Abstract
Dysregulated bone remodeling occurs when there is an imbalance between bone resorption and bone formation. In rheumatic diseases, including rheumatoid arthritis (RA) and seronegative spondyloarthritis, systemic and local factors disrupt the process of physiologic bone remodeling. Depending upon the local microenvironment, cell types, and local mechanical forces, inflammation results in very different effects on bone, promoting bone loss in the joints and in periarticular and systemic bone in RA and driving bone formation at enthesial and periosteal sites in diseases such as ankylosing spondylitis (AS), included within the classification of axial spondyloarthritis. There has been a great deal of interest in the role of osteoclasts in these processes and much has been learned over the past decade about osteoclast differentiation and function. It is now appreciated that osteoblast-mediated bone formation is also inhibited in the RA joint, limiting the repair of erosions. In contrast, osteoblasts function to produce new bone in AS. The Wnt and BMP signaling pathways have emerged as critical in the regulation of osteoblast function and the outcome for bone in rheumatic diseases, and these pathways have been implicated in both bone loss in RA and bone formation in AS. These pathways provide potential novel approaches for therapeutic intervention in diseases in which inflammation impacts bone.
Keywords: Inflammation, Rheumatoid arthritis, Ankylosing spondylitis, Osteoclast, Osteoblast, Boneremodeling, Bone erosions, Bone formation, Entheses, Wnt, DKK1, Sclerostin, IL-23, microRNAs
Introduction
Inflammatory rheumatic diseases have the ability to disrupt the normal process of coupled bone remodeling, in which osteoclasts resorb bone to maintain skeletal integrity and osteoblasts produce osteoid, which is subsequently mineralized to replace resorbed bone. In this review, we will focus on rheumatoid arthritis (RA) as a prototype of inflammatory arthritis that affects articular and periarticular bone, as well as bone in the axial and appendicular skeleton. We will compare and contrast the effects of inflammation on bone in RA with those in the rheumatic diseases categorized as seronegative spondyloarthritis (SpA). The effects of inflammation on bone in these latter diseases share some similarities with those in RA, especially at articular sites, but in axial SpA, the effects of inflammation on periosteal bone are quite distinct, raising important questions about the local bone microenvironments, contributing cell types and factors and the effects of local mechanical stressors to the ultimate outcome for bone.
Three forms of altered skeletal remodeling have been identified radiographically in patients with RA [1–3]. Joint margins are a site at which the inflamed synovium is in direct contact with bone, resulting in the bone “erosions” characteristic of RA. Similar articular erosions are seen in other inflammatory arthritides. Periarticular bone is also altered in RA, likely due to effects of cytokines and factors being expressed in neighboring joints. Finally, systemic osteopenia or osteoporosis involving the axial and appendicular skeleton remote from synovial inflammation is seen in patients with RA [4, 5]. Although systemic bone loss will not be covered in this review, it is likely that pro-inflammatory cytokines and other factors derived from inflamed synovial tissues enter the systemic circulation and mediate effects on bone at these distal sites.
RA presents several unique features that directly impact our understanding of bone remodeling in this disease. In RA patients, there is a phase of autoimmunity that may last 10 years or more, during which patients produce autoantibodies including rheumatoid factor (RF) and/or anti-citrullinated protein antibodies (ACPAs) [6, 7]. During this initial phase of autoimmunity, patients are asymptomatic and often unaware of their potential for developing RA. There is often a brief phase of arthralgia, and expression of serum cytokines increases prior to the onset of joint inflammation. In addition, increasing ACPA reactivity has been demonstrated prior to the onset of RA [8], and ACPAs are a strong predictor of joint erosion [9]. Finally, patients develop clinical disease with the typical symmetrical, inflammatory arthritis characteristic of RA. It has now been demonstrated that even during the pre-clinical phase of disease, prior to synovitis, systemic bone loss occurs in ACPA-positive patients [10], raising very interesting questions regarding the mechanistic role of ACPAs in bone resorption in this disease.
Over the past decade, a great deal has been learned about the factors and cell types driving osteoclastogenesis in RA and other inflammatory arthritides [11–15]. Our group and others have definitively demonstrated that osteoclasts are required for articular bone resorption in RA. Once formed, erosions do not typically repair [16], even in cases in which inflammation is well controlled clinically. Thus, attention has turned to the osteoblast and its role in the process of erosion and erosion repair [17]. It has become evident that the osteoblast is also a target of inflammation in the rheumatic diseases, playing a role in both the lack of repair of articular erosions, and in the bone formation seen in axial SpA.
Patients with seronegative SpA, including ankylosing spondylitis (AS), psoriasis, reactive arthritis, inflammatory bowel disease, and juvenile SpA are termed “seronegative” as they do not produce RF or ACPAs as seen in RA. These patients often develop erosion of articular bone, as well as in some cases erosion of the sacroiliac joints. However, in contrast to RA, patients with these diseases can also exhibit spinal features in response to inflammation that are quite distinct from those seen in RA [18]. These diseases have distinct genetic markers: HLA-B27 is the major genetic risk factor for AS [19] whereas in psoriatic arthritis, susceptibility associates with several HLA-B and C alleles, and different haplotypes seem to track with distinct disease manifestations, such as skin disease or arthritis [20]. In this review, we will focus on the outcomes for bone in AS as an example of diseases affecting the spine and sacroiliac joints and will discuss the mechanisms that may lead to these specific effects on bone.
In AS, inflammation affects the spine at enthesial sites where tendons and ligaments insert into bone. Over time, bone formation occurs, leading ultimately to bony fusion (ankylosis) of the sacroiliac joints and syndesmophyte formation. This process is associated with progressive chronic back pain, reduced mobility, and potentially kyphosis. Syndesmophytes, a hallmark of AS, are new areas of bony growth that are localized to intervertebral disks at the annulus fibrosis. Syndesmophytes may eventually bridge adjacent vertebral bodies (“flowing syndesmophytes”), leading to decreased spinal mobility and disability [21]. The pathobiologic mechanisms regulating bone in seronegative SpA will be addressed, along with a review of new areas of osteoblast biology that impact bone in these rheumatic diseases.
Bone Resorption in RA
Over the past decade, the osteoclast has become widely recognized as the cell responsible for bone erosions in RA. It was first noted that cells expressing an osteoclast marker, tartrate resistant acid phosphatase (TRAP) were present in subchondral bone in surgical samples from RA patients [22]. However, TRAP is not a definitive marker of the osteoclast, as it can be expressed in other cell types including macrophage polycarions. In order to demonstrate definitively that osteoclasts were present in surgical samples in RA, we used several markers to identify osteoclasts, including TRAP, cathepsin K and calcitonin receptor, the definitive marker of a bone-resorbing osteoclast [13]. Large, multinucleated cells were noted not only in subchondral bone, but also at the pannus-bone interface in these surgical samples. These cells expressed all of the osteoclast-specific markers examined, placing osteoclasts squarely at the site of bone erosion. Subsequent studies also showed that osteoclasts were present at similar sites within erosions in animal models of arthritis [11, 23–25]. Receptor activator of NF-κB ligand (RANKL) is also required for this process to occur. Inflamed synovial tissues provide several cellular sources of RANKL, including lymphocytes and fibroblast-like synoviocytes (FLS). RANKL synergizes with proinflammatory cytokines to promote the differentiation of osteoclasts from mononuclear precursors in synovial tissues.
We went on to demonstrate that osteoclasts are essential for bone erosion in the K/BxN serum transfer model of arthritis by demonstrating that mice deficient in RANKL, a factor required for osteoclast differentiation, do develop arthritis as well as cartilage destruction, but are protected from bone erosions in the absence of osteoclasts [11]. These findings were confirmed in a follow-up study in which mice lacking c-fos, a transcription factor essential for osteoclastogenesis, were crossed with transgenic mice expressing human TNF (hTNFtg) [12]. These mice are osteoclast deficient and develop a TNF-dependent inflammatory arthritis closely resembling RA. Despite the presence of inflammation, the arthritic joints of these mice were also protected from bone erosion and destruction. Furthermore, blocking the RANK-RANKL interaction using osteoprotegerin (OPG), the decoy receptor for RANKL, protected against systemic bone loss in hTNFtg mice [26], as well as arthritic bone loss in other animal models of arthritis [14, 27].
Inhibition of osteoclast activity in patients with RA has also demonstrated some efficacy in inhibiting the progression of bone erosion. The bisphosphonate zolendronic acid has been shown to reduce development of new erosions in the joints of both arthritic mice [28, 29] and in patients with RA [30]. Furthermore, neutralization of RANKL with the monoclonal antibody denosumab attenuated bone erosion and loss of hand bone mineral density (BMD) and systemic bone in RA patients [31–33]. Collectively, these studies have confirmed that osteoclasts mediate bone erosions in patients with RA and provide a target cell type for the prevention of articular bone loss.
