Abstract
BACKGROUND & AIMS
Analyses of outcomes after acute liver failure (ALF) have typically included all ALF patients regardless of whether they were listed for liver transplantation (LT). We hypothesized that limiting analysis to listed patients might provide novel insights into factors associated with outcome, focusing attention on disease evolution after listing.
METHODS
Listed adult ALF patients enrolled in the US Acute Liver Failure Study Group registry between 2000 and 2013 were analyzed to determine baseline factors associated with 21-day outcomes after listing.
RESULTS
We classified 617 patients (36% of overall ALF group) by 3-week outcome after study admission: 117 survived spontaneously (without LT, SS), 108 died without LT, and 392 underwent LT. Only 22% of acetaminophen (APAP) ALF patients were listed; however, this group of 173 patients demonstrated greater illness severity: higher coma grades, and more patients required ventilator, vasopressor or renal replacement therapy support. Only 62/173 (36%) of APAP patients received a graft, versus 66% for drug-induced liver injury patients, 86% for autoimmune and 71% for hepatitis B-related ALF. APAP patients were more likely to die than non-APAP patients (24% vs 17%), and the median time to death was sooner (2 vs 4.5 days). Despite greater severity of illness, the listed APAP group still had a SS rate of 40% vs. 11% for non-APAP causes (p < 0.001).
CONCLUSIONS
APAP outcomes evolve rapidly, mainly to SS or death. Patients with APAP ALF listed for LT had the highest death rate of any etiology, while more slowly evolving etiologies yielded higher LT rates, and consequently, fewer deaths. Decisions to list and transplant must be made early in all ALF patients, particularly in those with APAP ALF.
Keywords: Acetaminophen Hepatotoxicity, Acute Liver Failure, Liver Transplantation
Introduction
Acute liver failure (ALF) is a rare condition characterized by rapid onset of severe hepatocyte injury without prior liver disease that is associated with significant morbidity and mortality.1–3 While emergency liver transplantation (LT) can be lifesaving, the challenge remains to optimize patient selection. Drawbacks of transplantation include the need for lifelong immunosuppression and associated adverse events and utilization of a scarce resource, while patients who recover spontaneously are expected to have fully restored liver function with a minimum of long-term sequelae.4 Prognostic scores such as the Kings College Criteria (KCC), have been disappointing as they have high specificity (i.e., high probability of death if criteria met), but low sensitivity (i.e., low probability of survival if criteria not met).5
The wide variety of causes of ALF introduces tremendous heterogeneity in outcome. For example, acetaminophen (APAP)-related ALF is classically associated with the highest rate of spontaneous recovery, and lowest rate of death, of any etiology.3, 6 In contrast, other causes of ALF that evolve more slowly, such as autoimmune or drug-induced liver injury (DILI),7,8 are associated with a 15% and 27% likelihood of recovery, respectively, without transplantation.7,8 In addition to the etiology, the presence and severity of multi-organ failure diminishes the likelihood of spontaneous recovery in these critically ill patients. Lastly, host factors such as subject age, obesity, and other genetic factors are believed to influence the likelihood of adequate hepatocyte regeneration with supportive care that allows the native liver to recover. The challenge of predicting which patients should receive a transplant continues, and decisions to transplant remain somewhat subjective in the best of circumstances. The transplant team works to identify those clearly too sick or too healthy to be transplant candidates, and excludes those subjects not meeting the socioeconomic criteria for transplant (e.g., substance use, lack of insurance). Beyond these criteria, if there is uncertainty about whether the patient may spontaneously recover, or, conversely, any chance that the patient will stabilize by the time of liver availability, the patient is listed.
The widely accepted association of the etiology of ALF to outcome may not accurately reflect medical predictors of prognosis, however. Consequently, previous overall analyses of outcome based on etiology are likely to be highly biased. We hypothesized that limiting outcome analysis to patients who have been listed for LT, and therefore have the same starting point, the opportunity for surgical rescue, may reveal previously unrecognized associations of outcome with etiology, and with severity of liver injury and multi-organ failure, and the temporal course of the syndrome after admission to the hospital.
The Acute Liver Failure Study Group (ALFSG) has been prospectively identifying and studying the etiologies, presenting features, and clinical outcomes of adults with ALF over the past 17 years. The current analysis included 1,696 adult patients with ALF enrolled over a 14-year period, 617 (36%) of whom had been listed for liver transplantation. By focusing on subjects listed for transplant, we can evaluate those factors associated with surviving to transplantation or spontaneous recovery, without confounding due to socio-demographic or medical reasons for not listing, examining determinants of 21-day outcomes among listed ALF patients particularly among the four largest specific etiologies encountered: APAP, DILI, AIH and hepatitis B.
Patients and Methods
The main aim of this analysis was to evaluate survival with and without LT among US ALF patients listed for emergency LT, as per United Network for Organ Sharing (UNOS) guidelines, at one of the ALFSG participating sites.9 We assessed differences in demographics, etiology, and presenting clinical features in three groups of patients: those who eventually received LT, and those listed for LT who did not receive LT and survived or died by 21 days after enrollment.
Patient Selection
The ALFSG Registry was initiated in 1998 and has enrolled more than 2500 patients among the 32 participating clinical centers. All centers received institutional review board approval prior to enrolling patients in the registry. We restricted the present analysis to patients enrolled between January 1, 2000 and December 31, 2013, since only limited listing information for LT was collected between 1998 and 2000. The database comprises patients admitted with a clinical diagnosis of ALF, meeting the following criteria: duration of symptoms of jaundice or illness <26 weeks prior to admission, altered mental status in the absence of the previous use of sedatives, and INR ≥1.5. Patients with previously recognized cirrhosis and superimposed acute on chronic liver failure were excluded. Exceptions to the requirement of exclusion of cirrhosis were made for patients who presented with acute Wilson disease10 with liver failure and those with first presentation of autoimmune-related ALF. Written informed consent was obtained from the patient and or the patients’ legal next of kin to collect information on medical history and management data during the first seven days of enrollment including imaging, blood and urine samples, laboratory and clinical data. Case report forms (CRFs) during this period contained detailed information on whether patients were listed for transplantation and the reasons for not listing or withdrawing a patient from the LT list. We limited the dataset to those for whom 21-day follow up status was known as evidence of ‘immediate’ survival. Longer-term follow up data on this same patient group were reviewed in a separate publication.11 For patients to be included in the analysis, either a recorded death or, at minimum, a follow-up document had to be completed at least 21 days after enrollment. Patients listed for transplant were subsequently classified according to whether they received LT, and by their 21-day outcome. We grouped patients with ‘other’ and indeterminate etiologies together and analyzed only the largest patient groups based upon etiology. Diagnoses in the ‘Other’ patients group included ischemia/shock, pregnancy-related ALF, Budd-Chiari syndrome, mushroom toxicity, Wilson disease, hepatitis A, C and E and other viruses. Patients were considered as listed for LT regardless of whether the listing occurred prior to the end of the 7-day inpatient phase (N=411, 67%, range: −17 to 7 days) or later (N=25, 4%, range: 8 to 38 days); the specific date of listing for LT was not available for 181 patients (29%). Eighty percent of transplants occurred within the first 7 days of study enrollment and 19% occurred thereafter (range: 8 to 41 days). For all patients, 21-day status was defined as 21 days after enrollment in the registry.
