Abstract
Food intake is a complex behavior that can occur or cease to occur for a multitude of reasons. Decisions about where, when, what, and how much to eat are not merely reflexive responses to food-relevant stimuli or to changes in energy status. Rather, feeding behavior is modulated by various contextual factors and by previous experiences. The data reviewed here support the perspective that neurons in multiple hippocampal subregions constitute an important neural substrate linking the external context, the internal context, and mnemonic and cognitive information to control both appetitive and ingestive behavior. Feeding behavior is heavily influenced by hippocampal-dependent mnemonic functions, including episodic meal-related memories and conditional learned associations between food-related stimuli and postingestive consequences. These mnemonic processes are undoubtedly influenced by both external and internal factors relating to food availability, location, and physiological energy status. The afferent and efferent neuroanatomical connectivity of the subregions of the hippocampus is reviewed with regards to the integration of visuospatial and olfactory sensory information (the external context) with endocrine, gustatory, and gastrointestinal interoceptive stimuli (the internal context). Also discussed are recent findings demonstrating that peripherally-derived endocrine signals act on receptors in hippocampal neurons to reduce (leptin, glucagon-like peptide-1) or increase (ghrelin) food intake and learned food reward-driven responding, thereby highlighting endocrine and neuropeptidergic signaling in hippocampal neurons as a novel substrate of importance in the higher-order regulation of feeding behavior.
Keywords: obesity, reward, learning, feeding, ventral hippocampus, memory
1. Introduction
The rising prevalence of obesity in the U.S.A. is driven in part by a linear increase in average daily caloric consumption (1–3). Innovative pharmacological and other therapies that can reduce excessive food intake are urgently needed as behavior therapy offers limited success and gastrointestinal (GI) bariatric surgery, while effective, has serious adverse consequences (4). Basic science investigation of the systems neuroscience of feeding behavior has historically, and nearly exclusively, focused on neurons in the arcuate hypothalamic nucleus, and to a lesser extent, on neurons in other hypothalamic nuclei and the caudomedial medulla. Recent studies have expanded focus beyond these common targets, focusing attention on additional hindbrain, midbrain, and forebrain regions, including the parabrachial nucleus (5), ventral tegmental area (VTA) (6, 7), medial prefrontal cortex (mPFC) (8, 9), amygdala and extended amygdala (10), and nucleus accumbens (11–13). Neuroanatomically interconnected with several of these regions is the hippocampus; a forebrain structure historically associated with mnemonic control. Emerging evidence supports the view developed further here that hippocampal neurons contribute to the anatomically distributed neural control of feeding behavior. Our model proposes that hippocampal neurons integrate previous learned experience (episodic memories, conditional associative learning, incentive factors) with the external sensory context (visuospatial, olfactory, gustatory cues) and the internal context (interoceptive energy status cues) to influence decisions about when, where, what, and how much to eat. Below we highlight a diverse range of data that support this model.
2. Hippocampal-dependent mnemonic influences on feeding
2.1: Episodic Memory
The hippocampus is required for the formation and consolidation of declarative memory, which is comprised of semantic memory and episodic memory (14, 15). The former represents the conscious recollection of general factual information, whereas the latter represents autobiographical memories of events that can be explicitly recalled. Eating a meal or snack can become an episodic memory that is consolidated to long-term memory and recalled at a later time. The relevance of hippocampal-dependent episodic memory function to feeding behavior is supported by data showing that both meal initiation and meal size are influenced by the degree to which previous meals can be explicitly recalled. For example, amnesic patients with extensive bilateral hippocampal damage who show deficits in establishing new episodic memories will consume a second or even third meal offered only minutes later (16). Higgs and colleagues expanded these findings by showing that while patients with hippocampal damage persistently consume multiple successive meals, they demonstrate reduced liking of foods that were sampled vs. foods presented but not sampled (17), a phenomenon known as sensory-specific satiety. These findings indicate that sensory-specific hedonic modulation of feeding does not require hippocampal-dependent episodic memory of recent feeding occasions. In healthy human subjects Higgs’s group also demonstrated that priming the explicit recall of a recent meal decreases the amount of food that is consumed at the subsequent meal (18, 19).
The relevance of these latter findings to the control of normal feeding behavior is limited by the fact that individuals are rarely explicitly asked to recall a recent meal. An elegant study by Brunstrom and colleagues provided evidence that episodic meal-related memory influences appetite in neurologically intact subjects using procedures that did not involve specific instructions to recall a recent meal (20). The experimenters covertly manipulated the perceived vs. the actual amount of soup consumed during an experimental meal by refilling or drawing soup from a bowl during consumption. When assessed immediately after such a meal, hunger ratings were influenced more by the actual than by the perceived amount consumed. By contrast, the effect was reversed several hours after consumption; the perceived amount and not the actual amount consumed influenced hunger ratings. Collectively these results demonstrate that hippocampal-dependent episodic memory influences feeding via two mechanisms: [1] primed episodic recall of a recent meal reduces the amount subsequently consumed, and [2] the perceived amount and not the actual amount of food consumed during a recent meal influences hunger levels reported hours later.