Osteoclastogenesis is enhanced in the RA joint by several factors. Well known are the effects of pro-inflammatory cytokines on osteoclastogenesis, but recently, new factors have been demonstrated to contribute to this process, including autoantibodies and microRNAs. Cytokines including TNF and interleukin (IL)-1 and IL-17 promote the differentiation of osteoclast precursors to osteoclasts [34–36] through several mechanisms, including inducing the upregulated expression of RANKL in osteoblasts. IL-17 [37], as well as TNF in conjunction with IL-6/sIL-6R [38], induce RANKL expression in FLS. TNF also directly promotes the differentiation of osteoclast precursor cells, as well as expands the pool of these precursor cells [39]. In addition, IL-1 directly enhances the ability of osteoclasts to resorb bone [40] and is a mediator of TNF-induced osteoclastogenesis [34].
The role of IL-6 in osteoclastogenesis is more complex. Although IL-6 induces RANKL on FLS [38], the effect of IL-6 on osteoclast progenitors is inhibitory rather than stimulatory [41]. Yoshitake and colleagues demonstrated in vitro that IL-6 directly inhibits the differentiation of osteoclast progenitors via disruption of RANK signaling pathways [42]. The majority of in vivo data indicate, however, that the net effect of IL-6 is pro-inflammatory and pro-osteoclastogenic [43–46].
Clinical studies have helped to validate the impact of pro-inflammatory cytokines on osteoclastogenesis in RA. There is a strong relationship between the presence on MRI scanning of what has been termed “bone marrow edema” and the subsequent progression of bone erosions in RA patients [47–49]. The phrase bone marrow edema was coined to reflect the decreased fat and increased water content seen by MRI in the marrow of arthritic joints. Histologic analysis of areas of bone edema in animal models of inflammatory arthritis and of MCP joints from patients with RA has revealed that this MRI finding represents the replacement of bone marrow fat by inflammatory infiltrates, comprised largely of mature B cells and activated T cells [47]. Thus, bone marrow edema is better referred to as “osteitis.” This influx of inflammation into the marrow space introduces cell types that express RANKL, as well as pro-inflammatory cytokines that further induce osteoclastogenesis and promote the progression of articular bone erosions. In fact, MRI findings from MCP heads removed surgically from RA patients were correlated with histologic assessments, revealing that osteoclast density on trabecular bone was higher in samples showing osteitis on MRI than in those without osteitis [50]. The development of biologic agents that can retard or arrest radiographic progression in RA, including anti-TNF agents [51], IL-6R antagonists [52], and small molecule agents that block JAK/STAT signaling [53–55], among others, has revolutionized the treatment of this disease.
Periarticular Bone Loss
Periarticular bone loss is another important feature in patients with RA that results from an infiltrate of inflammatory cells, with a possible contribution from decreased mobilization. Periarticular bone lies deep to the “tide mark,” a specialized area of calcified cartilage that is easily identified by its metachromatic staining pattern, separating hyaline cartilage from the underlying subchondral bony plate. Deep to the subchondral bone is trabecular, or cancellous bone, which intercalates with the bone marrow. Periarticular osteopenia is typically seen in this region in RA and is often an early radiographic finding, present even before the onset of identifiable marginal joint erosions [56]. Digital X-ray radiogrammetry has revealed that periosteal bone changes may predict erosive disease [56]. Histomorphometry performed in RA patients undergoing arthroplasty has also revealed enhanced bone resorption as well as bone formation in this region, identifying this as a region of increased bone turnover [57].
Autoantibody-Mediated Osteoclastogenesis
Clinical studies have demonstrated that high titers of ACPAs are associated with radiographic progression in RA patients [58, 59]. Furthermore, ACPAs and rheumatoid factor together have been shown to have an additive effect on erosion size and number in patients with RA [60]. Since autoantibodies have been shown to promote inflammatory-mediated as well as inflammatory-independent bone loss in RA [61], there has been a great deal of interest in possible mechanisms. Stimulation of mononuclear cells or macrophages with immune complexes and ACPAs from RA patients results in the production of high levels of TNF by these cells, contributing to osteoclastogenesis [62–64]. Recent studies have shown that autoantibodies not only bind macrophages, but also bind osteoclast precursors. ACPAs bind citrullinated vimentin on the surface of osteoclast precursors, inducing these precursors to produce TNF, thus promoting their differentiation to mature osteoclasts [65]. In keeping with this finding, transfer of ACPAs into mice induced release of TNF from osteoclast precursors, driving osteoclastogenesis and resulting in osteopenia. In addition, Fc receptors on osteoclast precursors have been shown to influence osteoclastogenesis. For example, crosslinking FcγRIVon osteoclast precursors was shown to enhance osteoclast differentiation, whereas deletion of FcγRIV decreased osteoclast numbers and bone destruction in arthritic mice [66]. These studies demonstrate a role for the FcγRIV in inducing downstream signals that regulate the process of osteoclastogenesis.
The effect of immunoglobulin (IgG) sialylation on the interaction of immune complexes with osteoclasts has also been investigated [67]. Modification of IgGs by attachment of sialic acid residues to the Fc portions is known to mediate the anti-inflammatory effects of intravenous IgG [68]. Harre and colleagues have demonstrated that only non-sialylated immune complexes stimulate osteoclastogenesis in vitro and in vivo. Furthermore, administration of a sialic acid precursor results in elevated sialylation levels of IgG and decreased bone erosions in mice with collagen-induced arthritis [67]. Together, these studies emphasize the importance of autoantibodies in mediating bone loss in RA and suggest a new mechanism by which osteoclastogenesis in RA may be promoted independent of inflammation itself.
These findings are further supported by the surprising discovery that ACPA-positive healthy individuals show signs of bone loss compared to ACPA-negative individuals [10]. Micro-CT analysis was performed on MCP joints of age and gender-matched ACPA-positive individuals who showed no signs of synovitis and ACPA-negative healthy individuals. Although no evidence of bone erosion was seen in ACPA-positive individuals, bone volume per total volume and BMD were both significantly reduced in ACPA-positive individuals compared to ACPA-negative controls. Since ACPAs can be detected in serum years before the onset of RA [69, 70], it appears likely that bone damage in RA precedes the clinical onset of disease through mechanisms independent of inflammation.
Role of microRNAs (miRNAs) in Inflammation and Osteoclastogenesis
In addition to autoantibodies, recent studies have identified an important role for miRNAs in bone homeostasis. miRNAs are small non-coding RNAs 20–22 nucleotides in length that play an important role in the epigenetic regulation of gene expression [71, 72]. Each miRNA has the capacity to target several mRNAs for cleavage by binding to specific complementary sequences, typically in the 3′UTR of target mRNAs, suppressing target mRNA stability or, alternatively, by blocking protein translation and modifying cellular protein levels. This allows for the coordinated regulation of multiple gene targets within a pathway, or within several pathways that span biologic processes. Because miRNAs target existing mRNAs, they can act rapidly to regulate and fine tune gene expression. It is now appreciated that miRNAs are altered in their expression pattern between normal and diseased states, and thus may serve as therapeutic targets as well as provide biomarkers for the progression of disease.
miRNAs also play a role in cell–cell communication. They can regulate genes within the cell of origin or can be packaged in exosomes to mediate cell–cell interactions and the regulation of genes within target cell types [73, 74]. Exosomes are vesicles measuring 40–100 nm that are formed within secreting cells. Exosomal transfer of miRNAs to recipient cells occurs, and it has been shown that the transferred miRNAs are functional. Many examples of this type of cell–cell communication have been demonstrated [75], including a role for exosomal miRNA transfer in immune responses, transfer of miRNA-loaded exosomes from T cells to antigen presenting cells [76], and bone marrow mesenchymal stromal cell-derived exosomes promoting tumor growth in multiple myeloma [77].
miRNAs have been shown to play an important role in regulating inflammation and multiple lines of evidence implicate miRNAs in the pathogenesis of RA [78–80]. Expression of miR-23b is downregulated in arthritic synovium, and administration of miR-23b was shown to decrease synovitis in RA models by targeting multiple NF-κB signaling components, thus suppressing inflammation [81]. Conversely, miR-155, expressed in macrophages from RA patients, has been shown to promote inflammation by inducing expression of the pro-inflammatory cytokine TNF [82, 83]. In keeping with these findings, miR-155-deficient mice were protected from collagen-induced arthritis [82, 84]. Importantly, miRNAs have also been shown to play a critical role in the regulation of bone remodeling. For example, miR-155 and miR-21 have been implicated in the process of osteoclastogenesis [85, 86]. miRNA-dependent mechanisms can turn off genes that promote monocyte differentiation to instead promote the differentiation of monocytes to mature, bone-resorbing osteoclasts [87]. Interestingly, it has been shown that treatment of rats with antigen-induced arthritis with miR-124 not only suppressed measures of inflammation, but also targeted critical mRNAs involved in osteoclastogenesis, including NFATc1 [88]. Further elucidation of bone-related pathways regulated by miRNAs in rheumatic diseases may provide new insights into pathogenesis, as well as new targets for therapy.