Statistical Analysis
SAS software (version 9.3; Cary, NC) was used to perform statistical analyses. Baseline variables were described using counts and percentages for categorical data, or means and standard deviations (medians and interquartile ranges) for continuous normal (skewed) data. For variables identified as clinically relevant, statistical tests were performed using Chi-square, ANOVA, or Kruskal-Wallis tests. All statistical tests are reported as two-sided with a type I error rate of 5%.
Results
Listed patients: overall outcomes
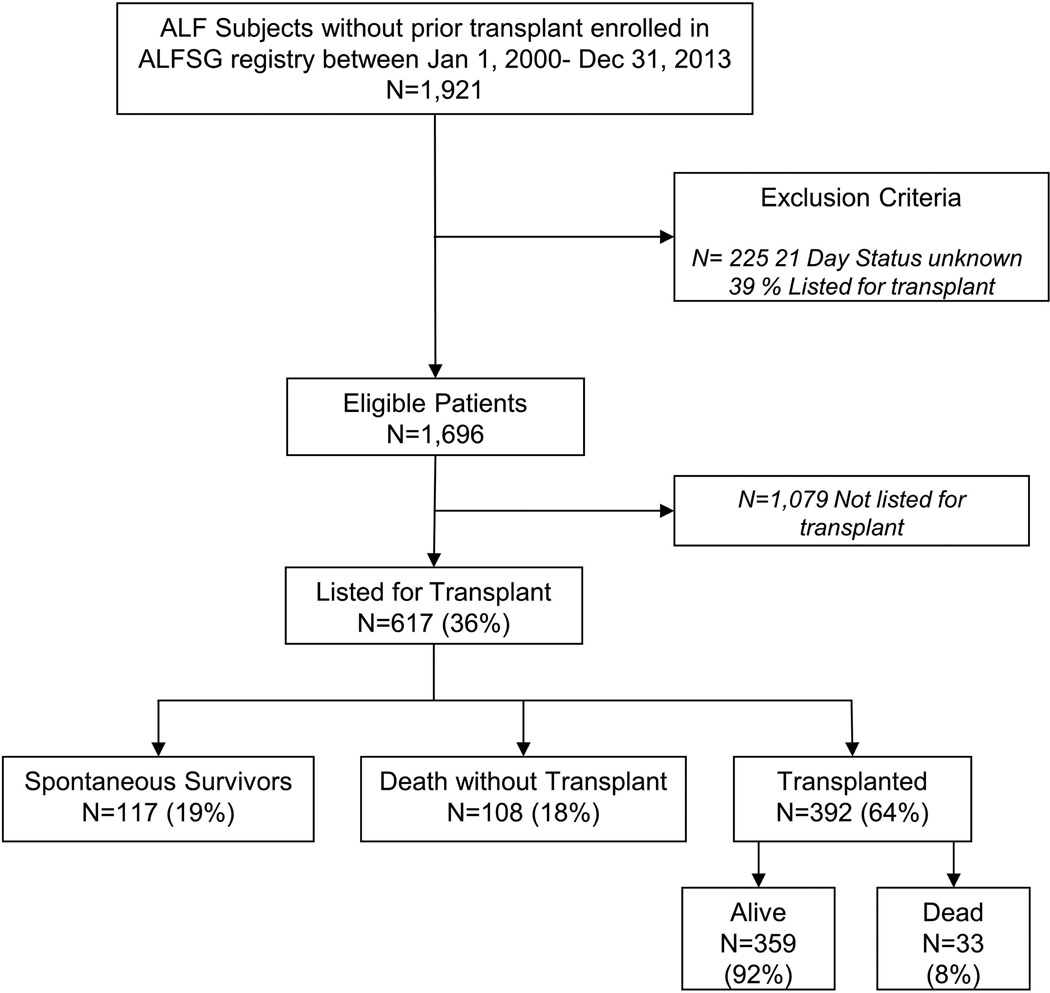
A total of 1,921 consecutive patients meeting ALF criteria were enrolled between January 1, 2000 and December 31, 2013. Of these, 1,696 patients had 21-day outcomes available (Figure 1), and 617 (36%) were listed for LT a median of 0 days after admission to the registry (interquartile range [IQR] 0,1). Demographic features, etiology of ALF, presenting clinical and laboratory features on admission and during hospitalization are presented in Table 1. The three-week clinical outcomes of the 617 listed patients were classified as those that survived without LT (spontaneous survivors; SS), those dying after listing (died), and those who received a LT.
Figure 1.
Flowchart describing construction of analysis dataset and three categories among the listed patients based on outcome at 21 days.
Table 1.
Demographic, clinical, and laboratory features of the 617 ALF patients who were listed for LT according to outcome at 21 days following admission to study. Data presented as Mean (SD), Median (IQR), or N (% of outcome) according to type of variable.
