1. Introduction
The retinal pigment epithelium (RPE) is essential for maintaining the health of the neural retina. RPE cell dysfunction plays a critical role in many common blinding diseases including age-related macular degeneration (AMD), diabetic retinopathy, and retinal dystrophies. The RPE is in close apposition to the neurosensory retina and is adherent to Bruch's membrane, a thin, pentalaminar matrix that has the choriocapillaris attached to its other side. Delineating the molecular function of each of these structures requires that they be isolated from one another. In large eyes like from the human, separating the RPE from both the retina and choroid is straightforward. Mouse models of ocular disease are commonly used to study these blinding diseases. In the mouse eye, separating the RPE from the choroid is challenging because of the small eye size with the resulting thin separation between the RPE and choroid, and the strong adhesion of the RPE to Bruch's membrane. For these reasons, traditional mechanical dissection strategies for isolating mouse RPE have included the choroid. The choroid contains a variety of cell types includes vascular endothelial cells, pericytes, fibroblasts, smooth muscle cells, melanocytes, and numerous immune cells. With traditional RPE/choroid extracts, the molecular signature of the RPE is in reality, the RPE/choroid, and thus potentially represents a mixed signature that might interfere with identifying an accurate RPE signal. Laser capture microdissection can be used to obtain pure population of RPE cells. However, this technique is labor intensive and time consuming. Herein, we describe a simple technique for isolating proteins from the RPE without getting contamination from the choroid or neural retina.
2. Materials and Supplies
2.1 Supplies
Primary antibodies for fluorescence immunohistochemistry: rabbit anti-Collagen VI antibody (1:50, Abcam Inc., Cambridge, MA) or mouse anti-RPE65 antibody (1:50, Santa Cruz Inc., Santa Cruz, CA).
Secondary antibodies for fluorescence immunohistochemistry: goat anti-rabbit IgG Alexa 594 or goat anti-mouse IgG Alexa 488 (1:1000; Thermo Fisher Scientific, Inc. Carlsbad, CA).
DAPI (1:1000; Sigma-Aldrich, Inc.).
Primary antibodies for Western blot analysis: goat anti-actin 1-19-HRP (1:5000; Santa Cruz, Inc.), rabbit anti-collagen VI (1:500, Abcam, Inc.), rabbit anti-collagen I (1:500; Abcam, Inc.), mouse anti-RPE65 (1:1000 Santa Cruz, Inc.), rabbit anti-Rhodopsin (1:1000, Santa Cruz, Inc.), rabbit anti-Best1 (1:500 MyBioSource, San Diego, CA), mouse anti-apo(a) monoclonal antibody (1:1000; LPA4; gift, Joe Witztum, MD, UC San Diego).
Secondary antibody for Western blot analysis: horseradish peroxidase conjugated secondary antibody (goat anti-mouse IgG (1:5000, Abcam, Inc.) or goat anti-rabbit IgG (1:5000, Abcam, Inc.).
Human Lipoprotein(a) (Biovision, Inc., Milpitis, CA)
Chemiluminescence detection system (Supersignal West Pico Chemiluminescence, Thermo Fisher Scientific, Waltham, MA).
2% paraformaldehyde (Sigma-Aldrich, Inc.)
RIPA lysis buffer with protease inhibitor, Sigma-Aldrich, Inc., St. Louis, MO
2.2 Equipment
ImageQuant LAS4000 scanner (GE Healthcare, Inc., Piscataway, NJ)
KCL™V3.4 BIO-TEK instrument (Bio-Tek Instruments, Inc. Winooski, VT)
Zeiss ZEN LSM 710 confocal microscope
Dumont forceps (Fine Science Tools, Inc., Foster City, CA) and Vannas scissors (Biomedical Research Instruments, Inc., Silver Spring, MD)
3. Detailed Methods
3.1 Animals and Care
Male and female C57BL6/129 and C57BL/6J mice (Jackson Labs, Bar Harbor, ME) were given standard rodent chow and water ad libitum, and kept in a 12-hour light-dark cycle. All studies were performed according to protocols approved by The Johns Hopkins University Animal Care and Use Committee in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals in Ophthalmic and Vision research.
