Abstract
FLT3 internal tandem duplication (FLT3-ITD) is an activating mutation found in 20%-30% of patients with acute myeloid leukemia (AML), which makes FLT3 an attractive target for the treatment of AML. Although FLT3-mutant patients respond to current FLT3 inhibitors, relapse usually happens due to the acquisition of resistant secondary mutations at the FLT3 catalytic domain, which is mainly on D835. In the search for compounds with broad FLT3 inhibition activities, we screened a kinase inhibitor library by using our unique FLT3 substrate and identified JAK3 inhibitor VI (designated JI6 hereafter) as a novel FLT3 inhibitor, which selectively targets FLT3 D835 mutants as well as FLT3-ITD. JI6 effectively inhibited FLT3-ITD-containing MV4-11 cells and HCD-57 cells transformed with FLT3-ITD and D835 mutants. Furthermore, administration of JI6 effectively targeted FLT3 signaling in vivo and suppressed the myeloproliferative phenotypes in FLT3-ITD knock-in mice and significantly prolonged the survival of immunodeficient mice implanted with the transformed HCD-57 cells. Therefore, JI6 is a promising candidate for development of next generation anti-AML drugs.
Keywords: AML, FLT3, kinase inhibitor, mouse model
Introduction
Acute myeloid leukemia (AML) is a malignant hematological disorder featured by impaired differentiation and uncontrolled proliferation of the hematopoietic progenitor cells (1). Although significant progress has been made in treating some young patients with cytotoxic chemotherapies, there is no effective treatment for old patients with adverse prognosis (2). Novel therapeutic methods are needed for the treatment of AML. With the intensive effort to define the pathogenesis of AML, several molecular lesions have been identified in the past decade (3). Among them, FMS-like tyrosine kinase 3 (FLT3) is one of the most attractive therapeutic targets identified in AML (4-6).
FLT3 is a receptor tyrosine kinase that plays a crucial role in the development of hematopoietic progenitor cells (7). The activating FLT3 internal tandem duplication (ITD) mutation occurs in 20%-30% of AML patients and represents a high risk factor for disease relapse (2, 8, 9). To date, several small molecule inhibitors and monoclonal antibodies against FLT3 have been developed (10-14). Although some clinical trials showed positive outcomes for some FLT3 inhibitors (15), most patients experienced drug-resistant relapse, which has become a major challenge in the field (15, 16). As a major mechanism underlying the FLT3 inhibitor resistance, acquired secondary point mutations in the FLT3 kinase domain have been identified in both preclinical and clinical studies (17, 18). These point mutations mainly affect the D835 amino acid residue at the catalytic domain of FLT3 and cause conformational changes that activate the enzyme and render it less accessible to inhibitors such as sorafenib and quizartinib (AC220). Therefore, novel FLT3 inhibitors that can overcome this acquired drug resistance need to be identified for the development of anti-AML therapies.
In this study, by screening a protein kinase inhibitor library using a protein substrate we recently developed, we identified JI6 as a potent FLT3 inhibitor. The potency and selectivity of this inhibitor was verified by using cells carrying FLT3-ITD and FLT3-D835 mutants. Furthermore, administration of JI6 inhibited FLT3-ITD-induced myeloproliferative diseases in knock-in mice and prolonged survival with a xenograft mouse model. We believe that JI6 may become a promising anti-AML drug.
Results
JI6 is a novel and selective FLT3 inhibitor
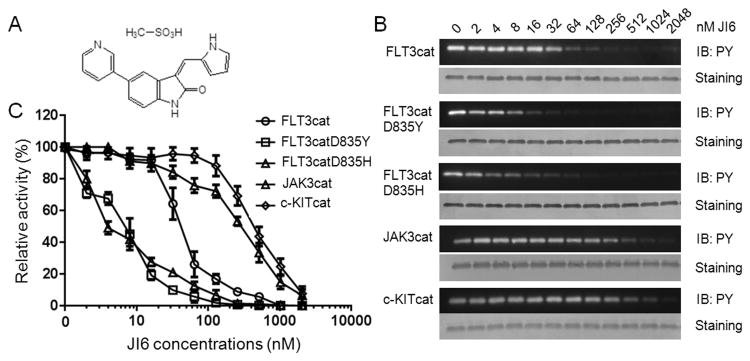
To screen novel FLT3 inhibitors, we developed a system containing a unique protein substrate to measure kinase activity of FLT3 (19). In this study, by screening a chemical library, we identified an oxindole-derived compound originally named JAK3 Inhibitor VI (CAS No. 856436-16-3) as a novel and selective FLT3 inhibitor (Figure 1A). For simplicity, we designated this compound JI6. As shown in Figure 1B and 1C, JI6 displayed potent inhibitory activities toward FLT3-WT, FLT3-D835Y, and FLT3-D835H with IC50 values of ∼40 nM, 8 nM, and 4 nM, respectively. In contrast, it was much less effective toward JAK3 and c-Kit with IC50 values ∼250 nM and ∼500 nM, respectively (Figure 1B and 1D). It should be pointed out that JI6 was initially defined as a JAK3 inhibitor and that c-Kit and FLT3 both belong to the class III receptor tyrosine kinase subfamily. Collectively, our data suggest that JI6 is a potent and selective FLT3 inhibitor. Importantly, it inhibits the D835 mutants more effectively than FLT3.
Figure 1. JI6 selectively inhibits FLT3 in in vitro kinase assays.
(A) Chemical structure of JI6. (B) Tyrosine kinase activities of recombinant proteins containing catalytic domains of FLT3, FLT3-D835Y, FLT3-D835H, JAK3, and c-KIT were analyzed with GST-FLT3S as a substrate in the presence of various concentrations of JI6. Tyrosine phosphorylation of GST-FLT3S was detected by using anti-phosphotyrosine antibody PY20, and its protein level, by Coomassie blue staining. (C) The relative kinase activity was calculated based on the density of the western blot bands normalized to the control group. Error bars denote standard deviation (n = 3).