The impact of episodic meal-related mnemonic information on feeding has been modeled indirectly in rodents. Parent and colleagues recently examined the impact of postprandial inactivation of hippocampal neurons on subsequent feeding behavior (21). After rats were trained to reliably and rapidly consume a 32% sucrose solution at a scheduled time daily, reversible inactivation of dorsal hippocampal neurons (via parenchymal GABA receptor agonist infusion) immediately following sucrose consumption decreased the latency to initiate feeding and increased the size of the subsequent chow meal. One interpretation of these results consistent with the human literature is that neural inactivation of hippocampal neurons disrupted consolidation for the memory of the meal, thereby decreasing the latency to initiate another meal and increasing the amount of food consumed. An alternative (yet not mutually exclusive) interpretation is that hippocampal inactivation disrupted processing of interoceptive satiation and/or satiety signals. This latter interpretation is discussed in more depth below.
2.2: Conditional associative learning
Food-related cues (visual, olfactory, and gustatory) become associated with rewarding or negative postingestive consequences, and these learned associations powerfully influence subsequent feeding behavior. The most classic example of this is conditioned flavor avoidance (or aversion) (CFA) learning, in which animals will avoid (or reject) flavor cues that have been previously associated with visceral malaise (22, 23). Neutral flavor cues can also become appetite-promoting based on their learned associations with nutritive consequences. For example, in flavor preference learning, nonnutritive orosensory flavors paired with gastric nutrient infusions are subsequently preferred compared to flavors associated with control conditions (24). In addition, taste stimuli such as quinine that evoke aversive taste reactivity responses can evoke ingestive oral responses when associated with a nutritive consequence (25).
The hippocampus is not required for CFA learning [except with long-trace delays (26)], nor is it necessary for most types of simple associative appetitive learning generally speaking (27). Rather, hippocampal neural processing is engaged when learned associations between stimuli and outcomes are conditional (28–32). For instance, rodents with complete hippocampal lesions perform as well as controls when learning a Pavlovian discrimination problem in which a discrete auditory cue (tone, or A) signals food reinforcement (A+ trials) and another auditory cue (white noise, or B) does not (B- trials). However, hippocampal lesions severely impair the ability to learn a conditional discrimination problem in which a third cue (light, or X) signals (or “sets the occasion for”) when the tone will not be reinforced (X->A- trials) (33).
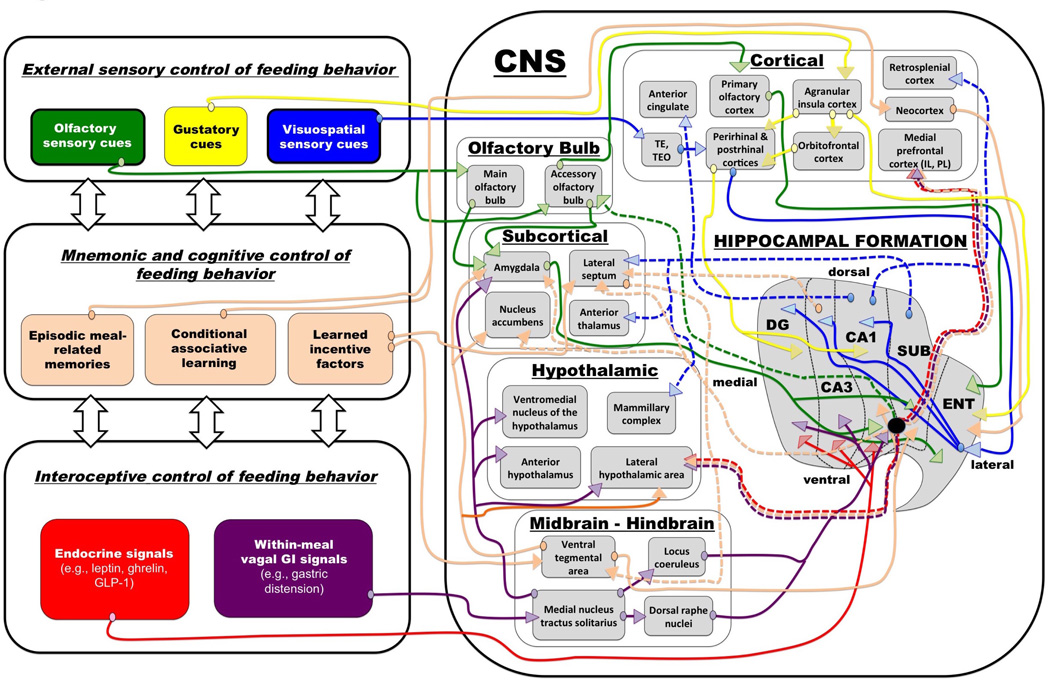
Davidson, Kanoski, and colleagues have previously argued that this type of hippocampal-dependent conditional discrimination learning captures the type of mnemonic process that influences many aspects of normal feeding behavior (34–38). As indicated above, food-related cues become appetite-promoting based on their learned postingestive nutritive outcomes. However, eating in response to these conditioned food cues is not always an appropriate or adaptive behavior, such as in the presence of a predator or during positive energy balance. The decision to eat or not to eat, or how much to eat, at any moment in time is modulated by various external and internal contextual cues, as well as cognitive factors (e.g., incentive motivation) that set the occasion for feeding behavior. Hippocampal-dependent neural integration of contextual and incentive factors and previous food-related experiences (episodic memories) contributes to the timing of eating and the amount consumed. Given its interconnectivity with neurons in various feeding-relevant regions, hippocampal neurons occupy prime neural real estate for the integration of learned incentive factors with the detection and utilization of food-relevant stimuli that inform about both external and internal contextual cues (Figure 1).
Figure 1.
