirectional relations between frontal EEG asymmetry and two complementary dimensions of temperament: activity level and fear. The uniquely important issue addressed herein, and the one central to the study of bidirectional effects, is that we explore the predictive relations between early biology and later temperament as well as early temperament to later biology. Moreover, we examine how these relations play out over the first four years of life.
Activity level and fear are fine-grained attributes that are prominently featured in multiple theories of temperament and personality (Depue & Collins, 1999; Eaton, McKeen, & Campbell, 2001; Kagan & Snidman, 2009; Rothbart & Ahadi, 1994; Rothbart, Ahadi, & Evans, 2000). Activity level is a facilitative behavior that is linked to interest- or approach-orientation, sensitive to context-related positive and negative emotional reactivity (Fox & Davidson, 1984; Rothbart et al., 2000), and ultimately loads onto the higher order dimension of Surgency in childhood and Extraversion in adulthood (Rothbart, Derryberry, & Posner, 1994; Rothbart et al., 2000). Across childhood, activity level is a foundational attribute of Surgency that helps drive stability in this broader temperament profile (Putnam, Rothbart & Gartstein, 2008). On the other hand, fear is typically linked to withdrawal-orientation, negative emotional reactivity, and loads onto the higher order dimension of Negative Affectivity in childhood and Neuroticism later in life (Rothbart et al., 1994, 2000). High activity level has been linked to externalizing problem behaviors in children (Bussing, Lehninger, & Eyberg, 2006; Fagot & O’Brien, 1994; Gjone & Stevenson, 1997; Ilott, Saudino, Wood, & Asherson, 2010), whereas high levels of fear have been linked to internalizing problems (Buss, 2011; Degnan & Fox, 2007; Fox et al., 2001; Rothbart, 2007).
Due to the clear links between activity level and fear and risk for later psychopathology, in addition to the high degree of salience to maternal reporters, we considered these dimensions of temperament ideal for longitudinal exploration. Further, our work is guided by the developmental psychopathology framework that highlights the importance of understanding typical developmental processes in order to better identify, prevent, and intervene in cases of atypical or maladaptive development (Cicchetti & Toth, 2009). An important first step in understanding the longitudinal relations between frontal EEG asymmetry and temperament is exploring their relations in a typically developing sample. Thus, unlike many previous studies of EEG asymmetry and temperament, we did not select for extreme reactivity in infancy. In this study, we explore the following research questions:
To what degree is frontal EEG asymmetry stable from 10 to 48 months of age in an unselected sample? Additionally, to what degree are activity level and fear stable over the same time period?
Are frontal EEG asymmetry and temperament bidirectionally related across the first four years of life?
Previous work that examined stability with children who were not selected for extreme reactivity found low to moderate stability in frontal EEG asymmetry across only two points in early childhood (Vuga, Fox, Cohn, Kovacs, & George, 2008). We thus expected to find little evidence of stability in asymmetry in our sample across four time points. Further, in accordance with the Fox model, we expected that greater left frontal EEG asymmetry would be related to higher levels of activity level and greater right frontal EEG asymmetry would be related to higher levels of fear, particularly at the early time points. It is worth noting that our study focuses on whether the continuous variable of frontal EEG asymmetry at earlier time points predicts frontal EEG at later time points, rather than examining whether a child has relatively greater left versus right frontal activation at one age predicting the next.
Method
Participants
Participants in our study included children and their mothers from a longitudinal study of individual differences in social-emotional and cognitive development. Mothers with infants were recruited through birth announcements featured in local newspapers and a commercial mailing list of new parents. All infants were healthy and full term with no prenatal, birth, or postnatal complications. Mothers and their children were invited for lab visits when children were 10, 24, 36, and 48 months old. Participants were seen in the lab in two waves of data collection that took place over eight years. The first cohort of children were seen in the lab between February 2003 and March 2008. The second cohort of children were seen between August 2007 and December 2011.
Children were selected for inclusion in our study if they had at least one usable data point of baseline EEG and at least one completed temperament questionnaire (n = 183, 98 girls and 85 boys). Mothers reported that their children were primarily Caucasian (91%) with 8% multiracial and 1% African American or other. One percent of mothers did not complete high school, 6% completed technical school, 22% had earned high school or technical degrees, and 71% were college graduates. The demographics of the sample reflect those of the relatively homogeneous population surrounding the small college town in rural Appalachia where the study was conducted.
Procedures
For the 10-month lab visit, infants and their mothers visited the research lab on or within two weeks after the infant’s 10-month birthdays. Baseline EEG was recorded for 1 minute while infants sat on their mothers’ laps. During the baseline recording, a research assistant manipulated a toy containing brightly colored balls on top of the testing table, 1.1 m in front of the infants. This procedure quieted the infants and yielded minimal eye movements and gross motor movements, thus helping infants to tolerate the EEG cap for the recording. Mothers were instructed not to talk to infants during the EEG recording. At the end of the assessment, mothers were paid for participating.