JI6 selectively inhibits FLT3-ITD-positive leukemia cells
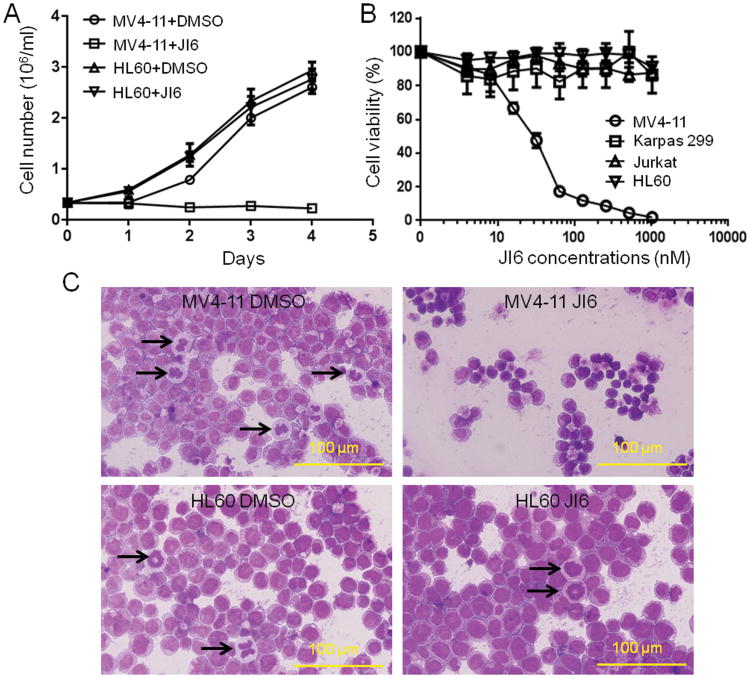
We then employed several existing cell lines to verify the inhibitory effects of JI6 on FLT3. These included FLT3-ITD-containing leukemia MV4-11 cells (20); naplastic large cell lymphoma Karpas 299 cells, which bear a mutation of tyrosine kinase Alk (21, 22); and two cell lines, HL-60 and Jurkat, which contain no known tyrosine kinase mutations. Upon treatment with 50 nM JI6, cell counting with trypan blue revealed that the growth of MV4-11 cells was totally halted while other cells were essentially unaffected (Fig. 2A). XTT-based cell viability assays demonstrated a dose-dependent inhibition of MV4-11 cells by JI6 with an IC50 value of ∼25 nM and no effects of JI6 on the three remaining cells at a concentration as high as 1 μM (Figure 2B). JI6-induced inhibition of MV4-11 cells is also manifested in morphology as revealed by Wright-Giemsa staining (Figure 2C). In comparison with the non-treated MV4-11 cells, JI6-treated cells were smaller with condensed nuclei that showed no mitotic activity. In contrast, HL-60 cells displayed normal morphology with many mitotic cells in the presence of JI6. The data demonstrate that JI6 specifically targets cells containing FLT3-ITD.
Figure 2. JI6 selectively inhibits FLT3-ITD-containing MV4-11 cell.
(A) MV4-11 and HL60 were cultured in the presence of 50 nM JI6. Viable cells were counted by using the trypan blue exclusion method. (B) MV4-11, HL60, Karpas 299, and Jurkat cells were cultured in the presence of various concentrations of JI6 for 48 hours. Cell viability was assessed by XTT assays. (C) Wright-Giemsa staining of MV4-11 and HL60 cells treated with 0 or 50 nM JI6 for 24 h. Black arrows point to mitotic cells. Error bars denote standard deviation (n = 3).
JI6 is potent against cells transformed with FLT3-ITD and D835 mutants
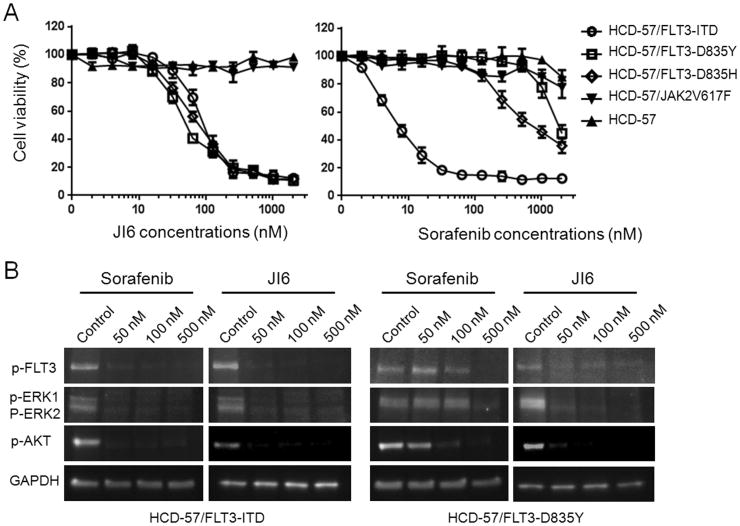
To evaluate if JI6 can effectively target drug resistant FLT3 D835 mutants in intact cells, we generated transformed HCD-57. HCD-57 cells are murine erythroleukemia cells that depend on erythropoietin (EPO) for survival. When infected with recombinant retroviruses carrying FLT3-ITD, FLT3-D835Y, FLT3-D835H, and JAK2V617F, they acquired ability to proliferate in the absence of EPO. In contrast, wild type FLT3 and JAK2 were not able to install EPO independency in these cells. We then performed cell viability assays to determine the inhibitory potency of JI6 together with sorafenib for comparison. As shown in Figure 3A, JI6 potently inhibited the viability of HCD-57 cells expressing FLT3-ITD, FLT3-D835Y, and FLT3-D835H with IC50 values of ∼40 nM, but it displayed essentially no effects on the parent HCD-57 or the cells transformed with JAK2V617F. As expected, sorafenib strongly inhibited the growth of HCD-57 cells transformed with FLT3-ITD and was far less active toward other cells. The data indicate that JI6 can effectively target FLT-3-ITD and D835 mutants in intact cells. We further investigated the effects of JI6 on cell signaling by performing western blot analyses with phospho-specific antibodies. As shown in Figure 3B, phosphorylation of FLT3 and its downstream signaling transducers including ERK and Akt were effectively inhibited by JI6 in both FLT3-ITD- and FLT3-D835Y-transfromed HCD-57 cells, whereas sorafenib showed a strong inhibitory effect on the FLT3-ITD cells and was much less effective toward the FLT3-D835Y cells.