| N | SS (N=117) |
Died (N=108) |
LT (N=392) |
P-Value | |
|---|---|---|---|---|---|
| Patient Demographics | |||||
| Age (years) | 617 | 35.5(12.5) | 40.1(14.9) | 39.8(13.9) | 0.008 |
| Gender (% Female) | 617 | 83(71) | 75(69) | 263(67) | 0.70 |
| Race | 617 | 0.014 | |||
| Caucasian | 91(78) | 86(80) | 261(67) | ||
| African-American | 11(9) | 10(9) | 75(19) | ||
| Other | 15(13) | 12(11) | 56(14) | ||
| Weight (kg) | 591 | 73.43(18.43) | 76.88(20.73) | 81.68(21.85) | <0.001 |
| Etiology (N=614; SS = 117, Died = 107‡, LT = 390) | |||||
| Acetaminophen | 173 | 70(60) | 41(38) | 62(16) | |
| Drug Induced Liver Injury | 95 | 11(9) | 21(20) | 63(16) | |
| Autoimmune Hepatitis | 83 | 6(5) | 6(6) | 71(18) | |
| Hepatitis B | 66 | 6(5) | 13(12) | 47(12) | |
| Indeterminate | 114 | 15(13) | 15(14) | 84(22) | |
| Other^ | 83 | 9(8) | 11(10) | 63(16) | |
| Admission Labs | |||||
| ALT (IU/L) * | 611 | 2950(5130) | 2476(4571) | 826(2076) | <0.001 |
| INR* | 605 | 2.6(2.2) | 3.0(2.7) | 3.2(2.3) | 0.06 |
| Bilirubin (mg/dL)* | 613 | 5.9(5.5) | 10.5(18.2) | 20.5(17.5) | <0.001 |
| Creatinine (mg/dL)* | 614 | 1.4(2.3) | 2.3(2.2) | 1.1(1.4) | <0.001 |
| MELD Score | 602 | 30.2(13.0) | 36.9(12.2) | 33.0(12.4) | 0.03 |
| Venous Ammonia* | 210 | 85.0(83.8) | 143.0(159.0) | 100.0(65.0) | 0.01 |
| Toxicology Screen Positive (% Yes) | 617 | 48(41) | 33(31) | 68(17) | <0.001 |
| Peak Labs | |||||
| ALT (IU/L) * | 617 | 3543(5484) | 2568(4780) | 904(2225) | <0.001 |
| INR* | 608 | 2.8(2.1) | 4.0(3.9) | 3.8(2.7) | <0.001 |
| Bilirubin (mg/dL)* | 617 | 11.0(11.1) | 16.6(19.3) | 23.1(15.9) | <0.001 |
| Creatinine (mg/dL) | 617 | 2.1(3.7) | 3.2(2.2) | 1.5(1.8) | <0.001 |
| MELD Score | 608 | 31.8(13.2) | 40.0(10.4) | 35.5(12.1) | 0.009 |
| Venous Ammonia* | 240 | 93.0(72) | 194.0(155) | 107.0(89) | <0.001 |
| Clinical Parameters at Admission | |||||
| Coma Grade (% 3/4) | 600 | 50(45) | 71(66) | 152(40) | <0.001 |
| Mechanical ventilation | 616 | 54(46) | 73(68) | 160(41) | <0.001 |
| Vasopressor Use | 616 | 20(17) | 32(30) | 55(14) | <0.001 |
| Renal replacement therapy | 616 | 31(26) | 42(39) | 74(19) | <0.001 |
| Clinical Parameters during Hospitalization | |||||
| Coma Grade (% 3/4) | 605 | 64(56) | 95(88) | 229(60) | <0.001 |
| Mechanical ventilation | 617 | 65(56) | 93(86) | 245(62) | <0.001 |
| Vasopressor Use | 617 | 31(26) | 70(65) | 111(28) | <0.001 |
| Renal replacement therapy | 617 | 44(38) | 66(61) | 128(33) | <0.001 |
Listed as median (IQR);
Other patients include the following etiologies: Shock/Ischemia, ALF in Pregnancy, Budd-Chiari, Mushroom toxicity, Wilson Disease, Hep A, Hep C, Hep E, Other Virus, Other;
1 patient who died is missing their etiology.
Overall, 58% (N=358) of listed patients were considered to have more severe disease at admission (coma grade 3/4 or requiring mechanical ventilation, vasopressors or renal replacement therapy [RRT]) of whom 30% (N=107) died. Conversely, fewer patients with less severe disease at admission died (13%, 34 out of 259), suggesting that more advanced coma grade and the need for ICU supportive measures portend poorer outcomes, independent of LT (P<0.001).
Role of etiology in listing for LT
As shown in Table 1, the likelihood of being listed and of receiving LT varied greatly according to etiology. Patients with APAP toxicity comprised 46% (774) of the overall ALF Registry, but only 22% (173) were listed. A much larger fraction of enrolled patients were listed in the other etiologies examined: 52% (95/181) of DILI ALF patients, 66% (83/126) of autoimmune ALF patients, and 54% (66/123) of hepatitis B patients. Thus, DILI, autoimmune and hepatitis B patients were 2 to 3 times more likely to be listed for LT than patients with APAP.
Table 2 presents an overall analysis of APAP vs. non-APAP patients. APAP patients were significantly younger (median age 35 vs. 41 years for non-APAP, p < 0.001), included a higher proportion of females (79 vs. 64% for non-APAP, p < 0.001), but more frequently had high coma grades on admission (64% vs. 38% in grade III/IV on admission, p < 0.001) and were twice as likely to require mechanical ventilation (70% vs. 37%), vasopressor support (29% vs. 12%) and RRT (36% vs. 19%) than the listed non-APAP patients.
Table 2.
Comparison of non-APAP and APAP ALF patients listed for LT.
| N | Non-APAP N=441 |
APAP N=173 |
P-Value | |
|---|---|---|---|---|
| Age (yrs) | 614 | 40.8(14.5) | 34.6(11.2) | < 0.001 |
| Gender (% Female) | 614 | 282(64) | 137(79) | < 0.001 |
| Weight (kg) | 588 | 82.27(21.55) | 71.49(18.64) | < 0.001 |
| Years Education | 388 | 13.3(2.9) | 13.1(1.9) | 0.51 |
| Platelet Count (× 1000/mm^3) | 605 | 142(116) | 127(110) | 0.03 |
| INR | 603 | 2.9(2.1) | 3.5(3.3) | 0.013 |
| ALT (IU/L) | 608 | 694(1540) | 4440(4706) | < 0.001 |
| Bilirubin (mg/dL) | 610 | 21.3(15.0) | 4.8(3.2) | < 0.001 |
| Creatinine (mg/dL) | 611 | 1.1(1.5) | 2.1(2.5) | < 0.001 |
| Tox Screen Positive (% Yes) | 614 | 65(15) | 84(49) | < 0.001 |
| Coma Grade (% 3/4) | 597 | 165(38) | 107(64) | < 0.001 |
| Mechanical ventilation | 613 | 164(37) | 121(70) | < 0.001 |
| Vasopressor Use | 613 | 54(12) | 51(29) | < 0.001 |
| Renal replacement therapy | 613 | 82(19) | 63(36) | < 0.001 |
Data presented as median (IQR) and N (%); three subjects with unknown etiology are omitted from this table. Data represents admission values.
Patients receiving a transplant
Among the 617 listed patients, nearly two-thirds (64%) underwent LT. However, the likelihood of actually receiving a graft again varied widely across etiologies, from 62/173 (36%) for APAP to 63/95 (66%) for DILI, 71/83 (86%) for autoimmune ALF and 47/66 (71%) for hepatitis B. Table 1 and Supplementary Table 1 compare clinical and admission laboratory parameters according to outcome. Those who underwent LT had higher median bilirubin levels and lower median aminotransferase and creatinine levels at enrollment than either those who died or survived without LT.
Of interest, however, those undergoing LT also had less advanced coma grades, less frequently required mechanical ventilation, use of vasopressors or RRT at admission than the other groups. Sixty six percent of those dying prior to LT were in advanced coma grades (grades 3/4) on admission to study, compared to 45% of spontaneous survivors and 40% of those who later received a graft. Similarly, 68% percent of those who died without LT received mechanical ventilation at admission, versus 46% of spontaneous survivors, and only 41% of those who underwent LT; those requiring mechanical ventilation at any time: 86, 56 and 62% for died, SS or transplanted respectively. Again, vasopressor support at any time was utilized in 65% of those who died but only 26 and 28% of survivors and those transplanted, while RRT at any time was given to 61% of those dying, and 38 and 33% of those surviving or requiring a graft.