Model for the central integration of information related to energy balance and its convergence within the hippocampal formation. Extrinsic energy-balance related connections of the hippocampal formation are represented by colored lines (solid lines = inputs, dashed lines = outputs, arrows = afferent targets, ovals = connection origin; color coded to represent different categories of information). The “flat-map” representation of the hippocampal formation depicts an unfolded rat hippocampus [adapted from (40)]. The black disk indicates the position of ventral CA1 / subiculum containing glutamatergic output neurons that process and transmit energy balance-relevant signals. Abbreviations: CA1,3 = field 1, 3 of Ammon’s horn, DG = dentate gyrus, SUB = subiculum, ENT = entorhinal areas.
3. Neuroanatomical Connectivity
3.1: External sensory food-relevant information
Visuospatial, olfactory, and gustatory cues arising from the external environment influences feeding behavior by: [1] facilitating spatial navigation to procure food, [2] indicating the presence and location of nonfood factors (e.g., social, life-threatening) that influence the likelihood of engaging in feeding vs. alternative behaviors, and/or [3] determining the safety or nutritive content of orally ingested foods. Within the hippocampus proper (CA fields/Ammon’s horn), external contextual visuospatial information is primarily processed in the “dorsal” (septal and anterior) subregion in rodents (analogous to caudal hippocampus in primates). Visuospatial information is communicated to dorsal hippocampus (dHP) neurons via cortical pathways involving communication from inferior temporal visual areas TEO and TE to the perirhinal and postrhinal corticies (39), and then to the caudolateral entorhinal cotex, which innervates all components of the hippocampal formation (CA fields, dentate gyrus, subiculum) (40–42). Visuospatial information is represented within the hippocampus primarily by the activity of pyramidal “place cell” CA1 and CA3 neurons that possess spatially selective firing fields to reflect the animal’s current spatial location. Place cell neurons are located across the dorsoventral hippocampal axis, however, the breadth of spatial representation increases linearly in rodents from less than 1 meter at the dorsal pole to ~10 meters at the ventral pole (43).
Consistent with dHP specialization for neural processing of fine-tuned spatial representation, dHP damage or inactivation typically produces greater deficits in spatial learning compared to “ventral” regions (temporal and posterior; anterior in primates) (44–46). However, ventral hippocampus (vHP) neurons also contribute to visuospatial navigation and external contextual learning (10, 38, 47–50). The functional distinction between dorsal and ventral hippocampal regions with regards to visuospatial learning is controversial and has been reviewed elsewhere (41, 51, 52).
Food-relevant visuospatial information is likely transmitted from dorsal CA1 (dCA1) neurons directly [and polysynaptically through dorsal subiculum (dSub)] via efferent projections to the anterior cingulate and retrosplenial corticies (53), two cortical regions that integrate visuospatial and mnemonic information to influence navigation and reward-based decision-making. Visuospatial information is also processed downstream of the hippocampus in the lateral septum, mammillary complex, and anterior thalamus, primarily via dSub efferent pathways (54). The importance of these dCA1 and dSub efferent pathways to cognitive processing of external visuospatial contextual features is exemplified by the fact that lesions or temporary inactivation to any of these individual output regions produces deficits in spatial or contextual learning that are comparable to hippocampal lesions (50, 55–60).
Olfactory cues also have a critical role in food procurement by providing information about the location of food and nonfood factors (e.g., predator, potential mate) that affect the likelihood of foraging or feeding. Olfactory information reaches the hippocampal formation through direct projections from the primary olfactory cortex to the medial band of the entorhinal cortex, which then projects to vHP neurons (41). Olfactory input from the main olfactory bulb also reaches the lateral entorhinal cortex and ventral CA1 (vCA1) and ventral subiculum (vSub) via an indirect pathway relayed through the posterior amygdala (40). Efferent pathways arising from the vCA1 and vSub directly innervate the accessory olfactory bulb (61), suggesting a bidirectional modulation of olfactory processing in vHP neurons. Consistent with the preferential olfactory innervation of vHP vs. dHP, vCA1 neurons respond more strongly to aversive/avoided olfactory contextual cues compared to dCA1 neurons (49).
Taste-relevant information is relayed to hippocampal neurons from the agranular insular gustatory cortex through perirhinal and lateral entorhinal cortices (62). Orbitofrontal cortical neurons also transmit taste-relevant information to parahippocampal regions through the rostral perirhinal and postrhinal cortices (63). There is also evidence that CA1 and dentate gyrus neurons are critical for learning the context specificity of taste–postingestive associations (64). However, much remains to be understood about the precise neuroanatomical pathways relaying gustatory information to the hippocampus and their functional significance.
3.2: Interoceptive energy-relevant information
The hippocampus, particularly the vHP, processes visceral energy status-relevant information and utilizes this information to control learned appetitive behavior. Consistent with this notion, human amnesic with bilateral hippocampal damage will consistently rate their hunger levels in the middle of the magnitude scale, regardless of when food was last consumed (16, 17). In rats, hippocampal lesions impair learning (or retaining) a discrimination problem in which different levels of food restriction (24hr vs. 1hr) are discriminative stimuli signaling a forthcoming food reinforcement (65).
Hippocampal neurons are activated by gastrointestinal satiation signals such as gastric distension (66) and vagus nerve electrical stimulation (67), and hippocampal neural processing directly contributes to meal size control (21, 68). Vagally-mediated satiation signals are likely transmitted to hippocampal neurons via yet unidentified polysynaptic pathways given that there are no known monosynaptic pathways between medial nucleus of the solitary tract (mNTS) and hippocampal neurons (68, 69). Two putative hindbrain relays connecting vagal input with vHP neurons are the locus coeruleus (LC) and dorsal raphe nuclei (DRN). Noradrenergic input from LC (70, 71) and serotonergic input from DRN (70) monosynaptically innervates ventral dentate gyrus and vCA1, and these projections have been identified as potentially relaying information from mNTS neurons (72).