3.2 Protocol for globe dissection
After sacrificing mice by CO2 asphyxiation under anesthesia, the eyes are enucleated and immediately placed in phosphate buffered saline (PBS) on ice.
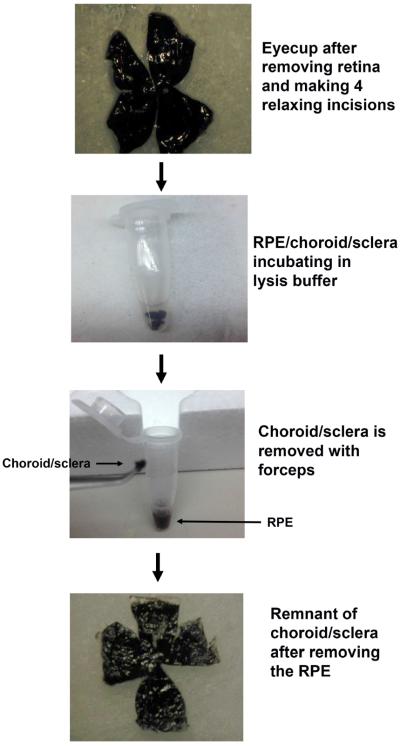
Using a dissecting microscope, the eye is incised with scissors at the posterior margin of the limbus and the cornea, and the iris and lens is removed (Figure 1).
Figure 1.
Schematic diagram of isolated RPE using the new protocol. With the posterior eye cup, the retina is removed, and the RPE/Bruch's membrane/choroid/sclera is cut into four parts, and then incubated in protein lysis buffer. After gently tapping on the microcentrifuge tube, the RPE is separated from Bruch's membrane/choroid/sclera.
The retina is carefully removed by cutting the optic nerve.
3.3 Traditional protocol for RPE/choroid dissection
With the neural retina removed, the RPE/choroid is dissected from the sclera, and placed in 200 μl protein lysis buffer, homogenized, and then placed on ice for 45 minutes.
3.4 New protocol for RPE isolation
Four small slits are cut with a scissors greater than half-way to the optic nerve from the peripheral edges into the RPE/choroid/sclera to flatten the tissue.
The RPE/choroid/scleral tissue is transferred RPE side up, to a 1.5 ml microcentrifuge tube containing 200 μl protein lysis buffer. The tissue is gently immersed in the buffer with a forceps and incubated from 10 minutes to 1 hour on ice, at which the tube is gently tapped over 50 times to release the RPE, seen as brown clumps, into the lysis buffer from the choroid/sclera.
The lysis buffer containing brown clumps of presumed RPE cells is transferred to a fresh microfuge tube and placed on ice for 5–60 minutes.
The remaining choroid/sclera is also placed on ice for 5–60 minutes and incubated in lysis buffer to extract protein.
3.5 Histology
The Bruch's membrane/choroid/scleral remnants were lightly fixed in 2% paraformaldehyde, cryopreserved, and OCT embedded. Sections (7 μm) were stained with Hematoxalin and eosin or assessed by confocal fluorescence immunohistochemistry.
3.6 Confocal fluorescence immunohistochemistry
For fluorescence immunohistochemistry, mouse cryosections (7 μm) were first blocked with 2% goat serum in PBS buffer for 1 hour at room temperature. Sections were then incubated with the primary antibody overnight at 4°C, washed with PBS, followed by incubation with labeled secondary antibody. DAPI was used to label nuclei. Appropriate mouse and rabbit IgG were use as isotype controls. Z stack images of tissue sections were imaged using a Zeiss ZEN LSM 710 confocal microscope.
3.7 Protein extraction
Proteins were extracted from the RIPA lysis buffer with protease inhibitor cocktail, EDTA-free (Sigma, Inc.) by first sonicating for 20 seconds, and then centrifuging for 15 minutes at 14000 rpm at 4°C. The supernatant was collected in new tubes and placed on ice. The protein concentration was measured using a KCL™V3.4 BIO-TEK instrument.