For the 24-, 36-, and 48-month lab visits, children were invited to the lab on or within two months after their respective birthdays, so that two months separated the youngest and oldest children at each yearly visit. Baseline EEG was recorded for 1 minute while the children sat in a chair and watched a clip of the film Finding Nemo (sea turtles riding the East Australian Current). Mothers sat in a chair to the right of their children. During the baseline recording, the TV monitor was 1.8 meters from the children, and mothers were instructed not to talk during the EEG baseline recording. At the end of each yearly visit, mothers were paid for participation and children were given a small toy to take home.
At each of the four visits, baseline EEG recordings were made. Based on publication guidelines for studies using EEG methodology (Keil et al., 2014), we report the following details. EEG was recorded from 16 left and right scalp sites: frontal pole (Fp1,Fp2), medial frontal (F3,F4), lateral frontal (F7,F8), central (C3,C4), temporal (T7,T8), medial parietal (P3,P4), lateral parietal (P7,P8), and occipital (O1,O2), referenced to Cz. EEG was recorded using a stretch cap (Electro-Cap, Inc., Eaton, OH: E1-series cap) with electrodes in the 10/20 system pattern. After the cap was placed on the head, a small amount of abrasive was placed into each recording site and the scalp gently rubbed. Following this, conductive gel provided by the cap manufacturer was placed in each site. Electrode impedances were measured and accepted if they were below 10K ohms. The electrical activity from each lead was amplified using separate James Long Company Bioamps (James Long Company, Caroga Lake, NY). During data collection, the high pass filter was a single pole RC filter with a 0.1 Hz cut-off (3 dB or half-power point) and 6 dB per octave roll-off. The low pass filter was a two-pole Butterworth type with a 100 Hz cut-off (3 dB or half-power point) and 12 dB octave roll-off.
Activity for each lead was displayed on the monitor of the acquisition computer. The EEG signal was digitized on-line at 512 samples per second for each channel so that the data were not affected by aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp., Southfield, MI) and the raw data were stored for later analyses. Prior to the recording of each subject a 10 Hz, 50 uV peak-to-peak sine wave was input through each amplifier. This calibration signal was digitized for 30 seconds and stored for subsequent analysis.
One week before each yearly assessment, temperament questionnaires were mailed to mothers who had agreed to participate. Mothers either brought the completed questionnaires with them when they came in for the lab visit or mailed them back in an envelope provided (mothers who had moved from the area were contacted about completing questionnaires).
Measures
EEG
Spectral analysis of the calibration signal and computation of power at the 9 to 11 Hz frequency band was accomplished. The power figures were used to calibrate the power derived from the subsequent spectral analysis of the EEG. Infant EEG data from each of the four lab visits were examined and analyzed using EEG Analysis System software developed by James Long Company. First, the data were re-referenced via software to an average reference configuration. Then, the average reference EEG data were artifact scored for eye movements using a peak-to-peak criterion of 100 uV or greater. The criterion for scoring movement artifact was a potential greater than 200 uV peak-to-peak. These artifact-scored epochs were eliminated from all subsequent analyses. The data were analyzed with a discrete Fourier transform (DFT) using a Hanning window of one-second width and 50% overlap. Participants with at least 50 DFT windows (i.e., 25 seconds of artifact-free baseline EEG data) were included in our study. Thirteen percent (n = 28) of the larger sample was excluded for insufficient artifact-free EEG data. Power was computed for the 6 to 9 Hz frequency band. Infants and young children have a dominant frequency between 6 to 9 Hz (Bell & Fox, 1994; Marshall, Bar-Haim, & Fox, 2002), and this particular frequency band has been correlated with patterns of emotion reactivity and emotion regulation during infancy (Bell & Fox, 1994; Buss et al., 2003; Dawson, 1994; Diaz & Bell, 2012) and early childhood (Fox et al., 2001). The power was expressed as mean square microvolts and the data were transformed using the natural log (ln) to normalize the distribution.
Frontal EEG asymmetry values were computed by first averaging the ln alpha power at two left frontal (F3 and F7) and corresponding right frontal sites (F4 and F8; Coan & Allen, 2004). Left mean power was then subtracted from the right mean power to create the composite asymmetry scores (F4/F8 – F3/F7 mean alpha power). Because power in the 6–9 Hz band has been shown to be inversely related to cortical activation during emotion reactivity and regulation in infants and young children (Fox, 1994), a negative asymmetry score reflects greater right frontal activation and a positive asymmetry score reflects greater left frontal activation.