The hippocampus also receives neural input communicating food reward-relevant incentive information. Receptors for dopamine (D1Rs and D2Rs), a key neurotransmitter that encodes the positive affective value of food and food-related stimuli, are expressed throughout the hippocampal formation (73, 74). Approximately 15–18% of DA neurons in the VTA innervate the vCA1 and vSub, while only half as many DA neurons innervate dHP neurons (75, 76). Hippocampal neurons also receive emotion- and other visceral-relevant information from the amygdala, with the posterior basal medial, medial, and central regions heavily innervating the ventral and dorsal dentate gyrus (77), and the lateral amygdala innervating vSub and vCA1 (40)
Hippocampal neurons likely transmit gastrointestinal and other interoceptive feeding-related information to other brain regions primarily via glutamatergic projections arising from vHP neurons. One important efferent pathway from vHP projects to the lateral hypothalamic area (LHA). LHA neurons express feeding-relevant neuropeptides [melanin-concentrating hormone (MCH), orexin, neurotensin] (78, 79) and LHA lesions produce aphagia and weight loss (80) whereas electrical stimulation of LHA neurons induces robust feeding (81, 82). The juxtadorsal, juxtadorsomedial, and juxtadorsomedial LHA subregions receive extensive input from vCA1 and vSub pyramidal neurons (83, 84). Neurons in other feeding-relevant hypothalamic nuclei, including the anterior and ventromedial nuclei, also receive direct projections from vCA1 and vSub neurons, albeit to a lesser extent than the LHA (84).
Extensive direct projections are also observed from the vCA1 and vSub neurons to the mPFC (infralimbic and prelimbic subregions) (53, 85), which has recently been linked to reward-driven feeding via D1R (8) and opioid receptor signaling (9). Nucleus accumbens core and shell neurons, other key targets of VTA dopamine signaling, receive substantial innervation from the vSub (86–88). The lateral septum, recently associated with the control of gastric motility (89, 90) and sucrose hyperphagia (91), is another potential feeding-relevant efferent pathway arising from vSub (92, 93) and dorsal CA3 neurons (94). We hypothesize that these monosynaptic outputs from the hippocampus to hypothalamic, striatal, prefrontal cortical, and septal outputs are critical downstream targets for hippocampal neural processing of interoceptive feeding-relevant information.
4. Endocrine communication
Various endocrine signals secreted from the periphery act on neurons throughout the brain to regulate food intake and body weight. The receptors for many of these signals are expressed in hippocampal neurons, including (but not limited to) cholecystokinin (CCK), insulin (95, 96), leptin (97), ghrelin (98), glucagon-like peptide-1 (GLP-1) (99), motilin (100), and amylin (101). Similarly, hippocampal neurons express receptors for several CNS-derived neuropeptides whose release potently modulates feeding, including Y1 receptor (102), melanocortin-4 receptor (103), orexin receptor 1 (104), and MCH1 receptor (105). The role of these hippocampus-expressed endocrine and neuropeptidergic receptors in feeding behavior remains largely unexplored. By contrast, in what follows data are reviewed for three endocrine signals (leptin, ghrelin, and GLP-1) recently shown to regulate food intake through action on hippocampal receptors. Interestingly, in addition to regulating food intake each of these hormonal signals up-regulates dynamic structural changes in hippocampal neurons that are purported to contribute to the formation and maintenance of new memories, including synaptic plasticity and neurogenesis (106–108). The neurotrophic functions of these endocrine signals have been reviewed elsewhere (109). Here we focus on the neural and behavioral mechanisms through which leptin, ghrelin, and GLP-1 communicate interoceptive energy-relevant signals to vHP neurons to influence feeding behavior.
4.1: Leptin
Leptin is a hormone produced principally from white adipocytes (110). Increased CNS leptin receptor (LepRb) signaling potently reduces food intake and body weight, while eliminating leptin signaling through mutation of leptin or LepRb results in hyperphagia and extreme obesity in both humans and rodents [(111) for review]. Leptin reaches the brain through blood-brain barrier transport from the peripheral circulation, with the highest transport observed in the hypothalamus and hippocampus (112). The effects of CNS LepRb signaling on energy balance control were initially thought to be mediated almost exclusively by LepRb signaling in the hypothalamic arcuate nucleus (113); however, subsequent research revealed that mNTS LepRb signaling plays an important endogenous role in food intake regulation (114–116). In addition, LepRb activation in the VTA reduces feeding and mesoaccumbens dopamine signaling (6, 117). Our recent data show that activation of hippocampal LepRbs also reduces food intake. Microinjections of leptin delivered to the dHP or vHP reduced 24-hr food intake in rats, with more potent effects observed following ventral than dorsal delivery (38). Collectively these findings support the view that leptin reduces food intake through a distributed circuitry involving engagement of LepRbs in multiple brain regions (118, 119), including the hippocampus.
In addition to reducing home cage chow intake, vHP LepRb activation also reduced learned appetitive, reward-related feeding behaviors (38). vHP leptin administration reduced food seeking in an environment that had been associated with consuming a palatable meal when tested in the absence of food. Moreover, vHP LepRb activation reduced the latency to obtain palatable food in an operant runway procedure and blocked memory consolidation for the spatial location of food. These data suggest that hippocampal leptin signaling modulates memory formation and retrieval in a manner that actively inhibits food-related features in the environment in favor of nonfood features, resulting in reduced appetitive responding in the presence of environmental food-associated cues.