Signaling Pathways that Regulate Osteoblast Differentiation
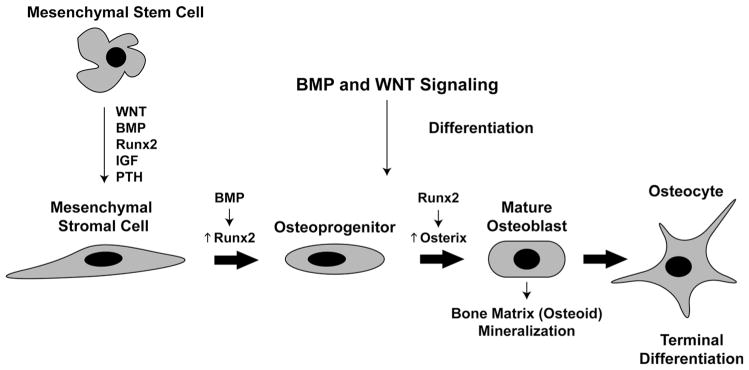
Despite the focus on osteoclasts at known sites of bone loss in RA, our laboratory and others have shown that inflammation not only induces osteoclastogenesis, but also inhibits osteoblast differentiation and function. This inhibition contributes to the development of arthritic bone loss in RA, as well as to the markedly diminished capacity of erosions to heal. For this reason, it is important to understand the pathways that promote or inhibit osteoblast differentiation. Unlike osteoclasts, osteoblasts derive from mesenchymal precursor cells. Several factors are known to regulate the stages of differentiation from mesenchymal stem cells (MSCs) to mature osteoblasts (Fig. 1). These include growth factors and hormones such as insulin-like growth factor (IGF) and parathyroid hormone (PTH) that aid in the transition of MSCs to mesenchymal stromal cells [89]. Upregulation of the pro-osteogenic transcription factor runt-related transcription factor 2 (Runx2) commits stromal cells toward an osteoprogenitor cell fate, while expression of the transcription factor osterix further promotes differentiation of the cell into a mature osteoblast. Mature osteoblasts produce type I collagen as well as non-collagenous proteins involved in bone mineralization, including osteocalcin and bone sialoprotein. This newly formed bone matrix eventually surrounds mature osteoblasts, embedding these cells within the bone matrix as terminally differentiated osteocytes.
Fig. 1.
Stages of osteoblast differentiation. Osteoblasts are derived from mesenchymal stem cells. Wingless (WNT) and bone morphogenic protein (BMP) pathways promote their differentiation to mesenchymal stromal cells, in conjuction with Runx2, insulin-like growth factor (IGF) and parathyroid hormone (PTH). Activation of BMP signaling promotes Runx2 expression in stromal cells, leading to further differentiation to osteoprogenitors. Runx2 subsequently induces expression of the transcription factor osterix, with further differentiation to a mature osteoblast. These ultimately become embedded in bone matrix as terminally differentiated osteocytes
The wingless (Wnt) [90] and bone morphogenetic protein (BMP) [91] pathways known to regulate skeletal development and organogenesis are also critical pathways regulating osteoblast differentiation. Wnt signaling includes the canonical Wnt/β-catenin pathway and two noncanonical pathways, the Wnt-calcium and the Wnt-planar cell polarity pathways [92]. In the canonical Wnt pathway, secreted Wnts, such as Wnt1 and Wnt3a, bind and activate a complex that includes the low-density lipoprotein receptor related proteins (LRP)5 and LRP6. These receptors complex with Frizzled co-receptors in the plasma membrane to promote the stabilization of cytosolic β-catenin, allowing its translocation to the nucleus to induce transcription of genes that promote osteoblast differentiation and bone formation.
Several antagonists to the Wnt pathway, including Dickkopf (DKK) and secreted frizzled-related protein (SFRP) family members, as well as sclerostin, have been identified. DKK-1 crosslinks LRP5/6, leading to the suppression of Wnt signaling in osteoblast precursors. Inhibition of DKK-1 expression has been linked to high bone mass [93] while overexpression of DKK-1 results in osteopenia in mice [94]. SFRPs inhibit Wnt signaling by binding directly to Wnt proteins. Deletion of the SFRP1 gene results in increased bone volume in mice [95], while its overexpression has been linked to decreased bone density [96]. Interestingly, inflamed synovial tissue has been found to be a source of DKK-1, which inhibits osteoblast-mediated bone formation in arthritic joints [97]. In contrast, the expression of DKK-1 is diminished in animal models and patients with AS [98]. The expression of SFRPs is also upregulated in arthritic synovial tissue [99] and likely contributes to the inhibition of osteoblast differentiation in inflammatory arthritis.
Sclerostin, a glycoprotein-secreted predominantly, if not exclusively, by osteocytes, inhibits the canonical Wnt signaling pathway by binding to the LRP5/6 receptor [100]. The effects of sclerostin on bone were originally brought to light when loss-of-function mutations in or near the sclerostin-encoding gene SOST were identified in patients with van Buchem’s disease [101–103] and sclerosteosis [104, 105], diseases associated with high bone mass. Deletion of the SOST gene increased bone formation in mice [106] while overexpression of SOST leads to significant bone loss [107].
Mesenchymal stem cells also require activation of BMP signaling to commit to the osteoblast lineage. BMPs belong to the transforming growth factor beta (TGF-β) superfamily and are secreted mainly by osteoblasts, chondrocytes, and endothelial cells [91]. Pro-osteogenic BMPs, such as BMPs 2, 4, and 7, bind membrane-bound receptors and result in phosphorylation of intracellular SMADs 1/5/8. These factors complex with SMAD4 and translocate to the nucleus to promote the transcription of BMP-responsive genes. A variety of secreted molecules, such as noggin and sclerostin itself, have been identified that sequester BMP ligands and inhibit their interaction with their receptor [108]. Dysregulation of BMP signaling has been associated with several skeletal disorders including heterotopic ossification, osteoporosis, and low and high bone mass diseases.
Role of miRNAs in Osteoblast Differentiation and Bone Formation
miRNAs have been shown to play a role not only in osteoclastogenesis, but also in the differentiation and function of osteoblasts [109, 110]. The Dicer enzyme is an endoribonuclease essential for processing of pre-miRNAs to their functional, mature form. Dicer null embryos do not survive, but conditional deletion of Dicer in skeletal cells has confirmed that miRNAs control bone turnover in the adult skeleton as well as regulating osteogenesis and bone mass. Deletion of Dicer in early osteoblast progenitors inhibits their maturation, demonstrating an essential role for miRNAs in bone formation. In contrast, deletion in mature osteoblasts leads to a marked increase in trabecular and cortical bone [109]. Specific miRNAs, including the miR-29 family, have been shown to target inhibitors of the Wnt signaling pathway to promote osteoblast differentiation [111]. In addition, the miR-23a-27a-24-2 cluster regulates the induction of osteogenesis and osteoblast differentiation [112]. Thus, it is reasonable to hypothesize that miRNAs play a role in the regulation of osteoblast function that results from inflammation in RA and other rheumatic diseases. This is an area of great interest, especially given the potential impact of a single miRNA on the regulation of an entire program of gene expression.
Limited Repair of Bone Erosions in the RA Joint
Clinical studies have shown that therapies that reduce joint inflammation can slow or halt the progression of osteoclast-mediated bone resorption in patients with RA. Despite treatment, however, repair of existing erosions is unusual [16]. These persisting erosions are associated with cartilage loss, as subchondral bone, which provides the scaffold for articular cartilage, is typically eroded. With erosion of subchondral bone, articular cartilage is lost. Persistent erosions have been shown to be associated with functional decline, and are associated with joint instability and likely also changes in mechanical forces across joints, which may further impact articular cartilage.
Dohn and colleagues investigated the frequency and extent of erosion repair in patients with RA given combination therapy with anti-TNF and methotrexate (MTX) [113]. After 12 months of therapy, high-resolution computed tomography (CT) of the wrist and MCP joints demonstrated that although erosion progression was halted, repair of erosions was rare. Subsequently, it was demonstrated that although biologic therapy significantly decreased synovitis scores in a cohort of RA patients, all patients had remaining synovitis after 12 months of treatment as determined by MRI [114]. This same study showed that erosion repair occurred in only 6 % of patients treated with adalimumab, suggesting that residual inflammation may impair osteoblast function and healing of erosions.