Asymmetry outliers were handled through Winsorization such that scores that fell outside ± 3 standard deviations of the mean for each time point were replaced by the next closest asymmetry score. This technique was applied to seven total outliers across the four time points of asymmetry data. Internal consistency for asymmetry scores across the four assessments was acceptable (α = .45, average rinter-item = .45). An average inter-item correlation between .15 and .50 is considered ideal and may be a more appropriate measure of the unidimensionality of a scale, particularly with so few items (Clark & Watson, 1995).
Temperament
Temperament was assessed with three developmentally appropriate parallel questionnaires developed by Rothbart and colleagues. Mothers completed the short forms of the Infant Behavior Questionnaire-Revised (IBQ-R; Putnam, Helbig, Gartstein, Rothbart, & Leerkes, 2013) before the 10-month visit, the Early Child Questionnaire (ECBQ; Putnam, Jacobs, Gartstein, & Rothbart, 2010) before the 24-month visit, and the Children’s Behavior Questionnaire (CBQ; Putnam & Rothbart, 2006) before the 36- and 48-month visits. Mothers responded to questions about their children’s typical behavior on a seven-point Likert-type scale on which 1 = never, 4 = about half the time, and 7 = always. The short forms of the questionnaires contain 91 to 107 items that comprise 14 to 18 temperament scales, with activity level and fear scales appearing on each. Coefficient alpha ranged from .66 to .71 for activity level and .65 to .76 for fear.
Data Analysis
Analyses were conducted in a structural equation modeling (SEM) framework in AMOS 19.0 software (Arbuckle, 2010). Missing data were accounted for through full information maximum likelihood, a technique that has been shown to yield less-biased estimates of missing data than listwise deletion (Enders & Bandalos, 2001). SEM is a flexible framework that allows variables to be modeled with multiple simultaneous relations. Thus, longitudinal models can allow for variables to be both predictors and outcomes, simultaneously.
To evaluate our first research question, we tested an unconditional autoregressive model. In autoregressive models, each variable is predicted by the same variable at the preceding time point. Thus, in our unconditional autoregressive model, 24-month asymmetry was predicted by 10-month asymmetry, 36-month asymmetry was predicted by 24-month asymmetry and so on, allowing for an examination of whether early asymmetry contributed to stability in later asymmetry. Because the amount of time between measurement occasions differed and because we would not necessarily expect stability estimates to be the same over time, autoregressive parameters were free to vary.
To evaluate our second research question, we built a series of cross-lagged autoregressive models to test the predictive relations within and between temperament and frontal EEG asymmetry across the first four years of life. The cross-lagged aspect refers to the ability to test bidirectional relations between two sets of variables across time. In cross-lagged autoregressive models, each endogenous variable has three sources of variance: an autoregressive aspect that captures stability over time, a cross-lagged aspect that tests the effect of one variable on a cross panel variable from the preceding time point, and a residual that is allowed to correlate with the residual of the cross panel variable at the same time point and is constrained to be equal across time (Ferrer & McArdle, 2003). In our models, we thus tested four groups of relations: (1) the effects of early asymmetry on later asymmetry, (2) the effects of early temperament on later temperament, (3) the effects of early asymmetry on later temperament, and (4) the effects of early temperament on later asymmetry.
We followed four model-building steps to test cross-lagged autoregressive models for each temperament variable, including: (1) an unconditional model that specified no cross-lagged associations, (2) a unidirectional cross-lagged model in which asymmetry at 10, 24, and 36 months predicted temperament at 24, 36, and 48 months, respectively, (3) a unidirectional cross-lagged model in which temperament 10, 24, and 36 months predicted asymmetry at 24, 36, and 48 months, respectively, and (4) a bidirectional cross-lagged model in which temperament and asymmetry predicted one another. After each step, a chi-square change test was conducted to determine whether the addition of lags improved model fit. Each unidirectional model (in steps two and three) was compared to the unconditional model to determine relative fit. If a unidirectional model fit the data significantly better than the unconditional model, the bidirectional model was then compared to the best-fitting unidirectional model. Although chi-square change tests are often considered the most informative assessments of relative model fit (Barrett, 2007), additional fit indices including the comparative fit index (CFI) and the root mean square error of approximation (RMSEA) were also evaluated to provide a more comprehensive sense of overall fit. Values higher than .90 on the CFI and values less than .08 on the RMSEA indicate satisfactory model fit (Bentler, 1990; Bentler & Bonett, 1980; Browne & Cudeck, 1993).
Results
Descriptive statistics for all study variables are provided in Table 1 (note: Winsorized EEG asymmetry data are displayed). The number of children with data for each variable is noted and although a total of 183 children were included in each model (based on our inclusion criteria of at least one usable data point of baseline EEG and at least one completed temperament questionnaire); the number of children with data at any given time point ranged from 88 to 177. Activity level and fear did not correlate at or across any time point except 10 months, r = .17, p < .05. There were no differences in levels of fear and activity level for children who did and did not provide usable EEG at any given time point, all ps > .50. Further, EEG asymmetry at 10 months did not predict whether a child was missing later asymmetry due to lack of participation or unusable data at later time points (χ2 (1) = 3.46, p > .05), nor did activity level or fear at 10 months predict whether a child left the study after the first assessment, ps > .20. The prediction success for missing EEG data and attrition was 0% for all 10-month predictors.