4.2: GLP-1
GLP-1 is an “incretin” hormone produced in the distal small intestines and in the hindbrain (NTS and reticular formation). Activation of peripheral or central GLP-1 receptors (GLP1-Rs) stimulates glucose-dependent insulin release and reduces food intake and body weight (120). Unlike leptin, which is a signal for longer-term energy status, GLP-1 acts primarily as a shorter-term, prandial satiation signal (121–123). GLP-1R activation in the hypothalamus (paraventricular nucleus or LHA) (124, 125) or in the mNTS (126–128) reduces food intake. Recently the anorectic effects of CNS GLP-1R activation were shown to involve action in the mesolimbic dopaminergic circuitry as well (129–131). GLP-1Rs are also expressed in hippocampal neurons, with the most dense expression patterns observed in the vCA1 and vCA3 pyramidal layers (99). Hsu, Kanoski, and colleagues have recently shown that vHP GLP-1R activation potently reduced food intake in rats, with ~40% reduction in 24hr chow intake and ~50% reduction in 24hr Western diet intake (68). These intake reducing effects are physiologically relevant to normal feeding, as vHP administration of the selective GLP-1R antagonist, exendin-(9–39), increased food intake.
In contrast to leptin, the anorectic effects of vHP GLP-1R signaling do not involve blocking incentive aspects of feeding, but rather, require prandial and/or postprandial mechanisms. For example, vHP GLP-1R activation reduced food intake via a specific effect on meal size without altering meal frequency, and also reduced motivated lever-press responding for palatable food under testing conditions that allowed for periodic food consumption, whereas no effects were observed on the expression of conditioned place preference for palatable food when tested without food access. Thus, these data are consistent with the hypothesis that longer-term energy status cues are communicated to hippocampal neurons, in part, through the adipose-derived hormone leptin, thereby reducing appetitive food-seeking/foraging behavior. On the other hand, shorter-term prandial and/or postprandial satiation cues are communicated to the hippocampus, in part, via GLP-1, thereby reducing meal size and appetitive responding during or immediately following food consumption. Interestingly, despite the fact that GLP-1Rs are robustly expressed on hippocampal neurons, NTS GLP-1 neurons do not innervate the hippocampus (68, 132), suggesting that under physiological conditions GLP-1 (of either peripheral or NTS origin) communicates to the hippocampus via humoral volume transmission through the cerbroventricular fluid (CSF) and/or vasculature. Indeed, active levels of GLP-1 peptides are present in both the CSF and vHP under physiological conditions (68), although the source (peripheral vs. central) is unclear.
4.2: Ghrelin
Ghrelin, a peptide hormone secreted from the stomach (133), communicates to the CNS to increase food intake and food-motivated behavior and is the only known circulating hormone with orexigenic properties. Like leptin and GLP-1, ghrelin receptors [growth hormone secretagogue receptor 1-A (GHSR1A)] are expressed throughout the brain and activation of GHSR1As in multiple different brain nuclei increases feeding, including the hypothalamus (134), VTA (7, 135) and mNTS (136). GHSR1As are expressed in hippocampal neurons with the most dense expression patterns observed in the vHP (137). We recently observed that activation of GHSR1A in vHP neurons approximately doubled food intake, whereas ghrelin delivered to the dHP had no effect on feeding (138).
Several of our findings suggest that vHP ghrelin receptor signaling increases both appetitive and consummatory aspects of feeding. First, vHP ghrelin increased feeding in rats by elevating both meal frequency and size (138). In further support of a role in appetitive control, vHP GHSR1A activation increased the frequency of meal initiation in response to discrete auditory cues that were previously conditioned to signal the availability of palatable food (138). In support of a role in consummatory control, a dose of ghrelin subthreshold for intake effects when administered to vHP alone attenuated the reduction in food intake following peripheral administration of the gut-derived satiation hormone CCK (unpublished data, Kanoski lab).
Collectively these findings indicate that vHP endocrine receptor signaling both stimulates and reduces feeding-relevant behaviors, with the direction of feeding effects being modulating by signals that inform about either positive (leptin, GLP-1) or negative (ghrelin) energy status.
5. Conclusions
Feeding is not always an appropriate or feasible behavior, even when animals are faced with severe nutrient deficiency. On the other hand, feeding can often occur in the absence of metabolic need based on incentive factors and/or exposure to conditioned reward-related cues. In addition to features in the external environment that influence feeding, interoceptive and cognitive factors, such as visceral malaise, satiation, or fear will decrease the likelihood of feeding or foraging, whereas other interoceptive or cognitive factors, such “hunger” arising from food deprivation (negative energy balance) or learned incentive motivation (often associated with positive energy balance) have the opposite effect. Importantly, the relationship between external sensory factors, internal states, and ingestive behavior is dynamic and can be modified with experience. Our model proposes that hippocampal neurons integrate: [1] episodic meal-related memories and food-relevant learned associations and incentives, [2] the external sensory food environment, and [3] interoceptive energy status-relevant cues (i.e., the internal context) to regulate decisions about when, where, what, and how much to eat. Visuospatial sensory information relating to food location is communicated primarily to dHP neurons, whereas olfactory cues are communicated primarily to vHP neurons. External contextual information is then transmitted to cortical regions that integrate visuospatial, olfactory, gustatory, and mnemonic information to influence navigation and reward-based decision-making. We speculate that the entorhinal cortex is a key site of neural integration of external contextual and episodic mnemonic information, as this region relays visuospatial, olfactory, and gustatory information to hippocampal neurons (40–42, 62), and is considered to be an important interface linking the neocortex and hippocampus to control for episodic memory formation and retrieval (139, 140).