Patients not transplanted
Approximately one third of patients that were listed for transplant were subsequently removed, inactivated or died. Since removal from the list (prior to death or recovery, for example) was not uniformly practiced (or information was missing regarding reasons for list removal), we grouped all patients by 21-day outcome, regardless of whether they actually were removed from the list. Information regarding reasons for list removal was provided for 191 (85%) of the 225 patients that either died or spontaneously survived. Of these, 112/191 (59%) were removed from the list because they were no longer considered viable LT candidates (irreversible brain damage, septicemia, ‘medically unsuitable’ for LT and ‘other’ reasons, not disclosed); 86/112 (77%) of whom died. Among the remaining 79 removed from the list due to clinical improvement (predicted recovery), after further adjudication all but one (99%) were alive at 3 weeks of follow-up.
Time from study admission to transplant/death; difference by etiologies
We examined time from listing to outcome for all three groups (Figures 2, 3a–d and Supplementary Table 2). Among those who received a LT, the median time from listing to LT was 3 days (IQR: 1 to 6 days) for the 359 patients who survived at least 21 days, and 2 days (IQR: 1 to 4 days) for the 33 who died post-LT, with time to death post-LT of 5 days (IQR: 3,11). Median time from study admission to list removal or inactivation for the 108 (18%) who died prior to LT was 2 days (IQR: 1,5), and time to death was 3 days (IQR: 2 to 6 days). The median time to list removal for those surviving without LT was 3 days (IQR: 2 to 8 days).
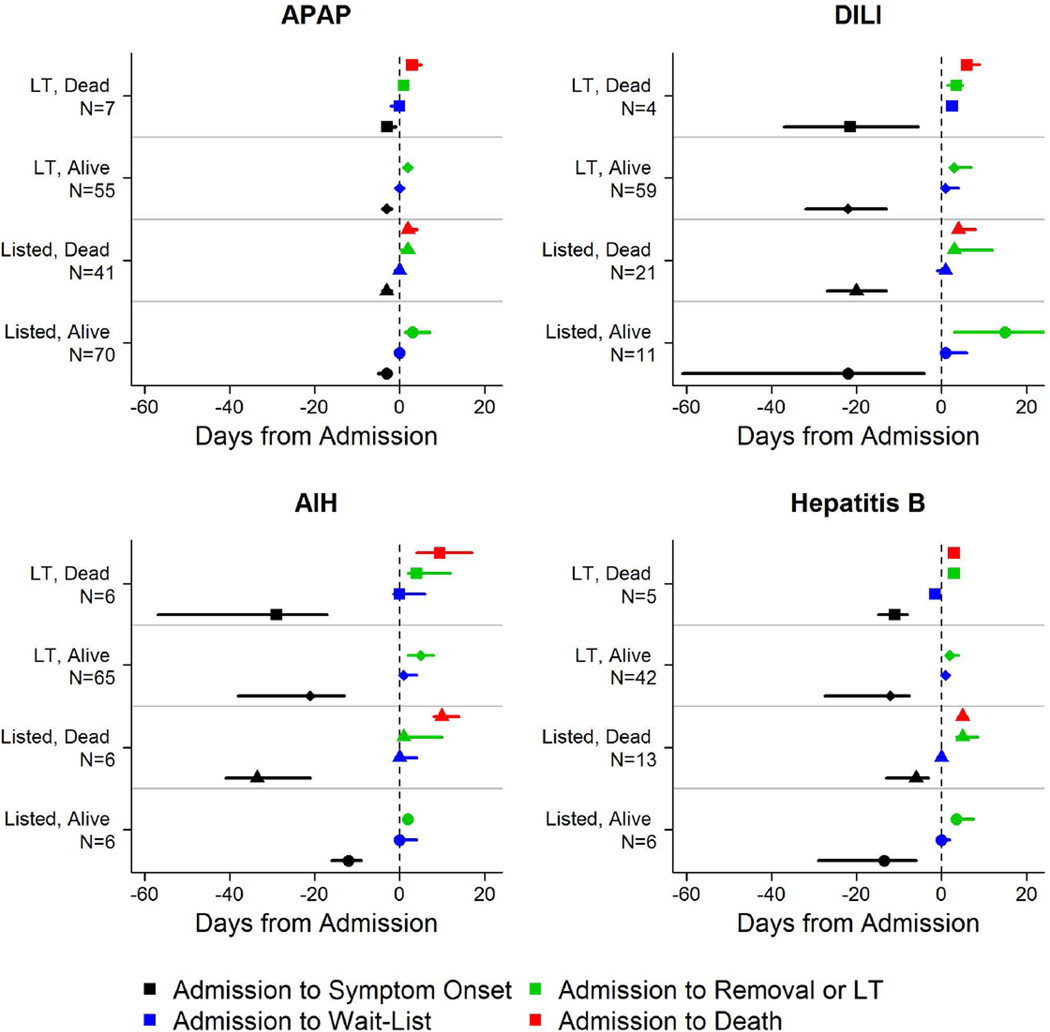
Figure 2.
Median time to event for disease progression by etiology. Symbol represents median time to event and surrounding bar represents interquartile range. (Black) Admission – Symptom Onset; (Red) Admission – List; (Green) Admission – Removal/Transplant; (Blue) Admission-Death.
Figure 3.