Table 1.
Temperament and EEG Asymmetry Descriptive Statistics by Age
| 10 Months | 24 Months | 36 Months | 48 Months | |
|---|---|---|---|---|
| EEG Asymmetry n | 163 | 105 | 111 | 88 |
| Mean (SD) | 0.02 (0.23) | 0.06 (0.34) | 0.03 (0.17) | 0.01 (0.18) |
| Range | −0.48–0.62 | −0.71–1.11 | −0.30–0.46 | −0.42–0.48 |
|
| ||||
| Temperament n | 177 | 150 | 131 | 121 |
| Activity Level | ||||
| Mean (SD) | 4.61 (1.01) | 4.84 (0.81) | 5.13 (0.71) | 5.22 (0.76) |
| Range | 2.67–7.00 | 2.50–6.63 | 3.43–6.86 | 3.43–6.86 |
| Fear | ||||
| Mean (SD) | 2.97 (1.09) | 2.53 (0.88) | 3.46 (1.26) | 3.70 (1.21) |
| Range | 1.00–5.67 | 1.14–5.57 | 1.00–6.67 | 1.00–6.80 |
Stability of Frontal EEG Asymmetry and Temperament
To answer our first research question, we assessed the degree of stability of frontal EEG asymmetry and temperament from 10 to 48 months of age. The unconditional autoregressive models provided stability estimates for both EEG asymmetry and temperament. The results for asymmetry indicated that asymmetry was only moderately stable at the trend level from 10 to 24 months (β = .17, p < .10) and was less stable beyond that. Additionally, the models showed that both temperamental activity level and fear were stable from 10 to 48 months and that early temperament became more predictive of later temperament level as children grew older (see estimates in Figures 1 and 2). Model fit statistics for these stability-only models indicated mediocre fit (Table 2).
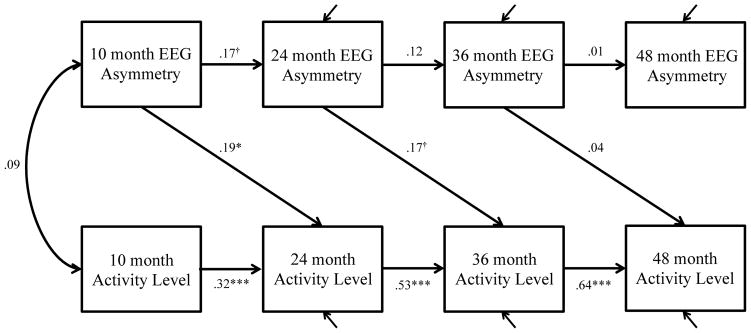
Figure 1. Final cross-lagged autoregressive model for frontal EEG asymmetry and activity level showing standardized estimates (first correlation only shown).
† p < .10, * p < .05, *** p < .001
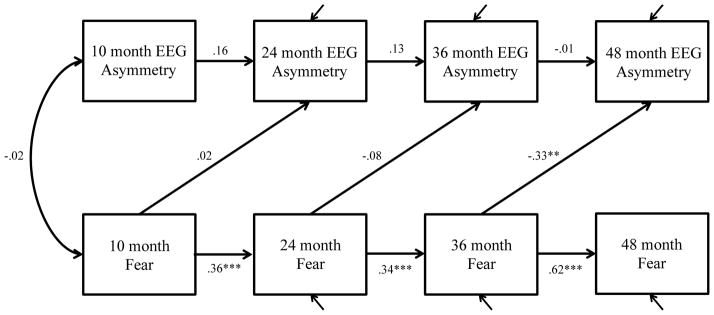
Figure 2. Final cross-lagged autoregressive model for frontal EEG asymmetry and fear showing standardized estimates (first correlation only shown).
** p < .01, *** p < .001
Table 2.
Model Comparisons Using Chi-squared Change Tests and Model Fit Statistics
| Model | χ2 | df | Δ χ2 | Δdf | p | CFI | RMSEA |
|---|---|---|---|---|---|---|---|
| Activity Level | |||||||
| Unconditional | 33.56 | 20 | – | – | – | .88 | .06 |
| * EEG Asym → Temp | 23.59 | 17 | 9.97 | 3 | .02 | .94 | .05 |
| Temp → EEG Asym | 31.35 | 17 | 2.21 | 3 | .53 | .88 | .07 |
| Bidirectional | 21.78 | 14 | 1.81 | 3 | .61 | .93 | .06 |
|
| |||||||
| Fear | |||||||
| Unconditional | 34.39 | 20 | – | – | – | .84 | .06 |
| EEG Asym → Temp | 32.39 | 17 | 2.00 | 3 | .57 | .83 | .07 |
| * Temp → EEG Asym | 25.31 | 17 | 9.08 | 3 | .03 | .91 | .05 |
| Bidirectional | 23.20 | 14 | 2.11 | 3 | .55 | .90 | .06 |
best fitting model, p <.05
Note. We the following acronyms and short forms in the table: EEG Asym for EEG asymmetry and Temp for temperament.