3.8 Western blot analysis
Western analysis was performed as described(Wang et al., 2014). Cell lysates (20μg protein) were separated on a 4% – 12% SDS-PAGE and electrophoretically transferred to a nitrocellulose membrane. Membranes were incubated with the primary antibody and then the appropriate horseradish peroxidase conjugated secondary antibody. Signal was detected with a chemiluminescence detection system. Blots were imaged with an ImageQuant LAS4000 scanner, and band intensity is reported as arbitrary densitometric units. Actin was used for signal normalization across samples.
3.9 Results
Due to the strong adhesion of the RPE to Bruch's membrane and choroid, and the thin Bruch's membrane due to the small globe size, prior attempts using mechanical debridement to isolate the RPE from choroid have been challenging and unsuccessful at obtaining pure populations of RPE cells(Xin-Zhao Wang et al., 2012). Therefore, we attempted a strategy that did not depend upon mechanical manipulation. After removing the anterior segment and neural retina, the remaining posterior eyecup comprised of the RPE-choroid-sclera was placed in lysis buffer for up to 60 minutes (Figure 1). After incubating in lysis buffer, the microcentrifuge tube was gently tapped to remove RPE debris from the posterior eye cup, and the eye cup was removed.
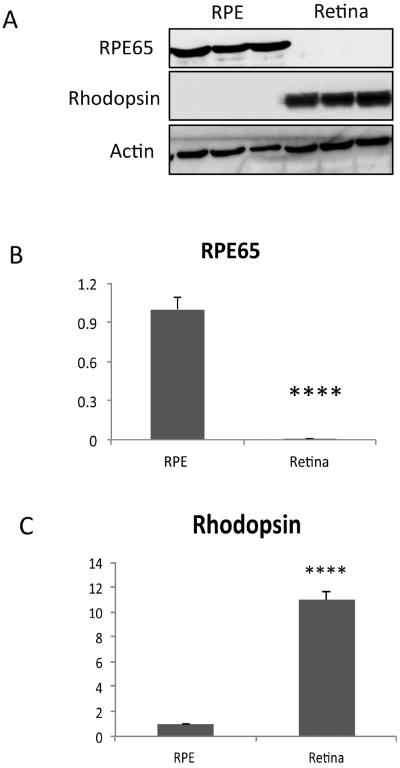
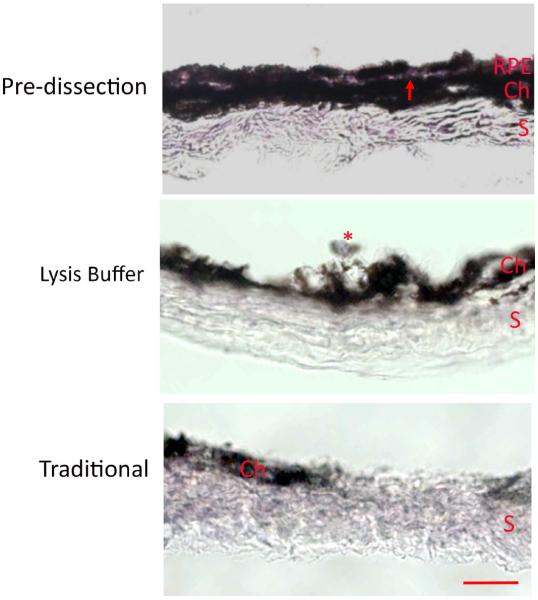
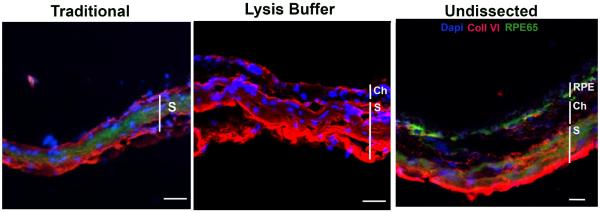
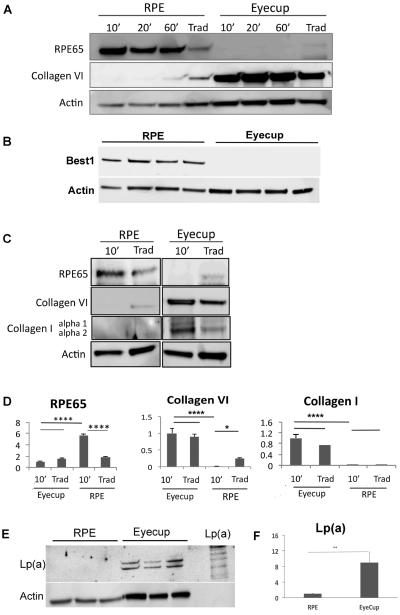
Using the new technique, we first show that the RPE cell lysates are free of neurosensory retinal contamination by finding an absence of rhodopsin, while as expected, abundant rhodopsin in neurosensory retinal extracts. The RPE lysates have abundant RPE65 protein, an RPE specific marker, using Western blot analysis (Figure 2). The Western blot in supplementary Figure 1 shows that endogenous immunoglobulins detected as a result of using an anti-mouse antibody to RPE65 did not interfere with interpretation of the RPE65 signal. We next show that the RPE is removed from the remaining eyecup (sclera) using either the traditional or lysis buffer technique (Figure 3). Using confocal fluorescence immunohistochemistry, we next looked at the separation of the RPE from the choroid using RPE65 and collagen VI as RPE and choroidal markers, respectively. RPE65 immunolabeling is absent after an eyecup is subjected to either the traditional or lysis buffer dissection protocol, in contrast to the undissected eyecup (Figure 4). Collagen VI is immunologically preserved in the choroidal layer of the eyecup after incubation in the lysis buffer, but not in the eyecup after using the traditional dissection protocol. Western analysis was used to confirm the fluorescence immunohistochemical studies. Figure 5A shows that the RPE lysates using the lysis buffer method had abundant RPE65 protein and compared favorably with the abundance of RPE65 extracted using the traditional method. Collagen VI was absent in the RPE lysates after 10–20 minute incubation, and minimally present