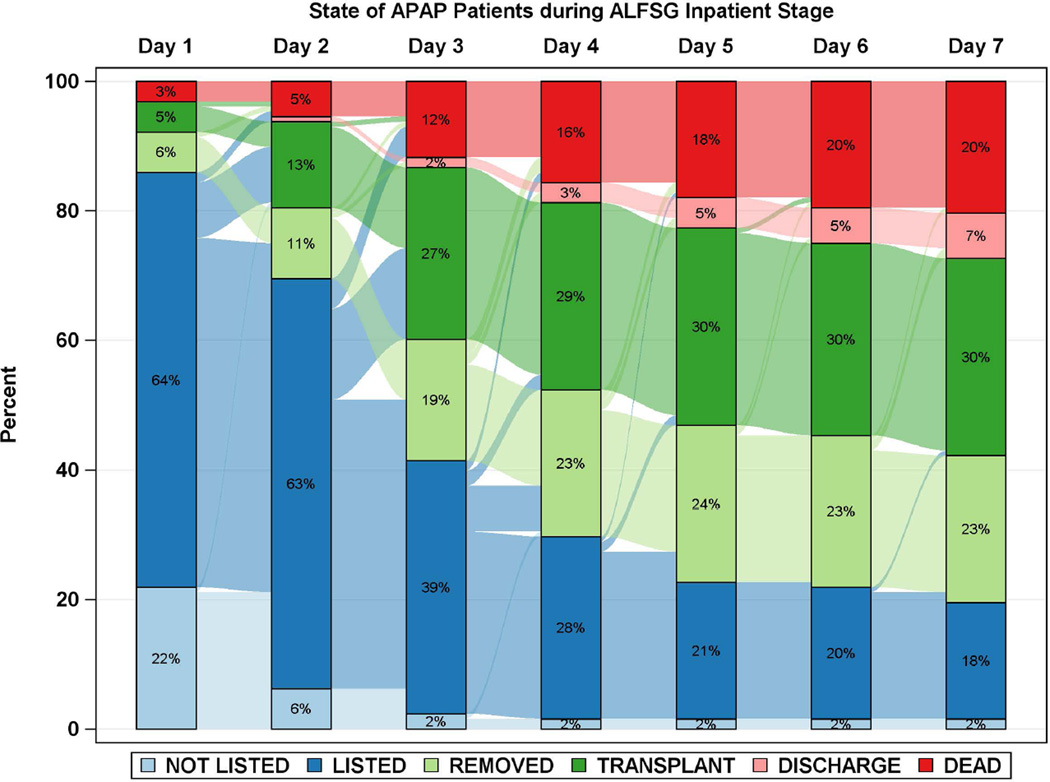
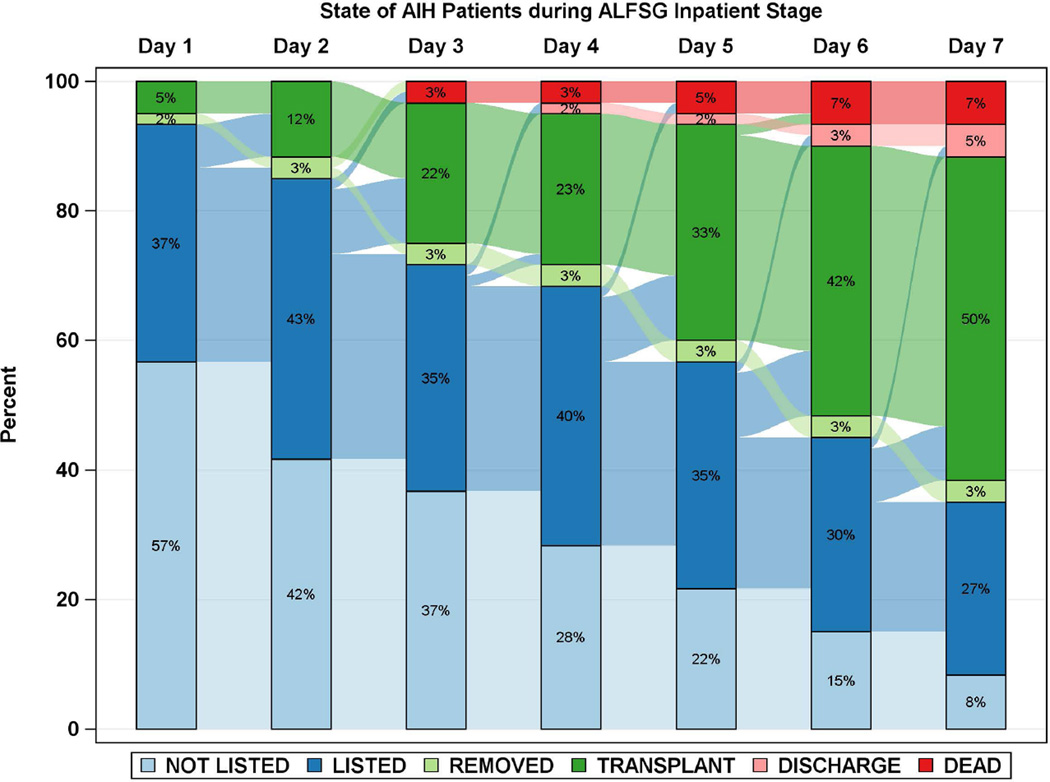
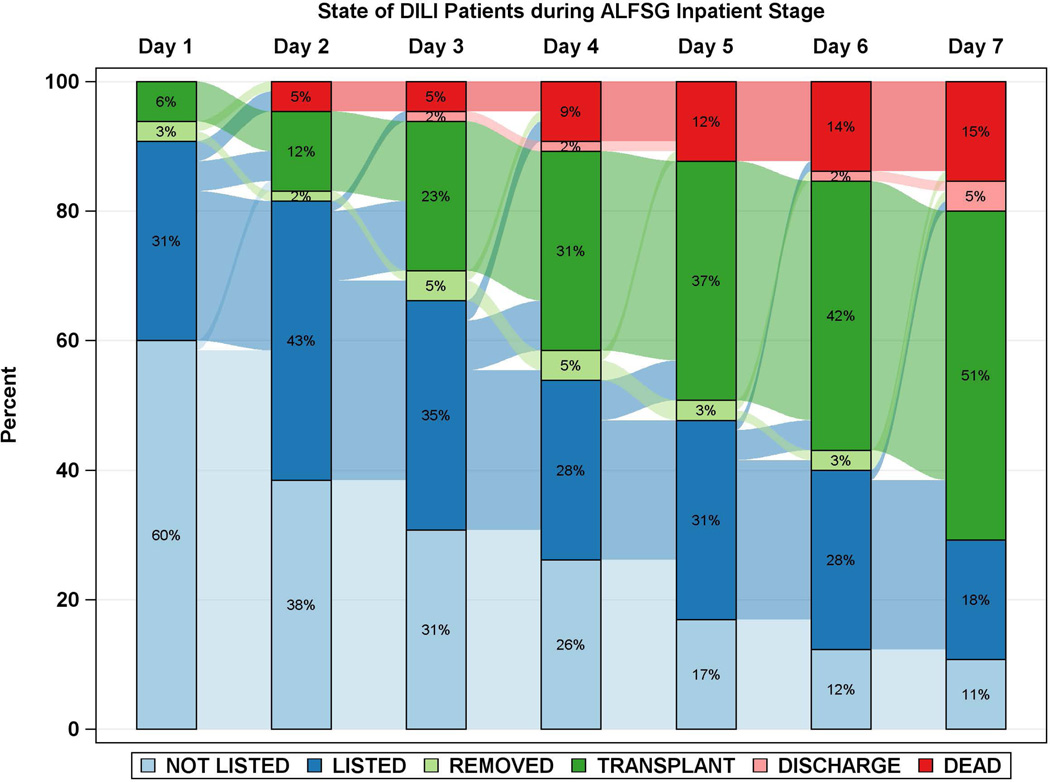
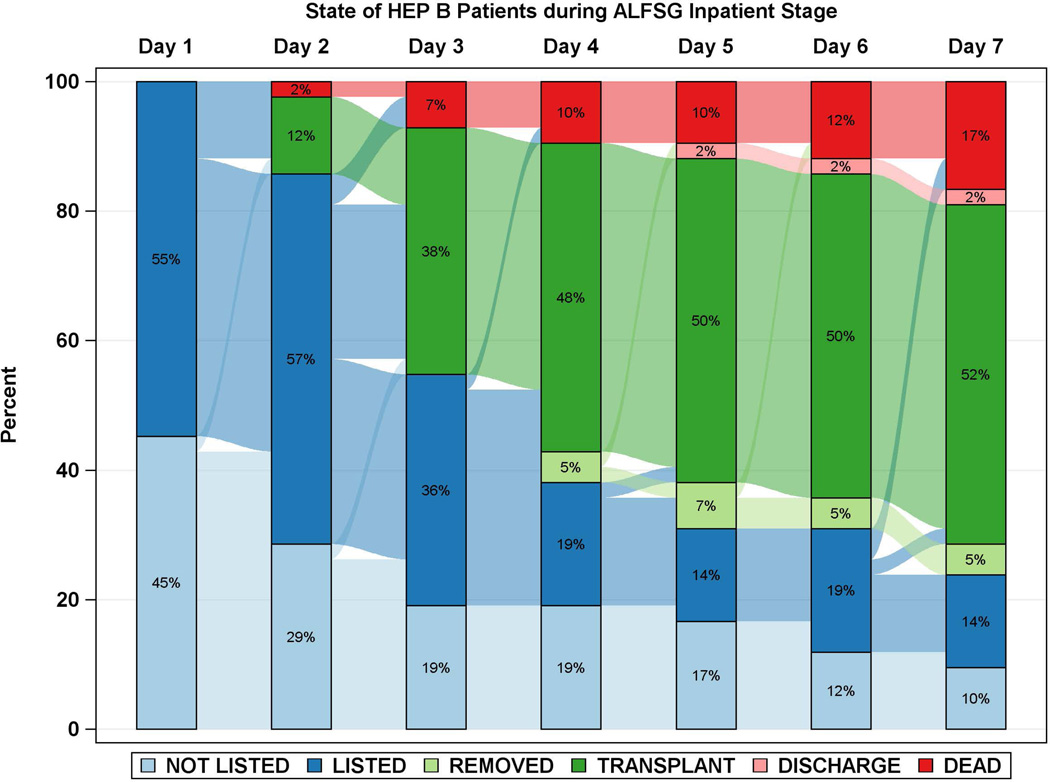
Diagrammatic representation of events by day after registry enrollment/listing according to etiology groups: a: APAP, b: Autoimmune Hepatitis (AIH), c: Drug-induced liver injury (DILI) and d: Hepatitis B. Most of the deaths and transplants in the APAP group (3a) took place within the first 48 hours, while both deaths and transplants evolved more slowly in the three non-APAP categories (3b–d). The remaining patients resemble the non-APAP groups. Figure is restricted to only those patients with a date of listing.
As noted above, the time to LT and the time to death were similar at 3 days across outcome groups, suggesting that timeliness of obtaining suitable donor organs is essential, as earlier LT by 1 or 2 days following listing would likely have rescued additional patients.
Time to LT and/or death for each of the 4 major etiologies was also reviewed (Figures 2 and 3a–d). Patients with APAP hepatotoxicity demonstrated uniformly rapid disease evolution and either were transplanted, died or recovered in a much shorter interval than other etiologies, median 1–2 days less than non-APAP cases. For example, APAP patients died a median of 2 days following listing versus 4.5 days for the non-APAP cases (Figure 2, 3a, Supplementary Table 2). By contrast, DILI, AIH and hepatitis B were all characterized by much slower onset of illness (Figure 2, black bars), relatively similar outcomes once LT listing occurred, and continued evolution in hospital over a longer time (4 to 7 days) than subjects with APAP hepatotoxicity Figure 3b–d). Thus, the majority of APAP cases either died or were transplanted by day 4, as compared to hepatitis B, DILI and AIH, where outcomes continued to evolve to 7 days and beyond. Non-APAP etiologies were more likely to receive a transplant (74%) and less likely to die without a transplant (15%), resulting in very few (11%) non-APAP cases as spontaneous survivors.
Post liver transplant survival
Among the 392 individuals who received a LT, 359 (92%) survived at least 21 days; the small number of deaths precluded us from identifying baseline predictors or differences in etiologies for early death following liver transplantation.
Discussion
This large, multi-center study focused solely on those ALF patients who were listed with UNOS for LT as Status 1 over a 14-year period, criteria for listing having remained constant during this interval.9 While just over a 1/3 of all ALF patients were listed, nearly 2/3 of those listed received a graft. Among listed patients who did not receive an organ, 52% recovered, which when compared to 92% of LT recipients surviving at least 21 days, appears to confirm the value of LT in saving lives. How many of these might have survived without a transplant we cannot know. Overall survival among listed patients in the current era was 77%, 58% the result of LT and 19% due to SS, a far cry from the 6% survival reported in the pre-transplant era.12 Of course, spontaneous survival might have been even higher if not for the ‘rescue’ of transplantation, driven by prognostic uncertainty.