Frontal EEG Asymmetry and Activity Level
Our second research question was whether there was evidence for bidirectional relations between frontal EEG asymmetry and temperament across the first four years of life. We focus first on the activity level dimension of temperament. Results of model building indicated that the single cross-lagged model, and not the bidirectional model, with asymmetry predicting later activity level was the best-fitting model (Table 2). With the inclusion of these cross-lags, the model provided adequate fit, CFI = .94, RMSEA = .05.
The final model is represented in Figure 1 and shows that asymmetry and activity level were not significantly correlated at 10 months, but there was a lagged predictive relation in which asymmetry predicted later activity level across the first three years of life. In particular, 10-month asymmetry predicted 24-month activity level (β = .19, p < .05) and 24-month asymmetry moderately, albeit at the trend level, predicted 36-month activity level (β = .17, p < .10). More left-frontal asymmetry at 10 and 24 months predicted greater activity level at 24 and 36 months, respectively. However, this predictive relation diminished to the point that there was no significant relation between 36-month asymmetry and 48-month activity level.
Frontal EEG Asymmetry and Fear
Like activity level, the unidirectional model provided the best fit, but in this case, the unidirectional model was from temperament to asymmetry (Table 2, Figure 2). This model yielded adequate fit, CFI = .91, RMSEA = .05.
Similar to the activity level model, asymmetry and temperament were not significantly correlated at 10 months. However, there was one predictive relation between fear and asymmetry in which 36-month fear significantly predicted 48-month asymmetry (β = −.33, p < .01). That is, greater fear at 36 months predicted more right frontal asymmetry at 48 months.
Discussion
The first goal of our study was to explore the degree of stability in resting frontal EEG asymmetry from 10 to 48 months of age in a sample of children who were not selected for extreme reactivity in infancy. Across our four measurement points, we found that frontal EEG asymmetry was only stable between the earliest two, i.e., from 10 to 24 months, although to a relatively low degree. As typically developing children grow older, the lack of asymmetry stability may be attributable to a greater sensitivity to experience and environment.
In addition, others have argued that the stability of frontal EEG asymmetry may be influenced by sex, handedness, parental history of depression, as well as the influence of normal brain development (Vuga et al., 2008). Thus, the shift from moderate early stability to later instability may be explained by the underlying development of neural networks. In particular, patterns of frontal EEG asymmetry in typically developing populations may be more susceptible to change due to the evolving prefrontal cortex, one of the last cortical regions to reach full structural development (Kanemura, Aihara, Aoki, Araki, & Nakazawa, 2003). It is further possible that frontal EEG asymmetry is a more robust predictor of temperament across development to the degree that children display more reactive temperaments. For children who fall outside the extremes, this relation is less likely to be observed. Our results highlight the need for additional longitudinal research of infants and children to increase our understanding of frontal EEG asymmetry stability in typically developing populations.
Another notable aspect of our findings is that maternal report of temperament from 10 months to 48 months of age was stable. Across the first four years of life, children displayed strong continuity in their behavioral tendencies towards activity level and fear as reported by their mothers. These results provide support for the use of the Rothbart temperament questionnaires in supporting other measures (e.g., physiological and observational) of temperament across time. Moreover, these longitudinal patterns of stability are important in elucidating the developmental mechanisms involved in the systems underlying temperament. As noted by Rothbart and colleagues (2000), temperament does not simply specify a set of behavioral tendencies that persist throughout development, but is also indicative of the underlying mechanisms involved in the development of individual differences. Therefore, additional research linking maternal report of temperament to neurophysiological underpinnings may provide support for the stability or instability of temperamental characteristics in later development.
The second goal of our study was to examine the bidirectional relations between frontal EEG asymmetry and the complementary dimensions of temperament, activity level and fear. Although asymmetry and temperament were never concurrently related at 10 months, significant lagged predictive relations emerged between asymmetry and each dimension of temperament. In relation to activity level, a unidirectional story emerged in which more left frontal asymmetry predicted higher activity level through 36 months. This finding confirms reports of a strong biological influence on activity level in early childhood (Saudino, 2012). It is likely that biology drives children to physically react to and explore their environments in early development, but as inhibitory control develops during the third year (Kochanska & Knaack, 2003), young children behave in more thoughtful and controlled ways.
