Abstract
The anteriorly based partial thickness sternocleidomastoid (SCM) muscle flap is among the various methods described to correct parotidectomy defects, but its indications and limitations are not clearly demonstrated in several reports. This study was done to test the aesthetic outcome of this method, its indications and limitations. At Dr. Babasaheb Ambedkar Memorial hospital, Mumbai, 20 patients presenting with benign parotid tumors underwent parotidectomy. 16 underwent superficial parotidectomy and 3 underwent adequate parotidectomy, 1 had total parotidectomy. The anteriorly based partial thickness SCM muscle flap was used to correct the contour deformity and to prevent Frey syndrome. The aesthetic result was evaluated by assessing and scoring the overall appearance of the scar, the degree of symmetry of the reconstructed parotid region and the site of the donor muscle in comparison to their contralateral normal sides. The overall aesthetic appearance was good in 17 patients, and moderate in 3 patients. 17/20 patients had an overall deep satisfaction with the result. The residual hollowness following total parotidectomy defect and the poor quality of scars were the main reasons affecting the aesthetic outcome. Superficial parotidectomy through modified Blair’s incision with immediate reconstruction with anteriorly based partial thickness SCM flap allows a satisfactory aesthetic outcome and minimal donor site morbidity. Scores of the above two parameters were accessed. Patients’ satisfaction was assessed by patients questionnaire.
Keywords: Parotidectomy, SCM muscle flap, Frey’s Syndrome
Introduction
Aesthetic goals in parotid surgery have seldom been addressed because oncologic concerns have largely overshadowed aesthetic issues for patients with parotid masses. Traditional parotidectomy incisions leave a visible scar on the neck as well as a visible hollow in the retromandibular region, which can extend onto the cheek [1]. Frey’s syndrome is an unpleasant phenomenon characterized by recurrent episodes of facial gustatory flushing and sweating limited to the cutaneous distribution of the auriculotemporal nerve. It occurs in up to 20 % of patients who have undergone parotid surgery and is thought to be due to misdirected regeneration of parasympathetic fibres normally supplying the parotid gland to innervate the cutaneous sweat glands [2–4]. The timing of its development ranged from 20 days to 22 months, median 11 months [5]. This study was designed to evaluate the aesthetic outcome and frequency of development of Frey’s syndrome after parotidectomy with surgical bed reconstruction using anteriorly based partial thickness SCM flap.
Materials and Methods
This non-randomized, prospective study was conducted at the ENT and HNS Department; Dr. Babasaheb Amedkar Memorial Hospital over a period of 3 years, started March 2011 till March 2014. Study included 20 patients, males accounted for 6 of the cases and 14 were females. The age ranged from 20 to 53 years (Table 1). Superficial parotidectomy was planned in 16 patients, adequate parotidectomy in 3 and total parotidectomy in 1 patient (Table 2). All tumors were FNAC proven benign; there was no palpable lymph node and MRI had shown no evidence of spread. With Ethics Committee approval, all patients were informed and consented for the anteriorly based partial thickness SCM flap operation after explanation and discussion of the procedure and possible complications of various surgical modalities. All operations were performed under general endotracheal anesthesia. The main trunk of the facial nerve was dissected about 1 cm inferomedially to the pointed end of the tragal cartilage of the ear, bisecting the angle between posterior belly of digastric muscle and the bony tympanic plate, a curved mosquito artery forceps was used for dissection of the superficial parotid from deep parotid in the plane of the facial nerve, this allowed the superficial parotid tissue to be lifted away, gradually exposing the full anatomical distribution of the facial nerve. The SCM muscle was then splitted as partial thickness about 1/3rd to 1/2 of the muscle thickness and upper 1/3rd (more or less) as length of flap depending upon the defect created and rotated anteriorly as anterior based flap preserving its blood supply. The flap is then sutured to the Superficial Musculo Aponeurotic System (SMAS), Parotid tissue with (3, 0) vicryl. The wound closed in layers with suction drain or corrugated drain with proper dressing. The drain was removed when collection minimal. The stitches were removed on 10th postoperative day. The FNAC findings were confirmed on histopathological examination (Fig. 1).
Table 1.
Age and sex distribution
| Age (years) | Male | % | Female | % |
|---|---|---|---|---|
| 0–20 | 0 | 0 | 1 | 5 |
| 20–40 | 2 | 10 | 5 | 25 |
| 40–60 | 4 | 20 | 8 | 40 |
Table 2.
Type of operative procedure
| Type of surgery | Male | % | Female | % |
|---|---|---|---|---|
| Superficial parotidectomy | 4 | 20 | 12 | 60 |
| Adequate parotidectomy | 1 | 5 | 2 | 10 |
| Total parotidectomy | 1 | 5 | 0 | 0 |
Fig. 1.
Modified Blair’s incision and main trunk of facial nerve with its branches with superficial lobe
Outcome Evaluation
The development of Frey’s syndrome and the aesthetic outcome depending on subjective satisfaction with the incision scar and depth of the retro mandibular dimple as assessed on a visual analogue scale ranged between 0 = unsatisfied and 10 = highly satisfied. The follow-up was performed as outpatient clinic visits 1, 4 weeks, 3 months, 12 months after surgery (Fig. 2).
Fig. 2.