Time to transplantation is a key determinant of overall outcome and the median time to transplantation in our study was 3.0 days, considerably longer than that reported in the United Kingdom (UK) where ALF patients overall received a donor organ within 1–2 days.13,14 Even shorter times were observed in a recent French study where median time from listing to transplant was 16 hours, with 89% transplanted by 48 hours.15 Of interest, in one UK study, 76% of listed patients received LT, higher than the 64% in our series, likely related to more rapid organ availability.13 In all studies including our own, time to LT did not differ between etiologies since it depends largely on organ availability once listing occurs. By contrast, time to death differed significantly, with APAP cases dying in a median of two days following listing compared to 4.5 days for the non-APAP etiologies. Sixty percent of APAP transplants and 90% of APAP deaths occurred by day 3. Etiology was clearly a very strong determinant of outcome after listing.
We purposely focused our attention solely on the events following listing to better understand who receives a graft and why. We divided patients between those with APAP hepatotoxicity and the main non-APAP etiologies, causes that differ in illness duration. While duration of illness has been thought determine outcome, this is inseparable from etiology. In terms of duration, only ischemic liver injury shares the ‘hyperacute’ (< 4 days) scenario with APAP. However, ischemia-related ALF cases rarely are listed for transplantation, only 3/97 in our series. (N.B.: after external adjudication, of 6 ischemia cases initially listed, 3 who were not transplanted had been listed or diagnosed incorrectly). Among the 3 transplants, one was non-ischemic etiology and two were transplanted for surgical misadventure, clamps across hepatic vessels). The vast majority of ischemia patients recover quickly in the short term if the cause of poor hepatic perfusion is addressed; if not, they are unsuitable for listing in any case.16 Thus, listed hyper-acute patients are virtually all APAP cases, the largest etiologic group overall. As illustrated in Table 2, biochemical and clinical features as well as outcomes differed vastly between APAP and the other etiologies. Unique characteristics of the APAP group (and ischemic patients) include very high aminotransferases, and low bilirubin levels as expected in a hyper-acute setting.17
Acetaminophen hepatotoxicity represents a unique form of liver injury that differs from most other etiologies with an expected good overall outcome.1,6 SS being more than twice as likely as the non-APAP etiologies. APAP patients are much less likely to undergo listing due to psychosocial reasons (and perhaps the clinician’s anticipation of their recovery).18 Indeed, Scandinavian centers list paracetamol candidates for only 72 hours, considering that the patient should recover or have died beyond that time interval.19 Thus, only 22% of APAP patients in our study were listed in the first place, compared to 52, 66 and 54% of DILI, AIH and hepatitis B patients.