after a 60 minute incubation in lysis buffer, but it was less than in the RPE/choroidal lysates recovered using the traditional method. The completeness of removing the RPE was assessed by evaluating the abundance of RPE65 in the remaining eyecup after dissection. Figure 5A also shows that minimal RPE65 and abundant collagen VI is seen in the eyecup after the lysis buffer dissection. Since RPE65 is a cytoplasmic protein, we next determined the extent that Best1, a cell membrane protein, is recovered after the lysis buffer dissection. Figure 5B shows that a 10 minute incubation resulted in significant Best1 protein recovery with minimal remnants in the remaining eyecup (p<0.001). While minimal RPE65 and Best1 remained in the eyecup after the traditional protocol as expected, we were surprised to identify significant collagen VI in the remaining eyecup, which suggests that dissection of the RPE/choroid is incomplete. To quantify these changes, we compared a 10 minute lysis buffer dissection to the traditional protocol. Figure 5C,D show that RPE65 was more abundant in the RPE lysates using the lysis buffer incubation than from the traditional protocol (p<0.0001). Collagen VI was less abundant in the RPE lysates derived from the lysis buffer dissection compared to the traditional dissection (p<0.05). In addition to collagen VI, minimal collagen I alpha 1 or alpha 2, a component of the inner collagenous layer of Bruch's membrane and sclera, was found in RPE cell lysates using the lysis buffer or traditional protocol while collagen I and collagen VI were abundant in the eyecups after either dissection protocol (Figure 5C,D). Since collagen VI is a structural protein in the choroid, we next wanted to show that a soluble protein in the choroid, such as albumin or immunoglobulins, do not leak into the RPE lysates using the lysis buffer protocol. Since these and other soluble proteins from the circulation are expressed by the RPE(Anderson and Anderson, 2002; Chen et al., 2012; Niu et al., 2013), we instead intravenously injected human plasma containing lipoprotein(a), composed of apolipoprotein(a) and apolipoproteinB, and after 5 minutes, assessed Lp(a) contamination in the RPE after lysis buffer dissection. We specifically selected Lp(a) because mice do not produce Lp(a). The Western blot in Figure 5E, F shows that Lp(a) remains in the eyecup, and does not appear in the RPE lysates using LPA4 antibody to detect apo(a). Finally, we compared the quantity of protein recovered from the RPE lysates using the two protocols. Since in pilot experiments, the amount of protein recovered from a 10 minute or 60 minute lysis buffer incubation was similar (data not shown), we compared a 10 minute incubation in lysis buffer to the traditional extraction technique. Figure 6 shows that the total protein recovery using a 10 minute lysis buffer incubation is significantly less than the traditional protocol. The lysis buffer technique requires two eyes to obtain an equivalent quantity of protein recovered from one eye with the traditional protocol.