GI and other visceral information is polysynaptically transmitted to vHP neurons (and dHP to a lesser extent) from hindbrain regions. Hippocampal neurons also receive energy balance-relevant information from circulating endocrine signals such as leptin, GLP-1, and ghrelin, and activation of their receptors in vHP neurons potently decreases (leptin, GLP-1) or increases (ghrelin) food intake and food-reward driven appetitive behaviors. We posit that the LHA, mPFC, nucleus accumbens, and the lateral septum are key efferent targets of vHP neurons that process interoceptive energy balance relevant information downstream of the hippocampus, although these hypotheses remain to be tested.
In conclusion, data reviewed here support the perspective that hippocampal neurons (particularly vHP) are an important neural substrate linking the external context, the internal context, and mnemonic and cognitive information to control both appetitive and ingestive behavior. Hippocampal endocrine and neuropeptidergic signaling is likely to attract considerably more attention as the field expands the scope of the neuroanatomical bases of energy balance beyond hypothalamic substrates.
Acknowledgements
The authors thank Dr. Joel Hahn and Ted Hsu for insightful input. We acknowledge our research support from the NIH; DK097147, DK102478, DK104897 (S.E.K.), and DK21397 (H.J.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Guyenet SJ, Schwartz MW. Clinical review: Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab. 2012;97:745–755. doi: 10.1210/jc.2011-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffey KJ, Popkin BM. Energy density, portion size, and eating occasions: contributions to increased energy intake in the United States, 1977–2006. PLoS Med. 2011;8:e1001050. doi: 10.1371/journal.pmed.1001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly MT, Wallace JM, Robson PJ, Rennie KL, Welch RW, Hannon-Fletcher MP, et al. Increased portion size leads to a sustained increase in energy intake over 4 d in normal-weight and overweight men and women. Br J Nutr. 2009;102:470–477. doi: 10.1017/S0007114508201960. [DOI] [PubMed] [Google Scholar]
- 4.Inabnet WB, 3rd, Winegar DA, Sherif B, Sarr MG. Early Outcomes of Bariatric Surgery in Patients with Metabolic Syndrome: An Analysis of the Bariatric Outcomes Longitudinal Database. J Am Coll Surg. 2012 doi: 10.1016/j.jamcollsurg.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD. NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci U S A. 2013;110:14765–14770. doi: 10.1073/pnas.1314137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, et al. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17:248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, et al. Endogenous Opioid Signaling in the Medial Prefrontal Cortex is Required for the Expression of Hunger-Induced Impulsive Action. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 12.Cone JJ, Roitman JD, Roitman MF. Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015;133:844–856. doi: 10.1111/jnc.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, et al. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J Neurosci. 2014;34:6985–6992. doi: 10.1523/JNEUROSCI.0115-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- 15.Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozin P, Dow S, Moscovitch M, Rajaram S. What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychol Sci. 1998;9:392–396. [Google Scholar]
- 17.Higgs S, Williamson AC, Rotshtein P, Humphreys GW. Sensory-specific satiety is intact in amnesics who eat multiple meals. Psychol Sci. 2008;19:623–628. doi: 10.1111/j.1467-9280.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 18.Higgs S. Memory for recent eating and its influence on subsequent food intake. Appetite. 2002;39:159–166. doi: 10.1006/appe.2002.0500. [DOI] [PubMed] [Google Scholar]
- 19.Higgs S. Cognitive influences on food intake: the effects of manipulating memory for recent eating. Physiol Behav. 2008;94:734–739. doi: 10.1016/j.physbeh.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Brunstrom JM, Burn JF, Sell NR, Collingwood JM, Rogers PJ, Wilkinson LL, et al. Episodic memory and appetite regulation in humans. PLoS One. 2012;7:e50707. doi: 10.1371/journal.pone.0050707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23:100–107. doi: 10.1002/hipo.22062. [DOI] [PubMed] [Google Scholar]
- 22.Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- 23.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 24.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 25.Breslin PA, Davidson TL, Grill HJ. Conditioned reversal of reactions to normally avoided tastes. Physiol Behav. 1990;47:535–538. doi: 10.1016/0031-9384(90)90122-k. [DOI] [PubMed] [Google Scholar]
- 26.Koh MT, Wheeler DS, Gallagher M. Hippocampal lesions interfere with long-trace taste aversion conditioning. Physiol Behav. 2009;98:103–107. doi: 10.1016/j.physbeh.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson TL, Jarrard LE. The hippocampus and inhibitory learning: a 'Gray' area? Neurosci Biobehav Rev. 2004;28:261–271. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Jarrard LE, Davidson TL. On the hippocampus and learned conditional responding: effects of aspiration versus ibotenate lesions. Hippocampus. 1991;1:107–117. doi: 10.1002/hipo.450010110. [DOI] [PubMed] [Google Scholar]
- 29.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- 31.Morris R. Theories of Hippocampal Function. In: Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. New York, NY: Oxford University Press; 2007. p. 872. [Google Scholar]
- 32.Gray JA, McNaughton N. The neuropsychology of anxiety. 2nd edition ed. Oxford: Oxford University Press; 2000. [Google Scholar]
- 33.Holland PC, Lamoureux JA, Han J-S, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 34.Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7:613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Kanoski SE. Cognitive and neuronal systems underlying obesity. Physiol Behav. 2012;106:337–344. doi: 10.1016/j.physbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benoit SC, Davis JF, Davidson TL. Learned and cognitive controls of food intake. Brain Res. 2010;1350:71–76. doi: 10.1016/j.brainres.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36:1859–1870. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webster MJ, Ungerleider LG, Bachevalier J. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. J Neurosci. 1991;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain research Brain research reviews. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 41.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol. 1998;398:49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, et al. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- 44.Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. Journal of Neuroscience. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- 47.de Hoz L, Knox J, Morris RG. Longitudinal axis of the hippocampus: both septal and temporal poles of the hippocampus support water maze spatial learning depending on the training protocol. Hippocampus. 2003;13:587–603. doi: 10.1002/hipo.10079. [DOI] [PubMed] [Google Scholar]