Similar findings were published in a study that examined erosion repair in RA patients treated with TNF inhibitors and MTX compared to matched patients treated with MTX alone [115]. The width and depth of erosions in MCP joints were measured by high-resolution microCT at baseline and after 1 year of treatment. Repair of erosions was shown to be very limited, and all erosions could still be identified following treatment. However, the mean depth of erosions decreased significantly by −0.1 mm (average erosion size was 2.4 mm) in patients treated with TNF inhibitors and MTX compared to controls. Deeper erosions showing signs of sclerosis at the base were found to be particularly prone to repair compared to more shallow erosive lesions.
These studies were extended to examine RA patients treated with IL-6R blockade [116]. Single bone erosions in MCP joints were imaged using microCT scanning at baseline and 1 year after treatment. Here, the width (as opposed to the depth) of erosions significantly decreased by −0.11 mm (average erosion size was 2.23 mm). Nevertheless, all erosions could be visualized 1 year later, again suggesting that IL-6R blockade does not lead to substantial healing of bone lesions within this time frame.
Inhibition of Osteoblast Function in RA Joints
Many studies now suggest that pro-inflammatory cytokines not only provoke osteoclastogenesis, but additionally contribute to bone loss by inhibiting osteoblast differentiation. For example, TNF is a potent inhibitor of osteoblast differentiation in cultured cells. Treatment of calvarial osteoblasts or the MC3T3 osteoblast-lineage cell line with TNF inhibited differentiation, as shown by a reduction in mineralizing nodules and osteocalcin secretion [117]. TNF also induces degradation of Runx2, a critical transcription factor for osteoblast differentiation [118]. This TNF-induced Runx2 degradation was mediated by upregulation of the ubiquitin ligases Smurf1 and Smurf2 [119]. In addition, high dose TNF treatment of osteoblast precursor cells induces their apoptosis [120]. Exposure of osteoblast cultures to IL-1 has also been shown to inhibit mineralizing nodule formation, as well as collagen protein synthesis and cellular replication [121], and IL-1 impairs the recruitment and migration of osteoblasts toward chemotactic factors [122]. Furthermore, the interaction of IL-6 with sIL-6R on osteoblasts upregulates prostaglandin E2 synthesis and reduces the ratio of OPG/RANKL, thus promoting osteoclast differentiation [123]. Thus, in vitro studies demonstrate that pro-inflammatory cytokines influence the osteoblast by impairing its differentiation and/or function and, in some cases, by promoting osteoblasts to induce osteoclast differentiation.
Our laboratory has demonstrated in an in vivo setting that arthritic inflammation inhibits osteoblast differentiation and function. Arthritis was induced in mice using the K/BxN model and dynamic histomorphometry was adapted to erosion sites to evaluate bone formation rates (BFRs) [124]. BFRs were significantly reduced at bone surfaces adjacent to inflammation compared to bone surfaces adjacent to normal marrow, demonstrating that inflammation inhibits osteoblast activity. Furthermore, in and around sites of articular erosion, there was a complete absence of cells expressing late-stage osteoblast lineage markers (mature osteoblasts). These findings demonstrate that synovial inflammation inhibits the capacity of osteoblasts to mature and form mineralized bone.
Studies in the hTNFtg model of RA have shown that cells within inflamed synovial tissues secrete the Wnt signaling pathway antagonist DKK-1, impairing osteoblast-mediated bone formation [97]. Furthermore, TNF was shown to upregulate DKK-1 expression in synovial fibroblasts, as well as in osteoblasts. Blockade of DKK-1, when given at the onset of inflammation, led to an absence of joint erosions in typical sites, despite the presence of arthritic inflammation. However, DKK-1 blockade also led to an upregulation of OPG, raising the possibility that erosions did not occur in this setting due to inhibition of RANKL activity. Histomorphometry performed on the periosteal surface of bone showed increased BFRs with DKK-1 blockade, as well as an increase in osteoblast numbers and osteoid deposition in the arthritic mice treated with the DKK-1 neutralizing antibody compared to controls. Thus, erosion was essentially absent after DKK-1 blockade, but periosteal bone formation occurred instead, which is not a usual feature of the hTNFtg mouse model, suggesting that periosteal bone formation is inhibited by DKK-1.
Indeed, clinical studies have validated the relevance of DKK-1 in arthritic joint remodeling. Serum levels of DKK-1 were significantly increased in patients with RA compared to healthy controls, and DKK-1 was expressed in the inflamed synovium from patients with RA compared to controls [97]. Recently, three DKK-1 single nucleotide polymorphisms (SNPs) were identified that are associated with production of increased levels of functional DKK-1 in sera, as well as with increased joint destruction over time in patients with RA [125]. These studies illustrate the impact of the Wnt antagonist DKK-1 on osteoblast inhibition in the setting of inflammatory arthritis.
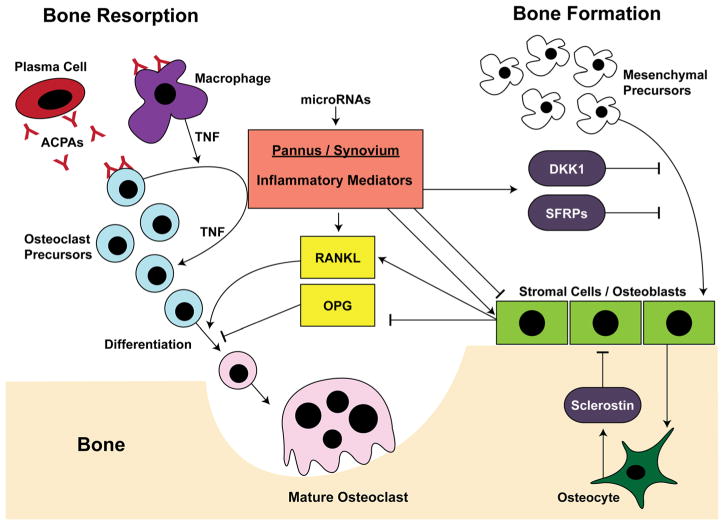
Our laboratory identified several Wnt signaling antagonists whose expression was upregulated in arthritic synovium using the K/BxN model, including members of the DKK family as well as SFRP1 and 2 [99, 124]. In addition, we induced arthritic inflammation and subsequently allowed the inflammation to resolve. Dynamic histomorphometry and microCT showed that upon resolution of inflammation, mature osteoblasts populated the eroded bone, and bone formation was induced at these sites, followed by repair of erosions. Notably, as synovial inflammation almost completely resolved, synovial expression of the Wnt antagonists sFRP1 and sFRP2 was downregulated and expression of the Wnt agonist Wnt10b was induced compared to arthritic synovium. These findings demonstrated that significant resolution of inflammation is necessary to promote Wnt signaling and erosion repair in the arthritic joint. Thus, cells within inflamed synovial tissues secrete factors that antagonize the Wnt signaling pathway and inhibit osteoblast differentiation and osteoblast-mediated bone formation. These effects are manifested clinically in the arthritic joint, where persistent inflammation likely contributes to the limited healing of erosions. Figure 2 provides a summary of pathways involved in the regulation of osteoclasts and osteoblasts in RA.
Fig. 2.
Cell types and factors regulating bone in rheumatic disease. Resorption: The inflamed synovium/pannus produces several inflammatory mediators that enhance osteoclastogenesis and inhibit osteoblast maturation in the joint, leading to the development and persistence of articular bone erosions. These mediators promote the differentiation of osteoclast precursors to mature osteoclasts, in part by the upregulation of receptor activator of NF-κB ligand (RANKL). Anti-citrullinated protein antibodies (ACPAs) can also promote osteoclastogenesis by binding to macrophages and/or osteoclast precursor cells and inducing TNF production, thus enhancing cellular expansion and differentiation. Formation: Bone formation occurs through the action of mature osteoblasts that produce organic bone matrix and orchestrate bone mineralization. These derive from mesenchymal precursors, whose differentiation is inhibited by antagonists of the Wnt signaling pathway, including Dickkopf (DKK) and secreted frizzled-related protein (SFRP) family members, and sclerostin, derived from osteocytes embedded in bone matrix. Inflammatory mediators also induce the production of RANKL, and inhibit the production of osteoprotegerin (OPG) by stromal cells/ osteoblasts. Furthermore, microRNAs can regulate these processes at several levels
Recently, agonists of Wnt signaling have been proposed as therapies for the treatment of joint destruction associated with aging and inflammation. One such example is Wnt4, an agonist of the non-canonical Wnt pathway. Yu and colleagues demonstrated a dual role for Wnt4 in increasing bone formation as well as in reducing bone resorption [126]. Transgenic mice overexpressing Wnt4 in osteoblasts were bred to arthritic hTNFtg mice (hTNFtg/OB-Wnt4). Importantly, hTNFtg/OB-Wnt4 mice had significantly less joint swelling and joint destruction than hTNFtg controls. Osteoblast counts as well as bone formation and mineral apposition rates were significantly elevated in hTNFtg/OB-Wnt4 compared to controls. Moreover, Wnt4 inhibited osteoclast formation and bone resorption by inhibiting NF-κB signaling in osteoclast precursors and macrophages. The ability of Wnt4 to diminish inflammation, decrease bone resorption, and increase bone formation makes it an attractive candidate for alleviating inflammatory joint destruction and further studies in this area are warranted.