Figure 2.
Rhodopsin and RPE 65 expression from RPE protein extracts isolated using lysis buffer digestion of C57BL/6J mice. A. Western blot of Rhodopsin, expressed by the photoreceptors of the neurosensory retina, is abundant in the retina, but not in RPE lysates. Western blot also of RPE65, an RPE specific marker, showing expression in the RPE, but not the retina. Expression was normalized to actin. B. Graph of RPE65 protein abundance in the RPE and retina using the lysis buffer protocol (n=5 mice). C. Graph of Rhodopsin protein abundance in the RPE and retina using the lysis buffer protocol (n=5 mice). ****p<0.0001
Figure 3.
Histological assessment of a C57BL/6J mouse eyes after lysis buffer and traditional dissection. Hematoxylin and eosin staining of the RPE/Bruch's membrane/choroid/scleral eyecup after the retina was removed. The eyecup pre-dissection shows an intact RPE layer. Note the thin clear line of Bruch's membrane (red arrow) that separates the choroid (Ch) from the RPE. The sclera (S) is also intact. After lysis buffer digestion, the RPE is gone, except for possibly a remnant of an RPE cell (*), with remaining choroid and sclera. After the traditional dissection, the sclera remains, with a remnant of the choroid (Ch). Bar=25 um.
Figure 4.
Confocal fluorescence immunohistochemistry of C57BL/6x129 mouse eyecup for RPE65 and collagen VI. The eyecup after the traditional dissection of the RPE/choroid shows nonspecific immunolabeling for RPE65 (green) and collagen VI (red) in the sclera (S) only. The eyecup after lysis buffer digestion has collagen VI immunolabeling present in the choroid (Ch) and sclera (S), but no RPE65 immunolabeling. The undissected eyecup shows prominent RPE65 immunolabeling (green) in the RPE and collagen VI labeling in the choroid. Nonspecific labeling for RPE65 and collagen VI is seen in the sclera. Nuclei are labeled with Dapi (blue). Bar=15um.
Figure 5.
Protein recovery by incubation time in C57BL/6x129 mice. RPE protein were collected after removing retina and choroid/sclera, incubated in protein lysis buffer for 10 min, 20min, and 60 min, and compared to protein recovered using the traditional method (Trad) that contained RPE-Bruch's membrane-choroidal proteins (n=5 each group). A. The lysis buffer incubation protocol results in abundant RPE 65, an RPE specific gene, and minimal choroidal contamination, as confirmed by low Collagen VI in RPE lysates. In comparison, collagen VI is abundant in the RPE/choroidal extracts using the traditional method. RPE65 is absent from the extracts recovered from the remaining eyecup after both the lysis buffer and traditional protocols. Collagen VI was abundant in the remaining eyecups after both the lysis buffer and traditional protocols. B. Western blot of RPE and eyecup lysates after a 10 minute incubation in lysis buffer showing Best1 in the RPE lysate, but not in the remaining eyecup (n=4 mice). C. Representative Western blot comparing a 10 minute incubation in lysis buffer to the traditional dissection of both the RPE lysates and of the remaining eyecup after dissection, for RPE65, collagen VI, and collagen I. Expression was normalized to actin. Note that the actin abundance is different between the RPE and eyecup. D. Graph showing the quantification of RPE65, collagen VI, and collagen I from the Western blots in C (n=5 mice). *p<0.05, ***p<0.001, ****p<0.0001. E. Western blot of Lp(a) using LPA4 antibody against human apo(a) in the RPE and remaining eyecup using the lysis buffer protocol (n=3 mice per group). An aliquot of Lp(a) is shown for comparison to illustrate the multiple bands which identify Lp(a). F. Graph quantifying the abundance of Lp(a) in the RPE and eycup lysates; **p<0.01.