- 48.Wilkerson A, Levin ED. Ventral hippocampal dopamine D1 and D2 systems and spatial working memory in rats. Neuroscience. 1999;89:743–749. doi: 10.1016/s0306-4522(98)00346-7. [DOI] [PubMed] [Google Scholar]
- 49.Keinath AT, Wang ME, Wann EG, Yuan RK, Dudman JT, Muzzio IA. Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus. 2014;24:1533–1548. doi: 10.1002/hipo.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beer Z, Chwiesko C, Sauvage MM. Processing of spatial and non-spatial information reveals functional homogeneity along the dorso-ventral axis of CA3, but not CA1. Neurobiol Learn Mem. 2014;111:56–64. doi: 10.1016/j.nlm.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience & Biobehavioral Reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 53.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain research reviews. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex, not ammon's horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- 55.Czajkowski R, Jayaprakash B, Wiltgen B, Rogerson T, Guzman-Karlsson MC, Barth AL, et al. Encoding and storage of spatial information in the retrosplenial cortex. Proc Natl Acad Sci U S A. 2014;111:8661–8666. doi: 10.1073/pnas.1313222111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YS, Danandeh A, Baratta J, Lin CY, Yu J, Robertson RT. Neurotrophic factors rescue basal forebrain cholinergic neurons and improve performance on a spatial learning test. Experimental neurology. 2013;249:178–186. doi: 10.1016/j.expneurol.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson AJ, Hindley EL, Pearce JM, Vann SD, Aggleton JP. The effect of retrosplenial cortex lesions in rats on incidental and active spatial learning. Frontiers in behavioral neuroscience. 2015;9:11. doi: 10.3389/fnbeh.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelson AJ, Vann SD. Mammilliothalamic tract lesions disrupt tests of visuospatial memory. Behav Neurosci. 2014;128:494–503. doi: 10.1037/bne0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warburton EC, Aggleton JP. Differential deficits in the Morris water maze following cytotoxic lesions of the anterior thalamus and fornix transection. Behav Brain Res. 1999;98:27–38. doi: 10.1016/s0166-4328(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 61.de la Rosa-Prieto C, Ubeda-Banon I, Mohedano-Moriano A, Pro-Sistiaga P, Saiz-Sanchez D, Insausti R, et al. Subicular and CA1 hippocampal projections to the accessory olfactory bulb. Hippocampus. 2009;19:124–129. doi: 10.1002/hipo.20495. [DOI] [PubMed] [Google Scholar]
- 62.Mathiasen ML, Hansen L, Witter MP. Insular projections to the parahippocampal region in the rat. J Comp Neurol. 2015;523:1379–1398. doi: 10.1002/cne.23742. [DOI] [PubMed] [Google Scholar]
- 63.Kondo H, Witter MP. Topographic organization of orbitofrontal projections to the parahippocampal region in rats. J Comp Neurol. 2014;522:772–793. doi: 10.1002/cne.23442. [DOI] [PubMed] [Google Scholar]
- 64.Chinnakkaruppan A, Wintzer ME, McHugh TJ, Rosenblum K. Differential contribution of hippocampal subfields to components of associative taste learning. J Neurosci. 2014;34:11007–11015. doi: 10.1523/JNEUROSCI.0956-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124:97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Min DK, Tuor UI, Chelikani PK. Gastric distention induced functional magnetic resonance signal changes in the rodent brain. Neuroscience. 2011;179:151–158. doi: 10.1016/j.neuroscience.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 67.Wang G-J, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40:327–337. doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyss JM, Swanson LW, Cowan WM. A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience. 1979;4:463–476. doi: 10.1016/0306-4522(79)90124-6. [DOI] [PubMed] [Google Scholar]
- 71.Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci. 1989;9:3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castle M, Comoli E, Loewy AD. Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience. 2005;134:657–669. doi: 10.1016/j.neuroscience.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 73.Martres MP, Bouthenet ML, Sales N, Sokoloff P, Schwartz JC. Widespread distribution of brain dopamine receptors evidenced with [125I]iodosulpride, a highly selective ligand. Science. 1985;228:752–755. doi: 10.1126/science.3838821. [DOI] [PubMed] [Google Scholar]
- 74.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 75.Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain research bulletin. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 76.Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668:71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- 77.Ohara S, Sato S, Tsutsui K, Witter MP, Iijima T. Organization of multisynaptic inputs to the dorsal and ventral dentate gyrus: retrograde trans-synaptic tracing with rabies virus vector in the rat. PLoS One. 2013;8:e78928. doi: 10.1371/journal.pone.0078928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown JA, Woodworth HL, Leinninger GM. To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Frontiers in systems neuroscience. 2015;9:9. doi: 10.3389/fnsys.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- 81.Coons EE, Levak M, Miller NE. Lateral hypothalamus: learning of food-seeking response motivated by electrical stimulation. Science. 1965;150:1320–1321. doi: 10.1126/science.150.3701.1320. [DOI] [PubMed] [Google Scholar]