In relation to fear, a unidirectional story emerged in which higher levels of fear at 36 months predicted more right asymmetry at 48 months. Given the stability of fear over time, our findings support the idea that early fear underpins later fear. Moreover, as patterns of fear became more stable in early childhood, we found that fearful three year olds displayed more right frontal EEG asymmetry at four years of age. Taken together, these results suggest that stable patterns of fearfulness may represent maladaptive patterns of dysregulated fear. For example, recent work by Buss and colleagues (Buss, 2011; Buss et al., 2013) suggests that an early pattern of dysregulated fear is a better predictor of risk than temperamental fear alone. In particular, they found dysregulated fear in toddlerhood was related to later socially anxious behavior and anxiety-related symptoms in early childhood. Thus, right frontal EEG asymmetry may be an index of this type of dysregulation and may explain why we observe the strongest relation between asymmetry and fear at a later time point—when measures of temperament capture maladaptive patterns of dysregulated fear.
The unidirectional findings for fear and asymmetry may best be explained through a self-organization framework. Derryberry and Rothbart (1997) propose that as children develop, frequently occurring patterns of internal motivation contribute to the organization of underlying cortical systems. In particular, the connections between active synapses are strengthened in response to interplay between a child’s motivational systems and the environment. In relation to fear, frequently occurring patterns of withdrawal motivation over the first four years of life may contribute to cortical organization such that a fearful temperament supports more right frontal EEG asymmetry by three years of age.
Further, it is likely that similar patterns of organization are taking place relative to activity level. We may see the predictive association between asymmetry and activity level disappear after three years of age due to a shift in children’s internal motivational systems. Due to the development of inhibitory control at this time, as well as increased demand from the environment for more controlled behavior, internal motivation may shift from reactive to more controlled approach. This evolving internal motivation in relation to activity level may account for the lack of bidirectional findings. It may be that if we were to model these relations beyond the fourth year of life, a bidirectional story would emerge in which activity level at 48 months or later would predict asymmetry. Future directions may include exploring these associations as well as the bidirectional relations between attention- and control-related dimensions of temperament.
Our study adds to the field through the unique contribution of a bidirectional longitudinal exploration of the complex relations between frontal EEG asymmetry and temperament with a sample of typically developing children who were not selected for extreme reactivity. Traditionally, researchers have examined concurrent associations between physiology and behavior or physiology as a predictor of behavior (Buss et al., 2003; Fox et al., 2005; McManis, Kagan, Snidman, & Woodward, 2002); a particular strength of our work is the investigation of bidirectional relations. In line with previous work with behavioral observations of temperament (Buss et al., 2003; Fox et al., 1994; Kagan & Snidman, 1999), our maternal report of temperament linked left asymmetry to more approach oriented behavior (activity level) and right asymmetry to more withdrawal oriented behavior (fear) at various time points.
Maternal report is particularly valuable because mothers observe their children in a variety of contexts and are able to accurately report on trait-linked behavior. Buss (2011) suggested assessing fear in multiple contexts as a way to alleviate the problem of inconsistent findings related to children with fearful temperaments that pervade the developmental literature due to the use of different measures across different studies. The use of maternal report is a valid and low cost way to address this issue and parent report of fear and activity level, in particular, have been found to be highly related to laboratory measures (Campbell & Eaton, 1999; Gartstein & Marmion, 2008).
Our study also highlights the value in examining stability in frontal asymmetric cortical activation, as unsustained patterns of functioning across time may reflect individual sensitivity to the environment and/or experiential forces that work to mitigate extreme traits. Additional research in this area may further elucidate the onset of underlying neurophysiological developments that contribute to individual differences in temperament. We view our relatively large sample as a strength of our study. Additional strengths include the fact that temperamental fear and activity level did not differ for children who did versus did not provide usable EEG nor did temperament predict attrition.
Taken together, our findings support the idea that early frontal EEG asymmetry is moderately stable, indicating a possible biological basis for the development of early individual differences in temperament. Our findings that frontal EEG asymmetry was predictive of later activity level and that fear was predictive of later temperamental fear suggest that more work is needed on the developmental processes of typically developing children and how physiological stability relates to observed behavior. Our work serves as an important first step toward that end and uniquely adds to the field through a bidirectional examination of these relations.
Acknowledgments
This research was supported by Grants HD049878 and HD043057 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research and to our research team for their assistance with data collection and coding.
Contributor Information
Grace Z. Howarth, George Mason University
Nicole B. Fettig, George Mason University
Timothy W. Curby, George Mason University
Martha Ann Bell, Virginia Tech.