Figure 6.
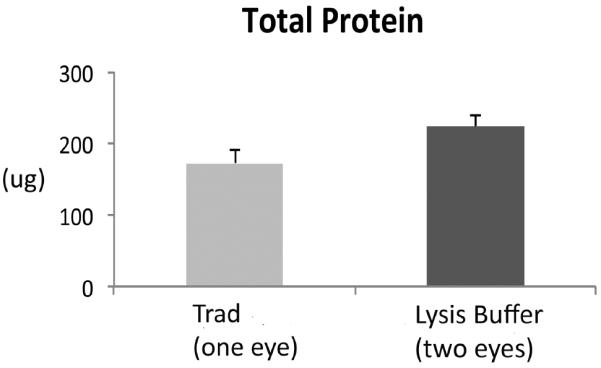
Total protein recovery. Graph of the protein (μg) recovered from the RPE lysates after a 10 minute incubation in lysis buffer and the traditional technique (n=5 mice). The lysis buffer technique required two eyes to obtain an equivalent quantity of protein from one eye using the traditional protocol.
4. Potential Pitfalls and Troubleshooting
It is difficult to separate the RPE from the choroid in the mouse due the small globe size that results in a thin Bruch's membrane of approximately 0.5 um thick(Ida et al., 2004), and the strong adhesion of the RPE to Bruch's membrane. Due to these factors, mechanical debridement is both time consuming and introduces contamination when separating the RPE. As a result, most laboratories dissect the RPE/Bruch's membrane/choroid and report their findings as RPE. While popular, this method can introduce potentially misleading information about the proteome. Other techniques for isolating RPE from mouse eyes have been reported. Claybon and Bishop(Claybon and Bishop, 2011) describe a technique for generating RPE flatmounts. Their technique uses an identical dissection to remove the anterior segment and neural retina, and the cuts into the RPE/choroid/sclera to flatten the eyecup. Boatright et al recently describe a similar technique for analyzing RPE sheets(Boatright et al., 2015). These reports did not dissect RPE from the choroid, and were not intended for protein isolation. Finally, Gu et al describe a technique to dissect apical microvilli and cell bodies from the RPE that was suitable for proteomic analysis(Gu et al., 2012). After removing the retina, the eyecup was incubated with WGA-microbeads, which were then scraped off the eyecups to isolate apical microvilli. Cell bodies were then mechanically debrided from the eyecup. While feasible in the rat eye, which is substantially larger than the mouse eye, it is not known if this technique would be achievable in a mouse eye.
Herein, we describe a new technique that is simple and can obtain pure RPE proteins that is free of contamination from the neurosensory retina and choroid. The technique is technically easy to perform because it utilizes standard dissection techniques. The procedure relies on timed lysis of the RPE so that RPE proteins are extracted before Bruch's membrane and sclera are digested, as indicated by a lack of recovering collagen I, a component of both inner Bruch's membrane and the sclera. This result suggests that collagen I is somewhat resistant to lysis buffer digestion using the incubation times of this study, and serves as a barrier to prevent choroidal contamination.
The new technique requires two eyes to recover a similar quantity of protein as the traditional RPE/choroid dissection. However, the RPE lysates using the new technique recovered relatively more RPE65 than when the traditional dissection was used, and importantly, the RPE lysates were free of collagen VI contamination in contrast to lysates recovered using the traditional protocol. Thus, despite the lower protein yield, the sensitivity and specificity is improved compared to the traditional method of using RPE/choroid lysates. Incubation periods longer than 10 minutes did not increase the quantity of protein recovered, and increased the possibility of introducing choroidal contamination after a 60 minute incubation. Based on our histological and Western blot analyses, a 10 minute incubation will extract RPE proteins with minimal choroidal contamination.