- 82.Stanley BG, Willett VL, 3rd, Donias HW, Dee MG, 2nd, Duva MA. Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol. 1996;270:R443–R449. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- 83.Hahn JD, Swanson LW. Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. J Comp Neurol. 2012;520:1831–1890. doi: 10.1002/cne.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. Journal of Comparative Neurology. 2006;497:101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- 86.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the "accumbens" part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 87.Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 88.Grace AA. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62:1342–1348. doi: 10.1016/j.neuropharm.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gong Y, Xu L, Wang H, Guo F, Sun X, Gao S. Involvements of the lateral hypothalamic area in gastric motility and its regulation by the lateral septum. General and comparative endocrinology. 2013;194:275–285. doi: 10.1016/j.ygcen.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 90.Gong Y, Xu L, Guo F, Pang M, Shi Z, Gao S, et al. Effects of ghrelin on gastric distension sensitive neurons and gastric motility in the lateral septum and arcuate nucleus regulation. Journal of gastroenterology. 2014;49:219–230. doi: 10.1007/s00535-013-0789-y. [DOI] [PubMed] [Google Scholar]
- 91.Mitra A, Lenglos C, Timofeeva E. Inhibition in the lateral septum increases sucrose intake and decreases anorectic effects of stress. Eur J Neurosci. 2015;41:420–433. doi: 10.1111/ejn.12798. [DOI] [PubMed] [Google Scholar]
- 92.Meibach RC, Siegel A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977;124:197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- 93.Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- 94.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Unger J, Livingston J, Moss A. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol. 1991;36:343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- 96.Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, et al. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. Journal of Biological Chemistry. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- 97.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 100.Depoortere I, De Clercq P, Svoboda M, Bare L, Peeters TL. Identification of motilin mRNA in the brain of man and rabbit. Conservation of polymorphism of the motilin gene across species. Peptides. 1997;18:1497–1503. doi: 10.1016/s0196-9781(97)00227-1. [DOI] [PubMed] [Google Scholar]
- 101.Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, et al. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiol Aging. 2014;35:793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 102.Caberlotto L, Fuxe K, Overstreet DH, Gerrard P, Hurd YL. Alterations in neuropeptide Y and Y1 receptor mRNA expression in brains from an animal model of depression: region specific adaptation after fluoxetine treatment. Brain research Molecular brain research. 1998;59:58–65. doi: 10.1016/s0169-328x(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 103.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 104.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 105.Lembo PM, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C, et al. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nature cell biology. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- 106.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. Journal of Neuroscience. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [see comment]. [DOI] [PubMed] [Google Scholar]
- 108.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 109.Moult PR, Harvey J. Hormonal regulation of hippocampal dendritic morphology and synaptic plasticity. Cell adhesion & migration. 2008;2:269–275. doi: 10.4161/cam.2.4.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [see comment][erratum appears in Nature 1995 Mar 30;374(6521):479]. [DOI] [PubMed] [Google Scholar]
- 111.Leshan RL, Bjornholm M, Munzberg H, Myers MG., Jr Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(Suppl 5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- 112.Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res. 2002;950:130–136. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- 113.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanoski SE, Zhao S, Guarnieri DJ, Dileone RJ, Yan J, De Jonghe BC, et al. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab. 2012;303:E496–E503. doi: 10.1152/ajpendo.00205.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 118.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 119.Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol. 2010;31:61–78. doi: 10.1016/j.yfrne.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 121.Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC. Incretins and Amylin: Neuroendocrine Communication Between the Gut, Pancreas, and Brain in Control of Food Intake and Blood Glucose. Annual review of nutrition. 2014 doi: 10.1146/annurev-nutr-071812-161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Williams DL. Minireview: finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology. 2009;150:2997–3001. doi: 10.1210/en.2009-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010;100:503–510. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1427–R1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 125.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol. 1998;274:R23–R29. doi: 10.1152/ajpregu.1998.274.1.R23. [DOI] [PubMed] [Google Scholar]
- 126.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS. Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. J Comp Neurol. 2013;521:2235–2261. doi: 10.1002/cne.23282. [DOI] [PubMed] [Google Scholar]
- 133.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 134.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 135.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 136.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 137.Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perello M, et al. Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J Comp Neurol. 2014;522:3644–3666. doi: 10.1002/cne.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin Signaling in the Ventral Hippocampus Stimulates Learned and Motivational Aspects of Feeding via PI3K-Akt Signaling. Biol Psychiatry. 2013;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leube DT, Weis S, Freymann K, Erb M, Jessen F, Heun R, et al. Neural correlates of verbal episodic memory in patients with MCI and Alzheimer's disease--a VBM study. International journal of geriatric psychiatry. 2008;23:1114–1118. doi: 10.1002/gps.2036. [DOI] [PubMed] [Google Scholar]
- 140.Insel N, Takehara-Nishiuchi K. The cortical structure of consolidated memory: a hypothesis on the role of the cingulate-entorhinal cortical connection. Neurobiol Learn Mem. 2013;106:343–350. doi: 10.1016/j.nlm.2013.07.019. [DOI] [PubMed] [Google Scholar]