Figure 3. JI6 selectively inhibits cell viability and FLT3 signaling of HCD-57 cells transformed by FLT3-ITDand FLT3-D835 mutants.
(A) Parental and oncogenic tyrosine kinase-transformed HCD-57 cells were cultured in the presence of various concentrations of JI6 or sorafenib for 48 hours. Cell viability was assessed by XTT assays. Error bars denote standard deviation (n = 3). (B) FLT3-ITD- and FLT3-D835Y-transformed HCD-57 cells were treated with the indicated concentrations of JI6 for 3 hours. Cell extracts were subjected to western blot analyses with antibodies against phosphorylated forms of FLT3 (pY591), ERK1/2 (pT202/pY204), and AKT (pS473). Equal protein loading was demonstrated by blotting with house-keeping gene product GAPDH.
JI6 induces apoptosis and cell cycle arrest in both FLT3-ITD- and FLT3-D835Y-transformed cells
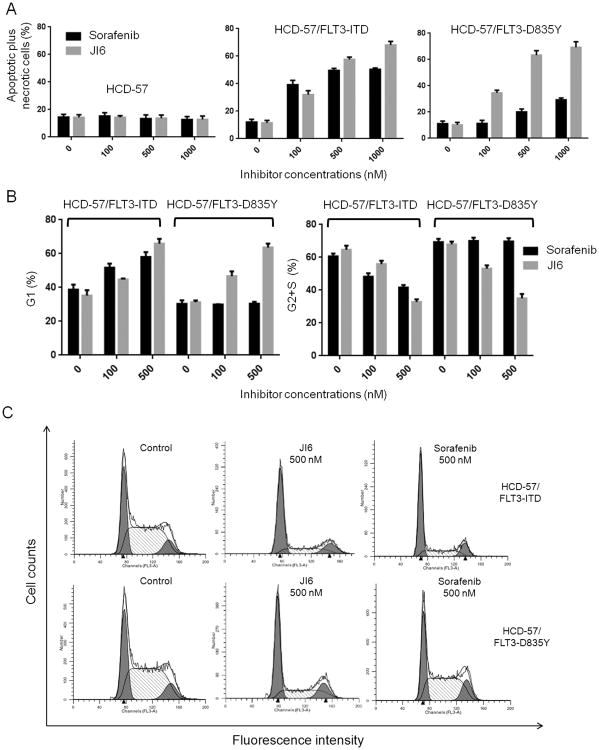
To further reveal how JI6 inhibits the growth of FLT3-mutant cells, we conducted apoptosis assays and cell cycle analyses. Apoptosis was demonstrated by staining cells with Annexin V and propidium iodide. As expected, in both FLT3-ITD- and FLT3-D835Y-transformed HCD-57 cells, the percentage of apoptotic and necrotic cells was increased following JI6 treatment. In contrast, sorafenib was potent to induce apoptosis and necrosis in the FLT3-ITD cells, but it had no effect on the FLT3-D835Y cells (Figure 4A). As expected, neither JI6 nor sorafenib displayed significant effects on the apoptosis of the parent HCD-57 cells that were cultured in the presence of EPO. The effects of JI6 and sorafenib on cell cycle followed a similar pattern. JI6 significantly reduced G2 and S phase cells and increased G1 phase cells in both FLT3-ITD and D835Y cells, while sorafenib exhibited such an effect only on the FLT3-ITD cells (Figure 4B and C). Together, the data indicate that JI6 effectively induces apoptosis and cell cycle arrest in both FLT3-ITD- and FLT3-D835Y-expressing HCD-57 cells with broader activity than sorafenib.
Figure 4. JI6 induces apoptosis and cell cycle arrest in both FLT3-ITD- and FLT3-D835Y transfromed HCD-57 cells.
(A) Apoptosis of parental and FLT3-ITD- or FLT3-D835Y-transformed HCD-57 cells induced by treatment with 0, 100, and 500 nM JI6 or sorafenib for 24 hours. Cells were stained with Cy5-labeled annexin V and propidium iodide, and data represent the percentages of apoptotic (annexin V-positive and propidium iodide-negative) plus necrotic (annexin V-positive and propidium iodide-positive) cells. (B) and (C) Cell cycle analyses of FLT3-ITD- and FLT3-D835Y-transformed HCD-57 treated with 0, 100, and 500 nM of JI6 or sorafenib for 24 hours. Percentages of cells in G1, S+G2 phases were calculated by using the ModFit software. Error bar denotes standard deviation (n=3).