Mechanisms of Bone Formation in AS
In contrast to the bone loss seen in patients with RA, inflammation affects the spine in AS at the entheses, sites at which tendons and ligaments insert into bone, leading ultimately to osteoblast activity and syndesmophyte formation. There is some debate as to whether bone formation follows an erosion event at these sites or occurs in the absence of erosion. If erosion does occur initially, factors released from the bone matrix, including TGF-β and BMPs, could promote this process. Syndesmophytes result from the process of endochondral ossification [21, 127, 128] with a cartilaginous phase initially, followed by remodeling and ultimately mineralization to form bone. Interestingly, in a recent study, the fate of murine hypertrophic chondrocytes was tracked from embryonic life, and it was demonstrated that these cells can become osteogenic and can survive into adult life. This has been termed a “chondrocyte-to-osteoblast lineage continuum” and may be relevant to the events leading to new bone formation in AS [129]. Studies of tissues from sacroiliac joints from patients with AS have shown localized areas of endochondral ossification [130]. These studies also demonstrated cellular expression of mRNA for pro-inflammatory cytokines, as well as TGF-β, which was expressed by cells in proximity to regions of bone formation. Significant advances have been made in further elucidating the pathways involved in bone formation in AS, including the Wnt, BMP, and IL-23/IL-22/ IL-17 signaling pathways.
Antagonists to the Wnt Signaling Pathway
Sclerostin is one antagonist of Wnt signaling that has been implicated in the process of bone formation in AS with dysregulated production demonstrated in this disease. Immunohistochemical staining of joints from AS patients showed significantly decreased sclerostin expression compared to healthy controls. In addition, lower levels of sclerostin were detected in the serum of AS patients compared to controls and were associated with increased syndesmophyte formation [131]. Interestingly, immune complexes composed of autoantibodies to sclerostin were also identified in the sera of patients with AS compared to healthy controls [132]. It is likely that these autoantibodies would interfere with the function of sclerostin as a Wnt signaling antagonist and would therefore lead to increased bone formation. However, the role of sclerostin blockade in AS is not entirely clear, as patients with AS can also develop articular erosions. In arthritic hTNFtg mice, sclerostin blockade was shown to inhibit the local bone and cartilage damage that occurred in inflamed joints [133]. Therefore, sclerostin blockade may alleviate the bone erosions seen in erosive inflammatory arthritis. These studies implicate sclerostin in contributing to bone remodeling in both AS and RA.
Expression of other Wnt pathway inhibitors, particularly DKK-1, is also decreased in mouse models and patients with AS. The inflammatory environment in hTNFtg mice typically provokes osteoclastogenesis and bone erosions. However, DKK-1 blockade in this model led to an increase in Wnt signaling and fusion of the sacroiliac joints [134], transforming the typically erosive phenotype to one of ankylosis. In another model of AS, the proteoglycan-induced spondylitis mouse, IHC staining, and mRNA expression levels showed significantly reduced levels of DKK-1 and SOST in the spine of inflamed mice compared with controls, again supporting the potential importance of Wnt inhibitors in the process of ankylosis [135].
In AS patients, DKK-1 levels were reported to be significantly lower than those in healthy controls and RA patients based on DKK1 binding to its LRP6 receptor [97]. Furthermore, functional DKK-1 levels were shown to be higher in the sera from AS patients who showed no syndesmophyte growth compared to those with bony spur growth [136]. These findings suggest that decreased activity of DKK-1 contributes to the osteoproliferation in AS. Wnt agonists have also been associated with AS bone formation. In a recent study, Wnt3a levels in the serum were found to be elevated in a cohort of 204 patients with AS compared to controls [137], a finding consistent with increased osteoblast activity. Taken together, these data implicate the Wnt signaling pathway as a contributor to the pathogenesis of bone accrual in AS.
The BMP Pathway and its Contribution to Bone Formation in AS
BMP family members were originally shown to promote endochondral bone formation and ankylosis in the DBA/1 spontaneous mouse model of SpA [127]. In this model, immunohistochemical stains revealed the expression of BMP2, BMP6, and BMP7 protein at sites of enthesitis. Overexpression of noggin, a broad-spectrum BMP antagonist, inhibited the progression of ankylosis in these mice, providing evidence that ankylosis can be inhibited by targeting the BMP pathway. In addition, active BMP signaling was confirmed in enthesial biopsies from patients with SpA, as shown by the presence of nuclear phosphorylation of Smads1/5.
BMPs have also been measured in the serum of AS patients. In one study, Chen et al. demonstrated that serum levels of BMP2, BMP4, and BMP7 were higher in AS patients with radiographic signs of ankylosis compared to patients without spinal fusion and healthy controls [138]. An additional study showed increased serum levels of BMP2 and BMP7 in 30 patients with AS compared to healthy controls. BMP2 levels correlated with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) questionnaire. BMP7 levels correlated with the Bath Ankylosing Spondylitis Radiology Index (BASRI) that takes into account syndesmophyte formation [139]. In contrast, a study by Wendling et al. found that although serum BMP-7 levels trended higher in AS patients compared to controls, the difference was not statistically significant [140]. Finally, the production of autoantibodies against the BMP antagonist noggin has been identified in the serum of AS patients. These autoantibodies may inhibit the effect of noggin and lead to increased BMP signaling [132]. Taken together, there is definite evidence that BMP signaling contributes to bone formation in AS, making this pathway an attractive candidate for therapeutic targets.
Inflammatory Pathways Mediating Bone Formation in AS
Newly identified pathways mediating inflammation and bone formation in AS suggest that innate immune mechanisms play a role. In addition to TNF, IL-23 and IL-17 have been implicated as cytokines central to pathogenesis [141, 142]. Early studies by Sherlock et al. have identified a role for IL-23, a member of the IL-12 family of cytokines, in both inflammation and bone formation in a model of SpA. These studies demonstrated that overexpression of IL-23 resulted in inflammation at enthesial sites as the initial manifestation of disease. Subsequently, synovial tissues also become inflamed, supporting the hypothesis that the initial lesion in SpA is likely to be localized to enthesial sites, with subsequent spreading of inflammation to joint tissues [143]. These investigators also demonstrated that IL-23 in enthesial tissues induced the production of IL-17A and F, and IL-22, by a unique and newly described subset of enthesial resident T cells, identified as RORγT+CD3+CD4−CD8−. These cells express the IL-23R and produce disease-promoting cytokines in response to IL-23. Interestingly, these unique T cells are found not only at enthesial sites, but also in the aortic ring, another site of inflammation in AS [142, 144]. Subsequent to these discoveries, there has been a great deal of interest in the biology of IL-23 and in identifying the potential source of this cytokine in SpA. There is mounting evidence for increased production of IL-23, potentially in the gut, and microscopic gut inflammation has been identified in patients with axial SpA [145]. In addition, there may be an altered responsiveness to this cytokine. HLA-B27 itself has also been shown to induce the production of IL-23 [146, 147], likely through activation of autophagy pathways. Finally, SNPs in the IL-23R are also associated with susceptibility or protection from AS, especially in Europeans [148], lending additional credence to the association of IL-23 in the pathogenesis of this disease.
Through cellular depletion and blocking antibody studies, it has been shown that although IL-17 plays a role in pathogenesis, it is not required, and IL-22 is a key pathogenic cytokine. Furthermore, IL-22 was found to induce the differentiation of osteoblasts from local precursor cells at the enthesial sites through induction of STAT3, which may promote the bone formation seen at these sites. Interestingly, IL-23 has also been shown to promote osteoclastogenesis in co-culture systems with osteoblasts [149] and to upregulate RANK expression on osteoclast precursor cells, to further induce osteoclastogenesis [150]. In vitro studies also suggest that IL-23 may act via IL-17 to induce osteoclastogenesis [151]. Therefore, the effects of IL-23 on bone appear to be pleotropic, and other mechanisms may be at play in the process of syndesmophyte formation in SpA.