References
- Arbuckle JL. IBM SPSS® Amos™19 user’s guide. Crawfordville, FL: Amos Development Corporation; 2010. [Google Scholar]
- Barrett P. Structural equation modeling: Adjudging model fit. Personality and Individual Differences. 2007;42:815–824. doi: 10.1016/j.paid.2006.09.018. [DOI] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. Brain development over the first year of life: Relations between EEG frequency and coherence and cognitive and affective behaviors. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York, NY: Guilford; 1994. pp. 314–345. [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037//0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–606. doi: 10.1037//0033-2909.88.3.588. [DOI] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Buss KA. Which fearful toddlers should we worry about? Context, fear, regulation, and anxiety risk. Developmental Psychology. 2011;47:804–819. doi: 10.1037/a002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Davis EL, Kiel EJ, Rrooker RJ, Beekman C, Early MC. Dysregulated fear predicts social wariness and social anxiety symptoms during kindergarten. Journal of Clinical Child & Adolescent Psychology. 2013;42:603–616. doi: 10.1080/15374416.2013.769170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Kiel EJ. Temperamental risk factors for pediatric anxiety disorders. In: Vasa RA, Roy AK, editors. Pediatric anxiety disorders: A clinical guide. New York: Springer Science4+Business Media; 2013. pp. 47–68. [Google Scholar]
- Buss KA, Malmstadt J, Dolski I, Kalin N, Goldsmith H, Davidson R. Right frontal brain activity, cortical, & withdrawal behavior in 6 months old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037/0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Bussing R, Lehninger F, Eyberg SM. Difficult child temperament and attention deficit hyperactivity disorder in preschool children. Infants and Young Children. 2006;19:125–131. doi: 10.1097/00001163-200604000-00005. [DOI] [Google Scholar]
- Campbell DW, Eaton WO. Sex differences in the activity level of infants. Infant and Child Development. 1999;8:1–17. doi: 10.1002/(SICI)1522-7219(199903)8. 1<1::AID-ICD186>3.0.CO;2-O. [DOI] [Google Scholar]
- Coan J, Allen J. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. The Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Constructing validity: Basic issues in objective scale development. Psychological Assessment. 1995;7:309–319. doi: 10.1037/1040-3590.7.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Dawson G. Frontal electroencephalographic correlates of individual differences in emotion expression in infants: A brain systems perspective on emotion. The development of emotion regulation: Biological and behavioral considerations. In: Fox NA, editor. Monographs of the Society for Research in Child Development. 2–3 Serial No 240. Vol. 59. Chicago: University of Chicago Press; 1994. pp. 135–151. [PubMed] [Google Scholar]
- Davidson RJ, Rickman M. Behavioral inhibition and the emotional circuitry of the brain: Stability and plasticity during the early childhood years. In: Schmidt LA, Schulkin J, editors. Extreme fear, shyness, and social phobia: Origins, biological mechanisms, and clinical outcomes. New York, NY: Oxford University Press; 1999. pp. 67–87. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Social Sciences. 1999;22:491–569. doi: 10.1017/S0140525X99002046. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/S0954579497001375. [DOI] [PubMed] [Google Scholar]
- Diaz A, Bell MA. Frontal EEG asymmetry and fear reactivity in different contexts at 10 months. Developmental Psychobiology. 2012;54:536–545. doi: 10.1002/dev.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WO, McKeen NA, Campbell DW. The waxing and waning of movement. Developmental Review. 2001;21:205–223. doi: 10.1006/drev.2000.0519. [DOI] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–457. doi: 10.1207/S15328007SEM0803_5. [DOI] [PubMed] [Google Scholar]
- Fagot BI, O’Brien M. Activity level in young children: Cross-age stability, situational influences, correlates with temperament, and the perception of problem behaviors. Merrill Palmer Quarterly. 1994;40:378–398. [Google Scholar]
- Ferrer E, McArdle JJ. Alternative structural models for multivariate longitudinal data analysis. Structural Equation Modeling. 2003;10:493–524. doi: 10.1207/S15328007SEM1004_1. [DOI] [Google Scholar]
- Fox NA. If it’s not left, it’s right: Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037/0003-066X.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. The development of emotion regulation: Biological and behavioral considerations. In: Fox NA, editor. Monographs of the Society for Research in Child Development. 2–3 Serial No 240. Vol. 59. Chicago: University of Chicago Press; 1994. pp. 152–166. [PubMed] [Google Scholar]
- Fox NA, Calkins SD, Bell MA. Neural plasticity and development in the first two years of life: Evidence from cognitive and socio-emotional domains of research. Development and Psychopathology. 1994;6:677–698. doi: 10.1017/S0954579400004739. [DOI] [Google Scholar]