JI6 effectively inhibits FLT3 signaling in vivo
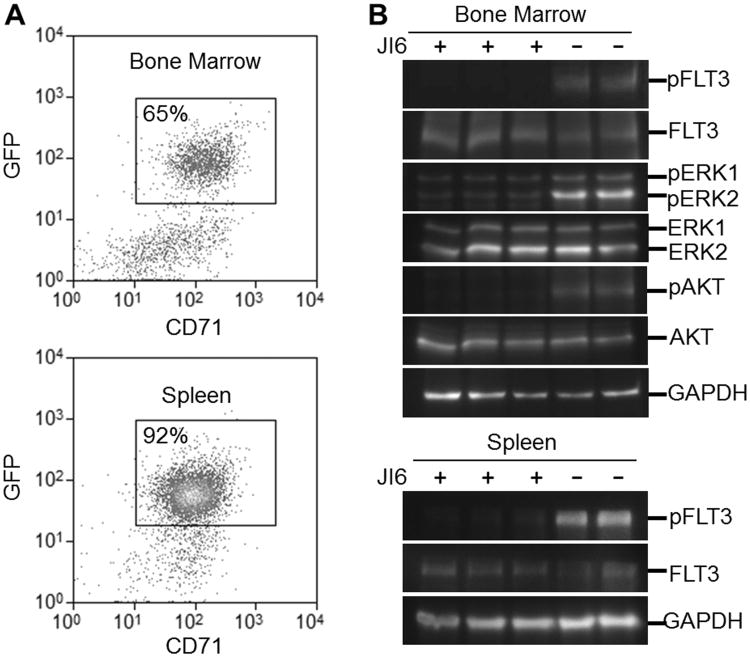
To investigate if JI6 can specifically target FLT3 mutants in vivo, we employed immunodeficient NSG mice engrafted with HCD-57 expressing FLT3-D835Y. The transformed HCD-57 cells were introduced to NSG mice intravenously. Three weeks after the implantation, the implanted cells occupied 65% and 92% of total nucleated cells in the bone marrow and spleen, respectively, as revealed by flow cytometeric analyses (Figure 5A). We then treated these mice intraperitoneally with a single dose of JI6 at 100 mg/kg and sacrificed the mice 4 hours later. Western blot analyses of bone marrow and spleen cell extracts with antibodies against phospho-forms of FLT3, ERK1/2, and Akt revealed that JI6 administration significantly inhibited phosphorylation of FLT3 and downstream signal transduction (Figure 5B). These data indicates the on-target effects of JI6 in vivo.
Figure 5. JI6 effectively inhibits FLT3 signaling in vivo.
NSG mice were engrafted with FLT3-D835Y-transformed HCD-57 cells for three weeks. (A) Flow cytometric analyses of bone marrow and spleen cells. Note that both tissues are infiltrated with FLT3-D835Y-transformed HCD-57 cells which are positive for CD71 and GFP. (B) Western blot analyses of bone marrow and spleen cell extracts from mice that were treated with vehicle control or JI6 through intraperitoneal injection at 30 mg/kg for 4 hours. Red blood cells were lyzed prior to analyses of bone marrow and spleen cells. Antibodies used were pFLT3 (pY591), FLT3, pERK1/2 (pT202/pY204), ERK1/2, pAKT (pS473) and AKT. Equal protein loading was demonstrated by blotting with house-keeping gene product GAPDH.
JI6 has anti-leukemic activity in mouse models
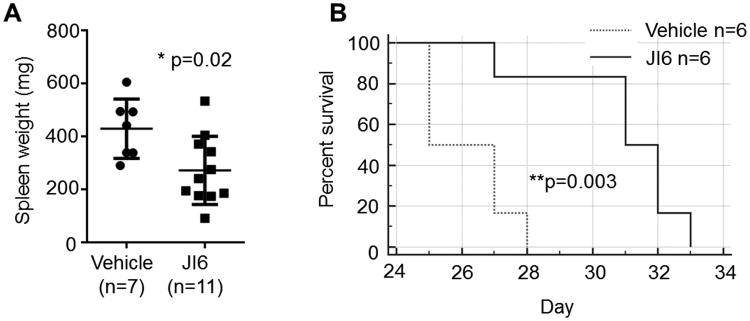
To evaluate the in vivo inhibitory effects of JI6 on drug-resistant FLT3 D835 mutants, NSG mice engrafted with HCD-57 expressing FLT3-D835Y were intraperitoneal injected with JI6 at a single daily dose of 15 mg/kg for 3 weeks. Upon transplantation of FLT3-D835Y-transformed HCD-57 cells, the spleen of NSG mice increased from 30 mg on average to over 300 mg due to infiltration of transplanted cells (see Figure 5A). Therefore, the spleen size can serve as a parameter to measure the effectiveness of drug treatment. Indeed, treatment of the mice with JI6 significantly reduced the spleen size in these mice, which indicate a strong anti-leukemia activity of JI6 in vivo. In addition, treatment with JI6 significantly prolonged the survival of these mice (Figure 6B).
Figure 6. JI6 effectively inhibit the proliferation of FLT3-D835Y-transformed HCD-57 in immunodeficient mice and prolongs the animal survival.
NSG mice were engrafted with FLT3-D835Y-transformed HCD-57 cells through intravenous injection. JI6 treatment was started 24 hours later by intraperitoneal injection of JI6 at a single daily dose of 15 mg/kg for 3 weeks. (A) Spleen weights of engrafted mice after 3 weeks of treatment with JI6 or vehicle. Error bars denote standard deviation (n≥7 as indicated). (B) Kaplan-Meier analysis of animal survival. Mice were treated with JI6 or vehicle from day 1 to day 21.