One such mechanism is almost certainly mechanical loading. In animal models, sites of periosteal bone formation occur at tendon and ligament insertion sites into bone, sites of mechanical stress, and it has long been suspected that mechanical forces are important in bone formation in SpA [21, 152, 153]. Recently, a pre-clinical model of SpAwas used in a “proof-of-concept” study to demonstrate that mechanical strain indeed regulates inflammation at enthesial sites, as well as bone formation at these sites [153]. In this study, the TNFΔARE model was used, a model that shares many of the skeletal features seen clinically in AS, and in which inflammation occurs initially at enthesial sites. Hind limbs were unloaded, and inflammation at the Achilles tendon was compared with the normally loaded, weight bearing controls. In the unloaded limbs, there was a significant suppression of both inflammation and new bone formation. Furthermore, ERK1/2 signaling played a critical role in inflammation associated with mechanical stress. This study confirms the importance of anatomic sites of inflammation, as well as the role of tissue injury via weight bearing and stress on both inflammation itself, and on outcomes for bone.
Therapeutic Intervention in AS
The development of biologic agents targeting TNF has considerably alleviated the spinal and joint inflammation and clinical disease activity parameters associated with AS. However, the prevention, and certainly reversal, of enthesial bone formation and structural damage remains a challenge. Clinical studies have evaluated whether TNF inhibition affects structural bone changes and radiographic progression in patients with AS over a 2-year period. In one such study, treatment with etanercept led to decreased spinal inflammation, but inhibition or reversal of radiographic progression was not detected by MRI [154]. Similar results were found using infliximab [155] and adalimumab [156]. These studies suggested that structural progression in the spine of AS patients is independent of TNF.
Recently, however, Haroon et al. analyzed the long-term effects of TNF inhibition on radiographic progression in a large cohort of AS patients [157] and did show a protective effect of TNF inhibitors on radiographic progression. Treatment was most beneficial when used early in disease (within 5 years of onset) and when given for >4 years. An additional study comparing AS patients treated with infliximab vs. historical controls also showed that treatment with a TNF inhibitor over a long period of time (8 years) may limit syndesmophyte formation [158]. In the treated group, 1.0± 0.6 new syndesmophytes/patient were identified vs. 2.7±0.8 syndesmophytes/patient in the historical controls. Nevertheless, bone formation was seen in both the untreated and treated patients over the 8-year period. Thus, biologic agents that block TNF activity successfully control spinal inflammation, but likely have a mild impact on radiographic progression in AS. Therefore, understanding the pathways and potential therapeutic targets that modulate bone formation in AS is of importance.
Conclusion
Tremendous progress has been made over the past decade in understanding the interactions of the immune system and bone in systemic rheumatic diseases. Emphasis has been placed on pathways and factors inducing osteoclastogenesis, as osteoclasts have been shown definitively to mediate local articular bone erosion, as well as loss of periarticular bone and bone in the axial and appendicular skeleton. However, it is now evident that inflammation also impacts osteoblast differentiation and function. In rheumatic diseases, activation or inhibition of the Wnt and BMP signaling pathways, both essential pathways for the differentiation and function of osteoblasts, results in very different outcomes for bone. Furthermore, the local anatomic microenvironment in which inflammation exists in these diseases is critical in determining whether bone is resorbed or formed. Specific cell types and factors present/ expressed in microenvironments, as well as mechanical forces that may be transduced locally, can clearly influence bone phenotypes. Finally, newly identified mechanisms, including the regulation of skeletal pathways by autoantibodies and miRNAs, shed further light on underlying processes. These insights into pathophysiologic mechanisms by which inflammation impacts bone thus provide potential novel approaches for the treatment of rheumatic diseases, the goal of which is to prevent alterations in bone homeostasis and thus to preserve patient function.
Footnotes
Conflict of Interest Rebecca Baum and Ellen Gravallese declare that they have no conflict of interest.
Contributor Information
Rebecca Baum, Email: rebecca.baum@umassmed.edu.
Ellen M. Gravallese, Email: ellen.gravallese@umassmed.edu.
References
- 1.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gravallese EM, Goldring SR. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum. 2000;43:2143–2151. doi: 10.1002/1529-0131(200010)43:10<2143::AID-ANR1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Gravallese EM, Goldring SR, Schett G. Osteimmunology: interaction of the Immune and skeletal systems. 2. Academic Press; London, Burlington MA, San Diego CA: 2015. The role of the immune system in the local and systemic bone loss in inflammatory arthritis. [Google Scholar]
- 4.Deodhar AA, Woolf AD. Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol. 1996;35:309–322. doi: 10.1093/rheumatology/35.4.309. [DOI] [PubMed] [Google Scholar]
- 5.Walsh NC, Gravallese EM. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol. 2004;16:419–427. doi: 10.1097/01.bor.0000127824.42507.68. [DOI] [PubMed] [Google Scholar]
- 6.Aho K, Heliovaara M, Maatela J, Tuomi T, Palosuo T. Rheumatoid factors antedating clinical rheumatoid arthritis. J Rheumatol. 1991;18:1282–1284. [PubMed] [Google Scholar]
- 7.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 8.Arkema EV, Goldstein BL, Robinson W, et al. Anti-citrullinated peptide autoantibodies, human leukocyte antigen shared epitope and risk of future rheumatoid arthritis: a nested case–control study. Arthritis Res Ther. 2013;15:R159. doi: 10.1186/ar4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jilani AA, Mackworth-Young CG. The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: a systematic literature review and meta-analysis. Int J Rheumatol. 2015;2015:728610. doi: 10.1155/2015/728610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleyer A, Finzel S, Rech J, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73:854–860. doi: 10.1136/annrheumdis-2012-202958. [DOI] [PubMed] [Google Scholar]
- 11.Pettit AR, Ji H, von Stechow D, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redlich K, Hayer S, Ricci R, et al. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110:1419–1427. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- 14.Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 15.Gravallese EM, Manning C, Tsay A, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Ideguchi H, Ohno S, Hattori H, Senuma A, Ishigatsubo Y. Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs. Arthritis Res Ther. 2006;8:R76. doi: 10.1186/ar1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301–312. doi: 10.1111/j.0105-2896.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- 18.Schett G, Coates LC, Ash ZR, Finzel S, Conaghan PG. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res Ther. 2011;13(Suppl 1):S4. doi: 10.1186/1478-6354-13-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsui FW, Tsui HW, Akram A, Haroon N, Inman RD. The genetic basis of ankylosing spondylitis: new insights into disease pathogenesis. Appl Clin Genet. 2014;7:105–115. doi: 10.2147/TACG.S37325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FitzGerald O, Haroon M, Giles JT, Winchester R. Concepts of pathogenesis in psoriatic arthritis: genotype determines clinical phenotype. Arthritis Res Ther. 2015;17:115. doi: 10.1186/s13075-015-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lories RJ, Luyten FP, de Vlam K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther. 2009;11:221. doi: 10.1186/ar2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromley M, Woolley DE. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984;27:968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Nishikaku F, Nakatuka M, Koga Y. Osteoclast-like cells in murine collagen induced arthritis. J Rheumatol. 1998;25:1154–1160. [PubMed] [Google Scholar]
- 24.Romas E, Bakharevski O, Hards DK, et al. Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum. 2000;43:821–826. doi: 10.1002/1529-0131(200004)43:4<821::AID-ANR12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Kuratani T, Nagata K, Kukita T, Hotokebuchi T, Nakasima A, Iijima T. Induction of abundant osteoclast-like multinucleated giant cells in adjuvant arthritic rats with accompanying disordered high bone turnover. Histol Histopathol. 1998;13:751–759. doi: 10.14670/HH-13.751. [DOI] [PubMed] [Google Scholar]
- 26.Schett G, Redlich K, Hayer S, et al. Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum. 2003;48:2042–2051. doi: 10.1002/art.11150. [DOI] [PubMed] [Google Scholar]
- 27.Romas E, Sims NA, Hards DK, et al. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol. 2002;161:1419–1427. doi: 10.1016/S0002-9440(10)64417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrak P, Gortz B, Hayer S, et al. Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor-mediated arthritis. Arthritis Rheum. 2004;50:2327–2337. doi: 10.1002/art.20384. [DOI] [PubMed] [Google Scholar]
- 29.Sims NA, Green JR, Glatt M, et al. Targeting osteoclasts with zoledronic acid prevents bone destruction in collagen-induced arthritis. Arthritis Rheum. 2004;50:2338–2346. doi: 10.1002/art.20382. [DOI] [PubMed] [Google Scholar]