But what happened to those APAP patients once they were listed? Listed APAP patients demonstrated more severe biochemical (Cr, INR) and clinical features (coma grade, requirements for mechanical ventilation, vasopressor and RRT) and were more likely to die without LT than non-APAP subjects (24% APAP dying vs. 7–22%, 15% overall, for the non-APAP causes [Table 1]). Though contrary to the presumption of better outcomes, the listed APAP subset had more severe illness, less chance of transplantation and died more frequently than the non-APAP group. Both in our study and in a UK cohort,20 those presenting with non-APAP ALF were twice as likely to receive LT compared to the APAP patients, confirming that sub-acute ALF patients are more likely to benefit from LT. Slower evolution of disease permits time to identify a liver, since if sub-acute patients deteriorate, they do so more slowly in the ICU setting. The lower apparent US organ availability and longer wait times lessen the likelihood of an APAP transplant while increasing the number of non-APAP patients that are transplanted. As a result, the very low number (11%) of SS patients within non-APAP etiologies may reflect ‘overtransplantation’ of would-be survivors as reflected in Figure 3. Thus, APAP ALF patients are both excluded from listing for psychosocial reasons and have a lower likelihood of receiving a graft—they are ‘sicker’ at listing and die more rapidly. Their short survival precludes, in the US at least, the timely receipt of an organ offer (Supplementary Table 2, Figures 2 and 3). Rescue for the APAP patient ideally must occur within 24 hours following listing, during which 54% of deaths in this group occurred (Figures 2,3). The study from Edinburgh, confirms this pattern: two times the number of APAP as non-APAP patients died after listing, even with more rapid transplant availability. These authors highlighted the greater severity of illness in the APAP group including higher likelihood of cerebral edema (63% of listed APAP patients) and higher requirements for pressor and ventilator support.14
One can argue, of course, that early access to organs is important across all etiologies. However, early access would also lead to more patients receiving an organ who otherwise might have made a spontaneous recovery, particularly for the non-APAP slowly evolving patients. In this light, improving patients in our study were removed from the list an average of 3 days after study admission for APAP and 4 days for non-APAP, while time to death for those not receiving LT was a median of 2 days after study admission for APAP and 4.5 days for non-APAP cases (Supplementary Table 2, Figures 2 and 3). Early (21-day) survival post-LT was similar for the APAP and non-APAP groups, as was observed in Edinburgh.14
A concerning finding in the present study was the less severe illness apparent at enrollment and thereafter in those eventually transplanted, when compared to those who died. Transplanted patients displayed features of clinical severity more similar to those SS patients (Table 1). Median MELD score was higher in the LT group, driven by higher bilirubin and INR levels and use of RRT, despite actual Cr levels being lower (high Cr level typically is associated with worse outcome).21 However, those that died awaiting LT were significantly more likely to have advanced HE and require mechanical ventilation, vasopressors and RRT, compared to the other two groups at initial presentation and during their hospital course (Table 2). For example, the group who eventually died demonstrated higher coma grades (66 and 88% at admission or any time) versus 45 and 56% for survivors and 40 and 60% for the transplanted group. Similar disparities were demonstrated for mechanical ventilation, vasopressor support and RRT, being given to 61% of those dying, and only 38 and 33% of those surviving or receiving a LT.
Traditionally, the urgency for listing for LT occurs at the threshold of stage II to III coma, around the time that intubation is needed. However, in the present study, early listing, prior to advanced coma grades occurred in 48%, since declaring someone to be Status 1 requires no specific score or state to be achieved.9 Given the current donor organ shortage, center behavior with respect to listing early may be driven by a perceived need for more lead time to identify a suitable donor organ. This strategy could have allowed for transplantation of patients who might have recovered spontaneously. A contrary argument is that these patients would have deteriorated eventually with sub-acute diagnoses--their ability to remain as viable candidates at the time of an organ offer was essential to their becoming an eventual transplant recipient in the first place. To achieve LT in the United States, therefore, ALF patients must survive several days, remaining ‘sick enough’ to justify priority status, but not ‘too sick’ for transplantation. Clearly, the 92% early survival of transplanted patients speaks to excellent patient selection as well as advancing technical capabilities.
We acknowledge several shortcomings of our study. First, the majority of ALFSG centers used similar but not identical management protocols.22 Variable decision-making to list a patient for LT or remove/inactivate them are recognized and may be site specific, and the random availability of donor organs contribute to the heterogeneity observed in times to LT but the large number of sites and wide geographic distribution mitigates against this. We were not able to demonstrate differences across US regions in regard to organ availability (data not shown), although such differences are considerable for patients with cirrhosis.23–25 Although our study took place over 14 years, the likelihood of undergoing LT did not change over time (data not shown). Within the ALFSG study sites, enrollment of new ALF patients is never universal, since some patients’ families do not agree to registry enrollment. Therefore, our study group may have some potential bias with reduced generalizability since all consecutive ALF patients seen in the participating centers were not enrolled.
Our conclusions support the value of LT for ALF across a variety of etiologies, and emphasize the importance of early referral and streamlining the listing process, particularly for APAP cases. The United States appears to be significantly behind Europe and the UK in organ availability. Since many characteristics of the LT group resembled those of the group that recovered without LT, future studies should consider whether over-transplantation occurs in certain circumstances in patients who could have made a full recovery, had a suitable donor organ not become available. Our findings substantiate previous work showing that post-LT survival for ALF patients has improved in recent years, possibly due to better selection of candidates as well as improved supportive care. The focus for the future should be on optimizing early transplant availability while considering more fully whether transplantation is truly needed in each instance. Identification of more accurate prognostic markers of recovery or liver regeneration and development of novel liver support devices might prolong short-term and, eventually, long-term survival without liver transplantation.