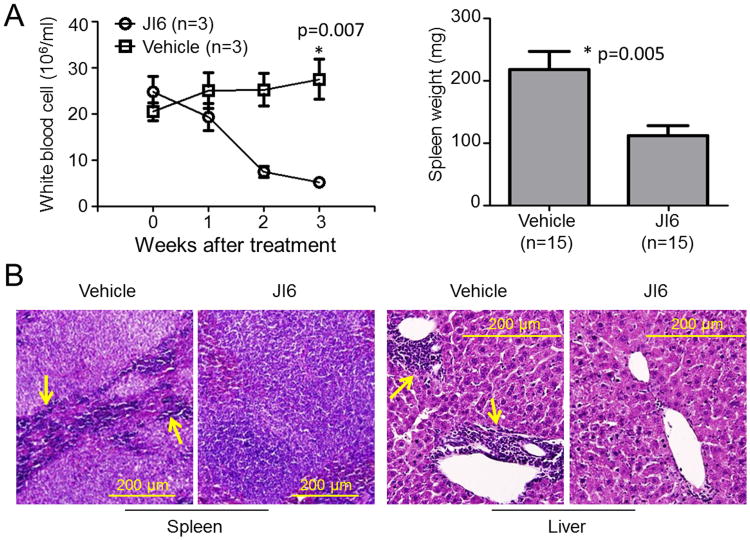
We further employed FLT3-ITD knock-in mice to assess anti-leukemic activities of JI6 in vivo. For this purpose, JI6 was administrated orally for 3 weeks at a single daily dose of 25 mg/kg. FLT3-ITD knock-in mice develop chronic myelomonocytic leukemia (CMML) phenotypes characterized by increased white blood cells (WBC), splenomegaly, and myeloid infiltration in spleen and liver (23). Comparing with the vehicle control, treatment with JI6 reduced white blood cells to the normal range and significantly shrunk the size of spleens (Figure 7A). Histochemical staining revealed disappearance of the myeloid infiltration in the spleen and liver upon JI6 treatment (Figure 7B). These data demonstrated the in vivo anti-leukemic activity of JI6. It should be noted that JI6 did not exhibit obvious toxicity at a daily dose of 25 mg/kg. However, at a daily dose of 50 and 100 mg/kg, death of treated mice occurred within two weeks with sharp declines in blood cell counts and clear liver damage. At low micromolar concentrations, JI6 did not exhibit general cell toxicity as determined in vitro by using mouse bone marrow stromal cells and human HT-1080 fibrosarcoma cells, but clear cell death occurred at concentrations over 10 μM (data not shown).
Figure 7. JI6 suppresses myeloproliferative phenotypes in FLT3-ITD knock-in mice.
(A) FLT3-ITD knock-in mice were treated with JI6 through oral gavage at a daily dose of 25 mg/kg for 3 weeks. White blood cells were analyzed weekly for selected mice, and spleen was weighted after all the mice were euthanized at the end of the treatment. Error bars denote standard deviation (n≥3 as indicated). (B) Hematoxylin and eosin staining of paraffin-embedded sections of spleen and liver from drug- or vehicle-treated mice. Arrows point to typical myeloid cells, which are essentially eliminated in the drug-treated mice.
Discussion
In the present study, we identified JI6 as an orally available FLT3 inhibitor with potent antileukemic activity in vitro and in vivo. Biochemical kinase assays revealed that JI6 potently and selectively inhibited FLT3. This potency and selectivity was further demonstrated by cell-based assays with leukemic cell lines and HCD-57 cells transformed by FLT3 mutants. Furthermore, in vivo administration of JI6 effectively suppressed malignant disease progression in FLT3-ITD-knock-in mice and xenograft models. More importantly, JI6 not only targets FLT3-ITD but also FLT3 D835 mutants that are resistant to existing inhibitors. These properties make JI6 a promising candidate for the development of next generation anti-leukemia drugs.
Identification of gain-of-function activation FLT3 mutations in a significant portion of AML patients has made FLT3 an ideal target for therapeutic drug development. Although many inhibitors have been identified, their clinical applications are disappointing. In large, a major challenge for currently available FLT3 inhibitors is the acquisition of secondary FLT3-TKD mutants that lead to drug resistance. Studies indicate that FLT3-TKDs, especially FLT3 D835 mutants, are associated with clinical resistance to sorafenib, quizartinib and ponatinib (17, 18). Based on their interactions with the catalytic domain of tyrosine kinases, TKIs can be classified into two types. Type I inhibitors bind to tyrosine kinases in both inactive and active conformations whereas type II inhibitors typically target the enzymes in their inactive conformation (24, 25). It is believed that mutations of FLT3 on the D835 site change the conformation of the kinase domain and stabilize it at the active stage thereby making it inaccessible by type II inhibitors such as sorafenib (18, 26). Our in vitro and in vivo data suggest that JI6 belongs to type I since it effectively targets FLT3 in active as well as inactive conformations. The ability of JI6 to inhibit the D835 mutants of FLT3 is particularly important. This is because the D835 mutation can not only be acquired as a secondary mutation upon treatment of FLT3-ITD-positive patients with TKIs but also is found in 7.7% of de novo AML patients independent of FLT3-ITD (1). Needless to say, novel FLT3 inhibitors targeting both FLT3-TKDs and FLT3-ITD are urgently needed. Recently, several such inhibitors including crenolanib, AMG925, G-749 and TTT-3002 have been identified (27-32), but further preclinical and clinical studies are required.
JI6 was initially identified as a JAK3 kinase inhibitor because it exhibited 16-fold greater selectivity over JAK2 (33). However, a recent study showed that JI6 inhibited rapamycin-induced autophagy inhuman breast carcinoma MCF7 cells although this effect was independent of JAK3 (34). To date, no other kinase targets have been reported. Our current biochemical assays indicate that JI6 is 6-fold more selective for FLT3 than its original target JAK3. Therefore, it is more appropriate to designate JI6 as a FLT3 inhibitor. Further supporting this is that fact that JI6 displayed 10-fold less inhibitory potency toward c-Kit, which is closely related to FLT3 in structure. This selectivity may be clinically important. In fact, much of the undesirable toxicity of FLT3 inhibitors may be attributed to the inhibition of c-Kit since combined knockout FLT3 and KIT caused profound hematopoietic defects in mice (35). JI6 belongs to the oxindole family. Oxindole-based compounds have been shown to exhibit selective inhibition activity to a variety of protein kinases (36-38). Interestingly, sunitinib, an FDA-approved anti-cancer drug that targets receptor tyrosine kinases is also a member of this family (39). Therefore, with further structural modifications to enhance potency and selectivity and to reduce toxicity, this family of inhibitors may find broader application in anticancer therapy.