- 30.Jarrett SJ, Conaghan PG, Sloan VS, et al. Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum. 2006;54:1410–1414. doi: 10.1002/art.21824. [DOI] [PubMed] [Google Scholar]
- 31.Deodhar A, Dore RK, Mandel D, et al. Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2010;62:569–574. doi: 10.1002/acr.20004. [DOI] [PubMed] [Google Scholar]
- 32.Cohen SB, Dore RK, Lane NE, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto T, Matsumoto T, Hosoi T, et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT) Osteoporos Int. 2015;26:765–774. doi: 10.1007/s00198-014-2964-2. [DOI] [PubMed] [Google Scholar]
- 34.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 36.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lubberts E, van den Bersselaar L, Oppers-Walgreen B, et al. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/ osteoprotegerin balance. J Immunol. 2003;170:2655–2662. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- 38.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008;47:1635–1640. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 39.Yao Z, Li P, Zhang Q, et al. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J Biol Chem. 2006;281:11846–11855. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 40.Jimi E, Nakamura I, Duong LT, et al. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp Cell Res. 1999;247:84–93. doi: 10.1006/excr.1998.4320. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki M, Hashizume M, Yoshida H, Shiina M, Mihara M. Intercellular adhesion molecule-1 on synovial cells attenuated interleukin-6-induced inhibition of osteoclastogenesis induced by receptor activator for nuclear factor kappaB ligand. Clin Exp Immunol. 2011;163:88–95. doi: 10.1111/j.1365-2249.2010.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008;283:11535–11540. doi: 10.1074/jbc.M607999200. [DOI] [PubMed] [Google Scholar]
- 43.Axmann R, Bohm C, Kronke G, Zwerina J, Smolen J, Schett G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009;60:2747–2756. doi: 10.1002/art.24781. [DOI] [PubMed] [Google Scholar]
- 44.Boe A, Baiocchi M, Carbonatto M, Papoian R, Serlupi-Crescenzi O. Interleukin 6 knock-out mice are resistant to antigen-induced experimental arthritis. Cytokine. 1999;11:1057–1064. doi: 10.1006/cyto.1999.0502. [DOI] [PubMed] [Google Scholar]
- 45.Kotake S, Sato K, Kim KJ, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- 46.Takagi N, Mihara M, Moriya Y, et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117–2121. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 47.Jimenez-Boj E, Nobauer-Huhmann I, Hanslik-Schnabel B, et al. Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow inflammation in rheumatoid arthritis. Arthritis Rheum. 2007;56:1118–1124. doi: 10.1002/art.22496. [DOI] [PubMed] [Google Scholar]
- 48.Haavardsholm EA, Boyesen P, Ostergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis. 2008;67:794–800. doi: 10.1136/ard.2007.071977. [DOI] [PubMed] [Google Scholar]
- 49.Hetland ML, Ejbjerg B, Horslev-Petersen K, et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA) Ann Rheum Dis. 2009;68:384–390. doi: 10.1136/ard.2008.088245. [DOI] [PubMed] [Google Scholar]
- 50.Dalbeth N, Smith T, Gray S, et al. Cellular characterisation of magnetic resonance imaging bone oedema in rheumatoid arthritis; implications for pathogenesis of erosive disease. Ann Rheum Dis. 2009;68:279–282. doi: 10.1136/ard.2008.096024. [DOI] [PubMed] [Google Scholar]
- 51.Smolen JS, Van Der Heijde DM, St Clair EW, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–710. doi: 10.1002/art.21678. [DOI] [PubMed] [Google Scholar]
- 52.Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–1167. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 54.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 55.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 56.Stewart A, Mackenzie LM, Black AJ, Reid DM. Predicting erosive disease in rheumatoid arthritis. A longitudinal study of changes in bone density using digital X-ray radiogrammetry: a pilot study. Rheumatology (Oxford) 2004;43:1561–1564. doi: 10.1093/rheumatology/keh385. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu S, Shiozawa S, Shiozawa K, Imura S, Fujita T. Quantitative histologic studies on the pathogenesis of periarticular osteoporosis in rheumatoid arthritis. Arthritis Rheum. 1985;28:25–31. doi: 10.1002/art.1780280105. [DOI] [PubMed] [Google Scholar]
- 58.Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 59.Saeki Y, Kudo-Tanaka E, Ohshima S, et al. Baseline anti-citrullinated peptide antibody (ACPA) titers and serum interleukin-6 (IL-6) levels possibly predict progression of bone destruction in early stages of rheumatoid arthritis (ERA) Rheumatol Int. 2013;33:451–456. doi: 10.1007/s00296-012-2397-1. [DOI] [PubMed] [Google Scholar]
- 60.Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205428. [DOI] [PubMed] [Google Scholar]
- 61.Harre U, Kittan NA, Schett G. Autoantibody-mediated bone loss. Curr Osteoporos Rep. 2014;12:17–21. doi: 10.1007/s11914-013-0185-9. [DOI] [PubMed] [Google Scholar]
- 62.Mathsson L, Lampa J, Mullazehi M, Ronnelid J. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res Ther. 2006;8:R64. doi: 10.1186/ar1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clavel C, Nogueira L, Laurent L, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58:678–688. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 64.Lu MC, Lai NS, Yu HC, Huang HB, Hsieh SC, Yu CL. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010;62:1213–1223. doi: 10.1002/art.27386. [DOI] [PubMed] [Google Scholar]
- 65.Harre U, Georgess D, Bang H, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122:1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seeling M, Hillenhoff U, David JP, et al. Inflammatory monocytes and Fcgamma receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc Natl Acad Sci U S A. 2013;110:10729–10734. doi: 10.1073/pnas.1301001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harre U, Lang SC, Pfeifle R, et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 2015;6:6651. doi: 10.1038/ncomms7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 69.Berglin E, Padyukov L, Sundin U, et al. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA- DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res Ther. 2004;6:R303–308. doi: 10.1186/ar1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 71.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 72.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Horizontal transfer of microRNAs: molecular mechanisms and clinical applications. Protein Cell. 2012;3:28–37. doi: 10.1007/s13238-012-2003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 76.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duroux-Richard I, Jorgensen C, Apparailly F. miRNAs and rheumatoid arthritis - promising novel biomarkers. Swiss Med Wkly. 2011;141:w13175. doi: 10.4414/smw.2011.13175. [DOI] [PubMed] [Google Scholar]
- 79.Nakasa T, Shibuya H, Nagata Y, Niimoto T, Ochi M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. 2011;63:1582–1590. doi: 10.1002/art.30321. [DOI] [PubMed] [Google Scholar]
- 80.Ceribelli A, Nahid MA, Satoh M, Chan EK. MicroRNAs in rheumatoid arthritis. FEBS Lett. 2011;585:3667–3674. doi: 10.1016/j.febslet.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 82.Kurowska-Stolarska M, Alivernini S, Ballantine LE, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A. 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tili E, Michaille JJ, Cimino A, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 84.Bluml S, Bonelli M, Niederreiter B, et al. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 2011;63:1281–1288. doi: 10.1002/art.30281. [DOI] [PubMed] [Google Scholar]
- 85.Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood. 2011;117:3648–3657. doi: 10.1182/blood-2010-10-311415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J, Zhao H, Chen J, et al. Interferon-beta-induced miR-155 inhibits osteoclast differentiation by targeting SOCS1 and MITF. FEBS Lett. 2012;586:3255–3262. doi: 10.1016/j.febslet.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 87.de la Rica L, Garcia-Gomez A, Comet NR, et al. NF-kappaB-direct activation of microRNAs with repressive effects on monocyte-specific genes is critical for osteoclast differentiation. Genome Biol. 2015;16:2. doi: 10.1186/s13059-014-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakamachi Y, Ohnuma K, Uto K, Noguchi Y, Saegusa J, Kawano S. MicroRNA-124 inhibits the progression of adjuvant-induced arthritis in rats. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206417. [DOI] [PubMed] [Google Scholar]
- 89.Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J Clin Invest. 2014;124:466–472. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanchez-Duffhues G, Hiepen C, Knaus P, Ten Dijke P. Bone morphogenetic protein signaling in bone homeostasis. Bone. 2015 doi: 10.1016/j.bone.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 92.Regard JB, Zhong Z, Williams BO, Yang Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb Perspect Biol. 2012;4:12. doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morvan F, Boulukos K, Clement-Lacroix P, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Sarosi I, Cattley RC, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 95.Bodine PV, Zhao W, Kharode YP, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 96.Yao W, Cheng Z, Shahnazari M, Dai W, Johnson ML, Lane NE. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J Bone Miner Res. 2010;25:190–199. doi: 10.1359/jbmr.090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 98.Daoussis D, Andonopoulos AP. The emerging role of Dickkopf-1 in bone biology: is it the main switch controlling bone and joint remodeling? Semin Arthritis Rheum. 2011;41:170–177. doi: 10.1016/j.semarthrit.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 99.Matzelle MM, Gallant MA, Condon KW, et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 2012;64:1540–1550. doi: 10.1002/art.33504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 101.Staehling-Hampton K, Proll S, Paeper BW, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- 102.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Lierop AH, Hamdy NA, van Egmond ME, Bakker E, Dikkers FG, Papapoulos SE. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res. 2013;28:848–854. doi: 10.1002/jbmr.1794. [DOI] [PubMed] [Google Scholar]