Supplementary Material
Acknowledgements
The authors acknowledge the support of NIDDK U-01 58369 to UT Southwestern and the strong support of NIDDK, the site coordinators and patients and their families.
| University of Texas Southwestern Medical Center | William M. Lee, M.D. |
| University of Washington | Iris Liou, M.D. |
| Washington University (closed) | Jeffrey Crippin, M.D. |
| University of California, San Francisco | Oren Fix, M.D., Bilal Hameed M.D |
| Mount Sinai (closed) | Lawrence Liu, M.D. |
| University of Nebraska (closed) | Timothy M. McCashland, M.D. |
| Mayo Clinic, Rochester (closed) | J. Eileen Hay, M.D. |
| Baylor University Medical Center (closed) | Natalie Murray, M.D. |
| University of Pittsburgh (closed) | Obaid Shakil Shaikh, M.D. |
| Northwestern University | Daniel R. Ganger, M.D. |
| Oregon Health Sciences Center (closed) | Atif Zaman, M.D. |
| University of California, Los Angeles | Steven Han, M.D. |
| University of Miami (closed) | Eugene Schiff, M.D. |
| University of Michigan | Robert Fontana, M.D. |
| Yale University | Michael Schilsky, M.D., Cary A. Caldwell M.D |
| University of Alabama, Birmingham | Brendan McGuire, M.D. |
| Massachusetts General Hospital (closed) | Raymond T. Chung, M.D. |
| Duke University (closed) | Don C. Rockey, M.D. |
| Columbia-Presbyterian (closed) | Robert Brown, MD |
| Mayo Clinic, Scottsdale (closed) | M. Edwyn Harrison, MD |
| Medical University of South Carolina | Adrian Reuben, M.B.B.S. |
| Albert Einstein Medical Center (closed) | Santiago J. Munoz, M.D. |
| University of Pennsylvania | K.Rajender Reddy, M.D. |
| Virginia Commonwealth University | R. Todd Stravitz, M.D. |
| University of California, Davis (closed) | Lorenzo Rossaro, MD |
| Mayo Clinic, Jacksonville, Jacksonville, FL (closed) | Raj Satyanarayana, M.D. |
| University of California, San Diego (closed) | Tarek Hassanein, M.D. |
| California Pacific Medical Center, San Francisco, CA (closed) | Timothy Davern, M.D. |
| * The Ohio State University, Columbus, Ohio | A. James Hanje, M.D. |
| * University of Kansas Medical Center, Kansas City, KS | Jody C. Olson, M.D. |
| * Emory University, Atlanta, GA | Ram Subramanian, M.D. |
| * University of Alberta, Edmonton, Canada | Constantine J. Karvellas, M.D. |
New sites
ABBREVIATIONS
- AIH
Autoimmune Hepatitis
- ALF
Acute Liver Failure
- ALFSG
Acute Liver Failure Study Group
- ALT
Alanine Aminotransferase
- ANOVA
Analysis of Variance
- APAP
acetyl-para-aminophenol
- CRF
Case Report Form
- CVVH
Continuous Veno-Venous Hemofiltration
- DILI
Drug Induced Liver Injury
- INR
International Normalized Ratio
- IQR
Interquartile Range
- KCC
King’s College Criteria
- LT
Liver Transplantation
- UNOS
United Network for Organ Sharing
- US
United States
Footnotes
AUTHOR CONTRIBUTIONS:
K. Rajender Reddy: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Caitlyn Ellerbe: acquisition of data; analysis and interpretation of data; statistical analysis
Michael Schilsky: analysis and interpretation of data, critical revision of the manuscript for important intellectual content
R. Todd Stravitz: analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Robert J. Fontana: analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Valeria Durkalski: acquisition of data; analysis and interpretation of data; statistical analysis
William M. Lee: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision
CONFLICT OF INTEREST STATEMENT: KRR, CE, MS, RTS, RJF, VED, and WML have no conflicts of interest regarding this work.
References
- 1.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–2534. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33:36–45. doi: 10.1055/s-0032-1301733. [DOI] [PubMed] [Google Scholar]
- 4.Lee WM, Squires RH, Nyberg SL, et al. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholongitas E, Theocharidou E, Vasianopoulou P, et al. Comparison of the sequential organ failure assessment score with the King's College Hospital criteria and the model for end-stage liver disease score for the prognosis of acetaminophen-induced acute liver failure. Liver Transplant. 2012;18:405–412. doi: 10.1002/lt.23370. [DOI] [PubMed] [Google Scholar]
- 6.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 7.Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: Results of a US multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stravitz RT, Lefkowitch JH, Fontana RJ, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53:517–526. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.OPTN Member Evaluation Plan Policy 9.1a Updated September 1, 2014. [Accessed online November 3, 2014]; http://optn.transplant.hrsa.gov/ContentDocuments/Evaluation_Plan.pdf.
- 10.Korman JD, Volenberg I, Balko J, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008;48:1167–1174. doi: 10.1002/hep.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana RJ, Ellerbe C, Durkalski VE, et al. Two-year outcomes in initial survivors with acute liver failure: results from a prospective, multicenter study. Liver Int. 2015;35:370–380. doi: 10.1111/liv.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakela J, Lange SM, Ludwig J, Baldus WP. Fulminant hepatitis: Mayo Clinic experience with 34 cases. Mayo Clin Proc. 1985;60:289–292. doi: 10.1016/s0025-6196(12)60534-5. [DOI] [PubMed] [Google Scholar]
- 13.Bernal W, Cross TJ, Auzinger G, et al. Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol. 2009;50:306–313. doi: 10.1016/j.jhep.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Simpson KJ, Bates CM, Henderson NC, et al. The utilization of liver transplantation in the management of acute liver failure: Comparison between acetaminophen and non-acetaminophen etiologies. Liver Transplant. 2009;15:600–609. doi: 10.1002/lt.21681. [DOI] [PubMed] [Google Scholar]
- 15.Saliba F, Camus C, Durand F, et al. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med. 2013;159:522–531. doi: 10.7326/0003-4819-159-8-201310150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Taylor RM, Tujios S, Jinjuvadia K, et al. Short and long-term outcomes in patients with acute liver failure due to ischemic hepatitis. Dig Dis Sci. 2012;57:777–785. doi: 10.1007/s10620-011-1918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khandelwal N, James LP, Sanders C, et al. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53:567–576. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee WM, Larson AM, Arenas J. Thinking through acetaminophen hepatotoxicity and liver transplantation: choosing worthy recipients. Liver Transpl. 2009;15:567–569. doi: 10.1002/lt.21728. [DOI] [PubMed] [Google Scholar]
- 19.Wei G, Bergquist A, Broome U, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. doi: 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 20.Bernal W, Hyyrylainen A, Gera A, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74–80. doi: 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Tujios SR, Hynan LS, Vazquez MA, et al. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol. 2015;13:352–359. doi: 10.1016/j.cgh.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: Recommendations of the Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–2501. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 23.Barshes NR, Lee TC, Balkrishnan R, et al. Risk stratification of adult patients undergoing orthotopic liver transplantation for fulminant hepatic failure. Transplantation. 2006;81:195–201. doi: 10.1097/01.tp.0000188149.90975.63. [DOI] [PubMed] [Google Scholar]
- 24.Yeh H, Smoot E, Schoenfeld DA, et al. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91:479–486. doi: 10.1097/TP.0b013e3182066275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentry S, Massie A, Cheek S, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant. 2013;13:2052–2058. doi: 10.1111/ajt.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.