In this study, JI6 was identified by performing FLT3 kinase activity assays with the protein substrate GST-FLT3S. GST-FLT3S is derived from the Y591 autophosphorylation site of FLT3 and has been proven as an excellent substrate for FLT3 kinase assays (19, 20). Interestingly, GST-FLT3S can also be effectively phosphorylated by JAK3 and c-Kit (Figure 1). The physiological meaning of this finding is not known. It will be interesting to find out if FLT3 can be phosphorylated by JAK3 and c-Kit on the same site. Nonetheless, our kinase activity assay results suggest that JI6 was very potent in inhibiting FLT3 than JAK3 and c-Kit. In the cell-based assays, we employed the erythroleukemia cell line HCD-57 instead of the commonly used Ba/F3 cells. HCD-57 cells require EPO for proliferation and survival (40), but they became EPO-independent when infected with retroviruses carrying oncogenic mutant kinases. In comparison with the IL-3-dependent Ba/F3 pro-B cells, HCD-57 cells may be more appropriate to study ontogenesis related to the myeloid linage. We believe this cell line should find broad applications in studying oncogenic potential of mutant genes and in cell-based inhibitor screening.
Materials and Methods
Reagents
Inhibitor Select Protein Kinase Library I containing 80 protein kinase inhibitors including JI6 was purchased from Calbiochem (CA, USA). Additional JI6 was synthesized with 97.7% purity by WuXi AppTec (Shanghai, China). Sorafenib was purchased from ChemieTek (IN, USA). All the kinase inhibitors were formulated in dimethyl sulfoxide (DMSO) for in vitro biochemical and cell-based assays. Monoclonal antibodies against pFLT3 (pY591), FLT3 (8F2), pERK1/2 (pT202/pY204), ERK, pAKT (pS473), AKT and GAPDH were from Cell Signaling Technology (MA, USA).
Cell culture
MV4-11, HL-60, and Jurkat cells were obtained from ATCC (VA, USA). Karpas 299 cells were kindly provided by Yi Zhao (University of Southern California) (21). MV4-11 cells were cultured in IMDM containing 10% FBS, and HCD-57 cells were maintained in IMDM with 20% FBS plus 1 unit/mL erythropoietin (Amgen, CA, USA) (41). The rest of the cells were cultured in RPMI medium supplemented with 10% FBS. All cells were cultured in a humidified atmosphere at 37°C with 5% CO2.
FLT3 kinase activity assays and inhibitor screening
Protein kinase activity assays and inhibitor screening were performed as previously described (19, 20, 42, 43). Briefly, the kinase substrate GST-FLT3S was purified from E. coli cells by using a glutathione-Sepharose column. Recombinant proteins that contained the catalytic domains of wild type and D835H/D835Y mutant forms of human FLT3 (aa573-993), human JAK3 (aa806-1124), and human cKit (aa558-976) were expressed by using the baculovirus expression system (Invitrogen) and isolated from recombinant baculovirus-infected Sf9 insect cells by using the NTA-Ni resin. Phosphorylation of GST-FLT3S by isolated recombinant protein kinases was carried out in a reaction buffer that contained 25 mM Tris–HCl (pH 7.5), 10 mM MgCl2, 0.2 mM ATP, and 2 mM dithiothreitol in the presence of various concentrations of protein kinase inhibitors. The level of GST-FLT3S tyrosine phosphorylation was determined by immunoblotting with antiphosphotyrosine antibody PY20 followed by horseradish peroxidase-conjugated secondary antibody. Detection and quantification of enhanced chemiluminescence signals were done by using FluorChem SP imaging system from Alpha Innotech (CA, USA).
Generation of mutant kinase-transformed HCD-57 cells
Retroviruses carrying mutant kinases were generated by using the pMSCV-IRES-GFP vector as previously described (44, 45). In brief, pMSCV-IRES-GFP plasmid containing the full length forms of FLT3-ITD, FLT3-D835Y, FLT3-D835H, and JAK2V617F were used to transfect GP2-293 cells together with the pVSV-G plasmid. Recombinant retroviruses were isolated by centrifugation at 20 000 × g for 2 h and then used to infect HCD-57 cells in the presence of 5 μg/ml polybrene (Sigma-Aldrich, MO, USA) under centrifugation at 1 800 × g for 2 h at room temperature. Infected HCD-57 cells were cultured in IMDM with 20% FBS in the presence of EPO for 24 hours and then seeded in a semi-solid medium containing IMDM, 20% FBS, and 1% methylcellulose in the absence of EPO. Single colonies were picked after 8-10 days of culture and further expanded in EPO-free liquid medium.
Cell proliferation, apoptosis and cell cycle assays
Cells were incubated with different concentrations of inhibitors for up to 4 days. Stock solutions of inhibitors were made in DMSO, and the final concentration of DMSO in the cell incubation system was controlled at 0.1%. For cell growth analysis, viable cells were enumerated using Trypan blue staining with a cytometer. For cell viability assay, 0.08 mg/ml XTT (2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide) and 8 μM Phenazine Methyl Sulfate (PMS) were added to the cells, and absorbance at 450 nm was measured after 3 hr incubation at 37°C. For apoptosis analysis, cells were stained with Cy5-annexin V and propidium iodide (Biovision, CA, USA). To assess cell cycle arrest, cells were fixed with ethanol overnight at 4°C and then stained with propidium iodide in the presence of RNase. Flow cytometric analyses were performed by using a FACSCalibur flow cytometer (BD Biosciences, CA, USA) at the Flow and Image Cytometry Laboratory of University of Oklahoma Health Sciences Center.