- 104.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 105.Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet. 2003;63:192–197. doi: 10.1034/j.1399-0004.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 106.Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 107.Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaur T, Hussain S, Mudhasani R, et al. Dicer inactivation in osteoprogenitor cells compromises fetal survival and bone formation, while excision in differentiated osteoblasts increases bone mass in the adult mouse. Dev Biol. 2010;340:10–21. doi: 10.1016/j.ydbio.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei J, Shi Y, Zheng L, et al. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol. 2012;197:509–521. doi: 10.1083/jcb.201201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lian JB, Stein GS, van Wijnen AJ, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hassan MQ, Gordon JA, Beloti MM, et al. A network connecting Runx2, SATB2, and the miR-23a~27a~24-2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci U S A. 2010;107:19879–19884. doi: 10.1073/pnas.1007698107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moller DU, Boonen A, Hetland ML, et al. Erosive progression is minimal, but erosion healing rare, in patients with rheumatoid arthritis treated with adalimumab. A 1 year investigator-initiated follow-up study using high-resolution computed tomography as the primary outcome measure. Ann Rheum Dis. 2009;68:1585–1590. doi: 10.1136/ard.2008.097048. [DOI] [PubMed] [Google Scholar]
- 114.Dohn UM, Ejbjerg B, Boonen A, et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis. 2011;70:252–258. doi: 10.1136/ard.2009.123729. [DOI] [PubMed] [Google Scholar]
- 115.Finzel S, Rech J, Schmidt S, et al. Repair of bone erosions in rheumatoid arthritis treated with tumour necrosis factor inhibitors is based on bone apposition at the base of the erosion. Ann Rheum Dis. 2011;70:1587–1593. doi: 10.1136/ard.2010.148395. [DOI] [PubMed] [Google Scholar]
- 116.Finzel S, Rech J, Schmidt S, Engelke K, Englbrecht M, Schett G. Interleukin-6 receptor blockade induces limited repair of bone erosions in rheumatoid arthritis: a micro CT study. Ann Rheum Dis. 2013;72:396–400. doi: 10.1136/annrheumdis-2011-201075. [DOI] [PubMed] [Google Scholar]
- 117.Gilbert L, He X, Farmer P, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 118.Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 119.Kaneki H, Guo R, Chen D, et al. Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts. J Biol Chem. 2006;281:4326–4333. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 121.Stashenko P, Dewhirst FE, Rooney ML, Desjardins LA, Heeley JD. Interleukin-1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res. 1987;2:559–565. doi: 10.1002/jbmr.5650020612. [DOI] [PubMed] [Google Scholar]
- 122.Hengartner NE, Fiedler J, Ignatius A, Brenner RE. IL-1beta inhibits human osteoblast migration. Mol Med. 2013;19:36–42. doi: 10.2119/molmed.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–1998. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 124.Walsh NC, Reinwald S, Manning CA, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24:1572–1585. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- 125.de Rooy DP, Yeremenko NG, Wilson AG, et al. Genetic studies on components of the Wnt signalling pathway and the severity of joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2013;72:769–775. doi: 10.1136/annrheumdis-2012-202184. [DOI] [PubMed] [Google Scholar]
- 126.Yu B, Chang J, Liu Y, et al. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-kappaB. Nat Med. 2014;20:1009–1017. doi: 10.1038/nm.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–1579. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lories RJ, Schett G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin North Am. 2012;38:555–567. doi: 10.1016/j.rdc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 129.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111:12097–12102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bollow M, Fischer T, Reisshauer H, et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis- cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59:135–140. doi: 10.1136/ard.59.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Appel H, Ruiz-Heiland G, Listing J, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2009;60:3257–3262. doi: 10.1002/art.24888. [DOI] [PubMed] [Google Scholar]
- 132.Tsui FW, Tsui HW, Las Heras F, Pritzker KP, Inman RD. Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing spondylitis. Ann Rheum Dis. 2013;73:1873–1879. doi: 10.1136/annrheumdis-2013-203630. [DOI] [PubMed] [Google Scholar]
- 133.Chen XX, Baum W, Dwyer D, et al. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann Rheum Dis. 2013;72:1732–1736. doi: 10.1136/annrheumdis-2013-203345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uderhardt S, Diarra D, Katzenbeisser J, et al. Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010;69:592–597. doi: 10.1136/ard.2008.102046. [DOI] [PubMed] [Google Scholar]
- 135.Haynes KR, Pettit AR, Duan R, et al. Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors. Arthritis Res Ther. 2012;14:R253. doi: 10.1186/ar4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heiland GR, Appel H, Poddubnyy D, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:572–574. doi: 10.1136/annrheumdis-2011-200216. [DOI] [PubMed] [Google Scholar]
- 137.Klingberg E, Nurkkala M, Carlsten H, Forsblad-d’Elia H. Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol. 2014;41:1349–1356. doi: 10.3899/jrheum.131199. [DOI] [PubMed] [Google Scholar]
- 138.Chen HA, Chen CH, Lin YJ, et al. Association of bone morphogenetic proteins with spinal fusion in ankylosing spondylitis. J Rheumatol. 2010;37:2126–2132. doi: 10.3899/jrheum.100200. [DOI] [PubMed] [Google Scholar]
- 139.Park MC, Park YB, Lee SK. Relationship of bone morphogenetic proteins to disease activity and radiographic damage in patients with ankylosing spondylitis. Scand J Rheumatol. 2008;37:200–204. doi: 10.1080/03009740701774941. [DOI] [PubMed] [Google Scholar]
- 140.Wendling D, Cedoz JP, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304–305. doi: 10.1016/j.jbspin.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 141.Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57:44–51. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 143.Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sherlock JP, Buckley CD, Cua DJ. The critical role of interleukin-23 in spondyloarthropathy. Mol Immunol. 2014;57:38–43. doi: 10.1016/j.molimm.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 145.Van Praet L, Van den Bosch FE, Jacques P, et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis. 2013;72:414–417. doi: 10.1136/annrheumdis-2012-202135. [DOI] [PubMed] [Google Scholar]
- 146.Ciccia F, Accardo-Palumbo A, Rizzo A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2014;73:1566–1574. doi: 10.1136/annrheumdis-2012-202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smith JA, Colbert RA. Review: The interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. 2014;66:231–241. doi: 10.1002/art.38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee YH, Choi SJ, Ji JD, Song GG. Associations between interleukin-23R polymorphisms and ankylosing spondylitis susceptibility: a meta-analysis. Inflamm Res. 2012;61:143–149. doi: 10.1007/s00011-011-0398-2. [DOI] [PubMed] [Google Scholar]
- 149.Kang YK, Zhang MC. IL-23 promotes osteoclastogenesis in osteoblast-osteoclast co-culture system. Genet Mol Res. 2014;13:4673–4679. doi: 10.4238/2014.June.18.10. [DOI] [PubMed] [Google Scholar]
- 150.Chen L, Wei XQ, Evans B, Jiang W, Aeschlimann D. IL-23 promotes osteoclast formation by upregulation of receptor activator of NF-kappaB (RANK) expression in myeloid precursor cells. Eur J Immunol. 2008;38:2845–2854. doi: 10.1002/eji.200838192. [DOI] [PubMed] [Google Scholar]
- 151.Yago T, Nanke Y, Kawamoto M, et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res Ther. 2007;9:R96. doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McGonagle D, Stockwin L, Isaacs J, Emery P. An enthesitis based model for the pathogenesis of spondyloarthropathy. additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol. 2001;28:2155–2159. [PubMed] [Google Scholar]
- 153.Jacques P, Lambrecht S, Verheugen E, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. 2014;73:437–445. doi: 10.1136/annrheumdis-2013-203643. [DOI] [PubMed] [Google Scholar]
- 154.van der Heijde D, Landewe R, Einstein S, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008;58:1324–1331. doi: 10.1002/art.23471. [DOI] [PubMed] [Google Scholar]
- 155.van der Heijde D, Landewe R, Baraliakos X, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 2008;58:3063–3070. doi: 10.1002/art.23901. [DOI] [PubMed] [Google Scholar]
- 156.van der Heijde D, Salonen D, Weissman BN, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther. 2009;11:R127. doi: 10.1186/ar2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65:2645–2654. doi: 10.1002/art.38070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis. 2014;73:710–715. doi: 10.1136/annrheumdis-2012-202698. [DOI] [PubMed] [Google Scholar]