RPE65 is a moderately abundant protein expressed by the RPE. Best1 is a representative cell membrane protein expressed by the RPE. We did not do an exhaustive survey of RPE and choroidal proteins so we do not know the full extent or limitations of isolating the RPE proteome. We acknowledge that this protocol might not extract RPE proteins of low abundance. As mentioned above, our protocol requires two eyes to obtain a similar quantity of protein obtained after using the traditional dissection technique, which will influence the number of mice that might be needed to complete a project. Gu et al reported a combined enzymatic digestion using hyaluronidase and mechanical debridement for isolating apical microvilli from cell bodies of RPE cells(Gu et al., 2012). This technique appears to be valuable for investigating subcellular organelles. Along with the work of Wang et al(Xin-Zhao Wang et al., 2012), who used enzymatic digestion to extract high quality RNA, we hope that our innovation will provide a valuable method for recovering RPE proteins that will improve our ability to study RPE cell behavior in both health and disease.
Supplementary Material
Western blot of RPE65. Expanded view of the Western blot shows nonspecific staining with this anti-mouse antibody that is suggestive of mouse IgG (55 and 25 KDa). Expression was normalized to actin.
Highlights.
-
-
A new RPE extraction technique using lysis buffer incubation is proposed.
-
-
The new technique was compared to traditional dissection of the RPE/choroid.
-
-
The new technique extracts RPE proteins from mouse eyes without choroidal proteins.
-
-
The sensitivity of recovering RPE proteins is increased over the traditional method.
Acknowledgements
Sonny Dike, Zhenhua Xu
Funding: NIH EY14005 (JTH), EY019044 (JTH), RPB Senior Scientist Award (JTH), unrestricted award from RPB to the Wilmer Eye Institute; P30EY001765 core grant, and a gift from the Merlau family and Aleda Wright. JTH is the Robert Bond Welch Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
References
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Dalal N, Chrenek MA, Gardner C, Ziesel A, Jiang Y, Grossniklaus HE, Nickerson JM. Methodologies for analysis of patterning in the mouse RPE sheet. Mol Vis. 2015;21:40–60. [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Chou HC, Lin ST, Chen YW, Lo YW, Chan HL. Effect of high glucose on secreted proteome in cultured retinal pigmented epithelium cells: its possible relevance to clinical diabetic retinopathy. J Proteomics. 2012;77:111–128. doi: 10.1016/j.jprot.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Claybon A, Bishop AJ. Dissection of a mouse eye for a whole mount of the retinal pigment epithelium. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Neric NJ, Crabb JS, Crabb JW, Bhattacharya SK, Rayborn ME, Hollyfield JG, Bonilha VL. Age-related changes in the retinal pigment epithelium (RPE) PLoS One. 2012;7:e38673. doi: 10.1371/journal.pone.0038673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida H, Ishibashi K, Reiser K, Hjelmeland LM, Handa JT. Ultrastructural Aging of the RPE-Bruch's Membrane-Choriocapillaris Complex in the D-Galactose-Treated Mouse. Invest Ophthalmol Vis Sci. 2004;45:2348–2354.. doi: 10.1167/iovs.03-1337. [DOI] [PubMed] [Google Scholar]
- Niu N, Zhang J, McNutt MA. Endogenous IgG affects the cell biology of RPE cells and involves the TLR4 pathway. Invest Ophthalmol Vis Sci. 2013;54:7045–7052. doi: 10.1167/iovs.13-12531. [DOI] [PubMed] [Google Scholar]
- Wang L, Kondo N, Cano M, Ebrahimi K, Yoshida T, Barnett BP, Biswal S, Handa JT. Nrf2 signaling modulates cigarette smoke-induced complement activation in retinal pigmented epithelial cells. Free Radic Biol Med. 2014 doi: 10.1016/j.freeradbiomed.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin-Zhao Wang C, Zhang K, Aredo B, Lu H, Ufret-Vincenty RL. Novel method for the rapid isolation of RPE cells specifically for RNA extraction and analysis. Exp Eye Res. 2012;102:1–9. doi: 10.1016/j.exer.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot of RPE65. Expanded view of the Western blot shows nonspecific staining with this anti-mouse antibody that is suggestive of mouse IgG (55 and 25 KDa). Expression was normalized to actin.