Cell signaling assays
Cells treated with various concentrations of inhibitors were extracted with a whole-cell extraction buffer containing 25 mM β-glycerophosphate (pH 7.3), 5 mM EDTA,2 mM EGTA, 5 mM β-mercaptoethanol, 1% TritonX-100, 0.1 M NaCl, 1 mM sodium vanadate, and a protease inhibitor cocktail (Roche Applied Science, IN, USA). Cell lysates were cleared by centrifugation in a microfuge at 13 000×g, and clear cell extracts containing equal amounts of total proteins were separated on SDS gels for western blot analyses with antibodies against pFLT3, FLT3, pERK, ERK, pAKT, AKT and GAPDH.
In vivo mouse models
FLT3-ITD knock-in (B6.129-Flt3tm1Dgg/J) and NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice were obtained from Jackson laboratory (ME, USA). Homozygous FLT3-ITD knock-in mice (20 weeks old, about equal numbers of male and female) were treated through oral gavage with JI6 suspended in PBS at a single daily dose of 25 mg/kg for 3 weeks. NSG mice (10-12 weeks old, male) were first implanted with 1×106 FLT3-D835Y-transformed HCD-57 cells by intravenous injection, and JI6 treatment was started 24 hours later by intraperitoneal injection of JI6 at a single daily dose of 15 mg/kg for 3 weeks. JI6 was dissolved in DMSO at 10 mg/ml and diluted 2-fold with PBS before intraperitoneal injection. Mice were monitored on a daily basis and considered dead when they became moribund and had to be euthanized. To study the effect of JI6 on FLT3 signaling, NSG mice implanted with FLT3-D835Y-transformed HCD-57 cells for 3 weeks were intraperitoneally injected with a single dose of 100 mg/kg JI6 and sacrificed in 4 hours. Collection of tissues and subsequent histochemical, flow cytometric and western blot analyses were performed as previously described (46). Mice were randomly chosen for control vehicle or JI6 treatment, and the observers were non-blinded. Power analyses were conducted to determine adequate number of mice for each experiment. This study was carried out under an approved protocol in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Statistics
The Prism software (Graphpad, CA, USA) was applied for the statistical analyses. A two-tailed t test was performed to compare the difference between two groups. Kaplan-Meier analysis and log rank test were used to analyze animal survival. A P value of less than 0.05 is considered significantly different.
Acknowledgments
This work was supported by grant HL079441 from the National Institutes of Health and a grant from Oklahoma Center for the Advancement of Science & Technology.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
References
- 1.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Estey E. Acute myeloid leukemia: 2014 update on risk-stratification and management. Am J Hematol. 2014;89:1063–1081. doi: 10.1002/ajh.23834. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi S. Current findings for recurring mutations in acute myeloid leukemia. J Hematol Oncol. 2011;4:36. doi: 10.1186/1756-8722-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swords R, Freeman C, Giles F. Targeting the FMS-like tyrosine kinase 3 in acute myeloid leukemia. Leukemia. 2012;26:2176–2185. doi: 10.1038/leu.2012.114. [DOI] [PubMed] [Google Scholar]
- 5.Leung AY, Man CH, Kwong YL. FLT3 inhibition-a moving and evolving target in acute myeloid leukaemia. Leukemia. 2012;27:260–268. doi: 10.1038/leu.2012.195. [DOI] [PubMed] [Google Scholar]
- 6.Annesley CE, Brown P. The Biology and Targeting of FLT3 in Pediatric Leukemia. Front Oncol. 2014;4:263. doi: 10.3389/fonc.2014.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 8.Gregory TK, Wald D, Chen Y, Vermaat JM, Xiong Y, Tse W. Molecular prognostic markers for adult acute myeloid leukemia with normal cytogenetics. J Hematol Oncol. 2009;2:23. doi: 10.1186/1756-8722-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124:3441–3449. doi: 10.1182/blood-2014-05-578070. [DOI] [PubMed] [Google Scholar]
- 10.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia(AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann M, Groβe-Hovest L, Nübling T, Pyż E, Bamberg ML, Aulwurm S, et al. Generation, selection and preclinical characterization of an Fc-optimized FLT3 antibody for the treatment of myeloid leukemia. Leukemia. 2012;26:1228–1237. doi: 10.1038/leu.2011.372. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 14.Levis M. Quizartinib for the treatment of FLT3/ITD acute myeloid leukemia. Future Oncol. 2014;10:1571–1579. doi: 10.2217/fon.14.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 16.Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD, et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res. 2004;64:6385–6389. doi: 10.1158/0008-5472.CAN-04-2148. [DOI] [PubMed] [Google Scholar]
- 17.Williams AB, Nguyen B, Li L, Brown P, Levis M, Leahy D, et al. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia. 2013;27:48–55. doi: 10.1038/leu.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target inhuman acute myeloid leukaemia. Nature. 2012;485:260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Guo Y, Han J, Ho WT, Li S, Fu X, et al. Generation and characterization of a highly effective protein substrate for analysis of FLT3 activity. J Hematol Oncol. 2012;5:39. doi: 10.1186/1756-8722-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y, Chen Y, Xu X, Fu X, Zhao ZJ. SU11652 Inhibits tyrosine kinase activity of FLT3 and growth of MV-4-11 cells. J Hematol Oncol. 2012;5:72. doi: 10.1186/1756-8722-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu FY, Johnston PB, Burke KA, Zhao Y. The Expression of CD30 in Anaplastic Large Cell Lymphoma Is Regulated by Nucleophosmin-Anaplastic Lymphoma Kinase–Mediated JunB Level in a Cell Type–Specific Manner. Cancer Res. 2006;66:9002–9008. doi: 10.1158/0008-5472.CAN-05-4101. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W, Du Y, Ho WT, Fu X, Zhao ZJ. JAK2V617F and p53 mutations coexist in erythroleukemia and megakaryoblastic leukemic cell lines. Exp Hematol Oncol. 2012;1:15. doi: 10.1186/2162-3619-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BH, Tothova Z, Levine RL, Anderson K, Buza-Vidas N, Cullen DE, et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12:367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blastcrisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 25.Burgess MR, Skaggs BJ, Shah NP, Lee FY, Sawyers CL. Comparative analysis of two clinically active BCR-ABL kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc Natl Acad Sci USA. 2005;102:3395–3400. doi: 10.1073/pnas.0409770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, et al. Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol. 2010;17:1241–1249. doi: 10.1016/j.chembiol.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Wang X, Eksterowicz J, Gribble MW, Jr, Alba GQ, Ayres M, et al. Discovery of AMG 925, a FLT3 and CDK4 dual kinase inhibitor with preferential affinity for the activated state of FLT3. J Med Chem. 2014;57:3430–3449. doi: 10.1021/jm500118j. [DOI] [PubMed] [Google Scholar]
- 28.Lee HK, Kim HW, Lee IY, Lee J, Lee J, Jung DS, et al. G-749, a novel FLT3 kinase inhibitor, can overcome drug resistance for the treatment of acute myeloid leukemia. Blood. 2014;123:2209–2219. doi: 10.1182/blood-2013-04-493916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith CC, Lasater EA, Lin KC, Wang Q, McCreery MQ, Stewart WK, et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc Natl Acad Sci USA. 2014;111:5319–5324. doi: 10.1073/pnas.1320661111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerman EI, Turner DC, Buaboonnam J, Hu S, Orwick S, Roberts MS, et al. Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia. Blood. 2013;122:3607–3615. doi: 10.1182/blood-2013-07-513044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma HS, Nguyen B, Duffield AS, Li L, Galanis A, Williams AB, et al. FLT3 kinase inhibitor TTT-3002 overcomes both activating and drug resistance mutations in FLT3 in acute myeloid leukemia. Cancer Res. 2014;74:5206–5217. doi: 10.1158/0008-5472.CAN-14-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Liu L, Liang L, Xia Z, Li Z, Wang X, et al. AMG 925 is a dual FLT3/CDK4 inhibitor with the potential to overcome FLT3 inhibitor resistance in acute myeloid leukemia. Mol Cancer Ther. 2015;14:375–383. doi: 10.1158/1535-7163.MCT-14-0388. [DOI] [PubMed] [Google Scholar]
- 33.Adams C, Aldous DJ, Amendola S, Bamborough P, Bright C, Crowe S, et al. Mapping the kinase domain of Janus Kinase 3. Bioorg Med Chem Lett. 2003;13:3105–3110. doi: 10.1016/s0960-894x(03)00657-7. [DOI] [PubMed] [Google Scholar]
- 34.Farkas T, Daugaard M, Jäättelä M. Identification of small molecule inhibitors of phosphatidylinositol 3-kinase and autophagy. J Biol Chem. 2011;286:38904–38912. doi: 10.1074/jbc.M111.269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 36.Bramson HN, Corona J, Davis ST, Dickerson SH, Edelstein M, Frye SV, et al. Oxindole-based inhibitors of cyclin-dependent kinase 2 (CDK2): design, synthesis, enzymatic activities, and X-ray crystallographic analysis. J Med Chem. 2001;44:4339–4358. doi: 10.1021/jm010117d. [DOI] [PubMed] [Google Scholar]
- 37.Wood ER, Kuyper L, Petrov KG, Hunter RN, 3rd, Harris PA, Lackey K. Discovery and in vitro evaluation of potent TrkA kinase inhibitors: oxindole and aza-oxindoles. Bioorg Med Chem Lett. 2004;14:953–957. doi: 10.1016/j.bmcl.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Lockman JW, Reeder MD, Robinson R, Ormonde PA, Cimbora DM, Williams BL, et al. Oxindole derivatives as inhibitors of TAK1 kinase. Bioorg Med Chem Lett. 2011;21:1724–1727. doi: 10.1016/j.bmcl.2011.01.077. [DOI] [PubMed] [Google Scholar]
- 39.Papaetis GS, Syrigos KN. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. 2009;23:377–389. doi: 10.2165/11318860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Wen D, Boissel JP, Showers M, Ruch BC, Bunn HF. Erythropoietin structure-function relationships Identification of functionally important domains. J Biol Chem. 1994;269:22839–22846. [PubMed] [Google Scholar]
- 41.Zhao W, Zou K, Farasyn T, Ho WT, Zhao ZJ. Generation and Characterization of a JAK2V617F-Containing Erythroleukemia Cell Line. PLoS One. 2014;9:e99017. doi: 10.1371/journal.pone.0099017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Xu M, Xing S, Ho WT, Ishii T, Li Q, et al. Erlotinib effectively inhibits JAK2V617F activity and polycythemia vera cell growth. J BiolChem. 2007;282:3428–3432. doi: 10.1074/jbc.C600277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z, Xing S, Wang S, Ho WT, Zhao ZJ. Characterization of a highly effective protein substrate for analysis of JAK2(V617F) Activity. Exp Hematol. 2007;35:1624–1632. doi: 10.1016/j.exphem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu MJ, Sui X, Zhao R, Dai C, Krantz SB, Zhao ZJ. PTP-MEG2 Is Activated in Polycythemia Vera Erythroid Progenitor Cells and Is Essential for Growth and Expansion of Erythroid Cells. Blood. 2003;102:4354–4360. doi: 10.1182/blood-2003-04-1308. [DOI] [PubMed] [Google Scholar]
- 45.Moreno-Miralles I, Pan L, Keates-Baleeiro J, Durst-Goodwin K, Yang C, Kim HG, et al. The inv(16) Cooperates with ARF Haploinsufficiency to Induce Acute Myeloid Leukemia. J BiolChem. 2005;280:40097–40103. doi: 10.1074/jbc.M506855200. [DOI] [PubMed] [Google Scholar]
- 46.Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]