- Fox NA, Davidson RJ. Hemispheric substrates of affect: A developmental model. In: Fox NA, Davidson RJ, editors. The psychobiology of affective development. Hillsdale, NJ: Erlbaum; 1984. pp. 353–381. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Marmion J. Fear and positive affectivity in infancy: Convergence/discrepancy between parent-report and laboratory-based indicators. Infant Behavior and Development. 2008;31:227–238. doi: 10.1016/j.infbeh.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjone H, Stevenson J. A longitudinal twin study of temperament and behavior problems: Common genetic or environmental influences? Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:1448–1456. doi: 10.1097/00004583-199710000-00028. [DOI] [PubMed] [Google Scholar]
- Ilott N, Suadino KJ, Wood A, Asherson P. A genetic study of ADHD and activity level in infancy. Genes, Brain and Behavior. 2010;9:296–304. doi: 10.1111/j.1601-183X.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Early childhood predictors of adult anxiety disorders. Biological Psychiatry. 1999;46:1536–1541. doi: 10.1016/S0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. The long shadow of temperament. Cambridge, MA: The Belknap Press of Harvard University; 2004. [Google Scholar]
- Kanemura H, Aihara M, Aoki S, Araki T, Nakazawa S. Development of the prefrontal lobe in infants and children: a three-dimensional magnetic resonance volumetric study. Brain and Development. 2003;25:195–199. doi: 10.1016/S0387-7604(02)00214-0. [DOI] [PubMed] [Google Scholar]
- Keil A, Debener S, Gratton G, Junghofer M, Kappenman ES, Luck SF, Yee CM. Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology. 2014;51:1–21. doi: 10.1111/psyp.12147. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Knaack A. Effortful control as a personality characteristic of young children: Antecedents, correlates, and consequences. Journal of Personality. 2003;71:1087–1112. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- McManis MH, Kagan J, Snidman NC, Woodward SA. EEG asymmetry, power, and temperament in children. Developmental Psychobiology. 2002;2:169–177. doi: 10.1002/dev.10053. [DOI] [PubMed] [Google Scholar]
- Neppl TK, Donnellan MB, Scaramella LV, Widaman KF, Spilman SK, Ontai LL, Conger RD. Differential stability of temperament and personality from toddlerhood to middle childhood. Journal of Research in Personality. 2010;44:386–396. doi: 10.1016/j.jrp.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E. Development and assessment of short and very short forms of the Infant Behavior Questionnaires-Revised. Journal of Personality Assessment. 2013 doi: 10.1080/00223891.2013.841171. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Jacobs J, Gartstein MA, Rothbart MK. Development and assessment of short and very short forms of the Early Childhood Behavior Questionnaire. Poster presented at International Conference on Infant Studies; Baltimore, MD.. 2010. [Google Scholar]
- Putnam SP, Rothbart MK. Development of the short and very short forms of the Children’s Behavior Questionnaire. Journal of Personality Assessment. 2006;87:103–113. doi: 10.1177/1073191113508809. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Rothbart MK, Gartstein MA. Homotypic and heterotypic continuity of fine-grained temperament during infancy, toddlerhood, and early childhood. Infant and Child Development. 2008;17:387–405. doi: 10.1002/ICD.582. [DOI] [Google Scholar]
- Rothbart MK. Temperament, development, and personality. Current Directions in Psychological Science. 2007;16:207–212. doi: 10.1111/j.1467-8721.2007.00505.x. [DOI] [Google Scholar]
- Rothbart MK, Ahadi SA. Temperament and the development of personality. Journal of Abnormal Psychology. 1994;103:55–66. doi: 10.1037//0021-843X.103.1.55. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-354.78.1.122. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 6. New York: Wiley; 2006. pp. 37–86. [Google Scholar]
- Rothbart MK, Derryberry D, Hershey K. Stability of temperament in childhood: Laboratory infant assessment to parent report at seven years. In: Molfese VJ, Molfese DL, editors. Temperament and personality development across the life span. Hillsdale, NJ: Erlbaum; 2000. pp. 85–119. [Google Scholar]
- Rothbart MK, Derryberry D, Posner MI. A psychobiological approach to the development of temperament. In: Bates JE, Wachs TD, editors. Temperament: Individual differences at the interface of biology and behavior. Washington, DC: American Psychological Association; 1994. pp. 83–116. [Google Scholar]
- Saudino KJ. Sources of continuity and change in activity level in early childhood. Child Development. 2012;83:266–281. doi: 10.1111/j.1467-8624.2011.01680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Bell MA. Stability in infant frontal asymmetry as predictor of toddlerhood internalizing and externalizing behaviors. Developmental Psychobiology. 2010;52:158–167. doi: 10.1002/dev.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter CA, Putnam S, Jahromi LJ. Exuberant and inhibited toddlers: Stability of temperament and risk for problem behavior. Development and Psychopathology. 2008;20:401–421. doi: 10.1017/S0954579408000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, George CJ. Long-term stability of electroencephalographic asymmetry and power in 3 to 9-year-old children. International Journal of Psychobiology. 2008;67:70–77. doi: 10.1016/j.ijpsycho.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]