Anteriorly based partial thickness SCM muscle flap sutured to the SMAS
Results
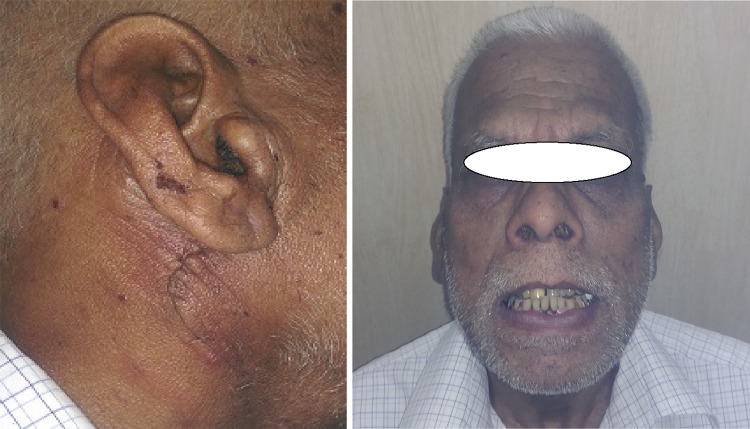
A total of 20 patients (6 males and 14 females) with a mean age of 41 years were included in this study (Table 1). Postoperative pathology revealed clear margins and complete resection of all tumors. Pleomorphic adenoma represented the commonest histopathology in 16 patients (80 %). Warthins tumor was found in the remaining four (20 %) specimens (Table 3). Transient facial nerve dysfunction occurred only in one patient (2.5 %) who recovered spontaneously within 1 month. No evidence of injury to the spinal accessory nerve was reported. Numbness of ear lobule was seen in 10 patients (50 %) out of 10, 6 patients (30 %) recovered in 3 months and 4 (20 %) developed permanent numbness (Table 4). The overall aesthetic appearance was assessed by the two surgeons was good in 18 patients (90 %), moderate in 2 patients (10 %) and no patient had an overall poor score (Table 5). The asymmetry between the external appearances of site of the donor muscle to the contralateral side was minimal in 17 patients (85 %) and moderate in 3 (15 %) (Table 6). On the other hand, patients satisfaction questionnaire about the aesthetic outcome showed that 17 patients (85 %) had an overall deep satisfaction with the result (Fig. 3).
Table 3.
Histopathological examination
| Histopathology | No. of patients | % |
|---|---|---|
| Pleomorphic adenoma | 16 | 80 |
| Warthin tumor | 4 | 20 |
Table 4.
Complications following the Procedures
| Complications | No. of patients | % |
|---|---|---|
| Transient VII nv.Paresis | 1 | 5 |
| Injury to spinal accessory nerve | 0 | 0 |
| Numbness of ear lobule | 10 | 50 |
| Temporary | 6 | 30 |
| Permanent | 4 | 20 |
| Salivary fistula | 0 | 0 |
| Freys syndrome | 0 | 0 |
| Local tumor recurrence | 0 | 0 |
Table 5.
Appearance of scar
| Appearance of scar | No. of patients | % |
|---|---|---|
| Good | 18 | 90 |
| Average | 2 | 10 |
| Poor | 0 | 0 |
Table 6.
Symmetry of reconstructed parotid site to contralateral site
| Symmetry of face | No. of patients | % |
|---|---|---|
| Minimal asymmetry | 17 | 85 |
| Moderate asymmetry | 3 | 15 |
| Marked asymmetry | 0 | 0 |
Fig. 3.
Postoperative picture showing appearance of scar and symmetry of face
Discussion
The time honoured Blair’s incision was first introduced in 1912, and was then modified by Bailey in 1941 and is commonly referred to as modified Blair’s incision and is one of the commonest incisions that have been used for decades [1, 6–9]. The extent of the contour deformity following parotidectomy varies greatly among patients depending on the tumor size, tumor location and the body habitus [3, 8, 10]. Several designs of the sternocleidomastoid muscle have been used to correct this deformity. The muscle can be used based inferiorly on its vascular supply from lower pedicle (a branch from the thyrocervical trunk) [5, 11, 12]. It can be partially splitted along its whole length and mobilized as an open book to be advanced medially as a bipedicle flap with superior arterial supply by occipital artery [13, 14]. Kim and Mathog [15] used the superficial portion of the sternocleidomastoid with the parotid fascia connected to it anteriorly and the platysma connected to it inferiorly; the flap was then reflected forward and rotated and sutured to the temporal fascia, filling the defect. Some authors took the whole muscle in very large defects [16]. In the current study we have used the anteriorly based, partial thickness muscle flap of upper 1/3rd of SCM to correct medium sized depressions following superficial parotidectomies as well in total and adequate parotidectomy. In terms of postoperative symmetry between both parotid regions, all this subset of patients had a good score (score of 3). Similar results were reported by others [9, 13]. The technique is easy, simple and adds few extra minutes to the operative time. Chow et al. [12] have recommended using the whole muscle in reconstructing total parotidectomy defects. Harvesting the whole sternocleidomastoid muscle bulk would leave an unsightly donor site defect in the neck and creates an obvious asymmetry between both sides of the neck [16]. Asymmetry was only minimal in most patients in the current study as only the upper 1/3rd partial thickness of the muscle was harvested. Similar results were reported by others [13]. Moreover, with partial thickness sternocleidomastoid flap, the risk of injury to the spinal accessory nerve is minimized than if the whole muscle is taken. There was neither an injury reported in the current study nor in other studies using either the superficial inferiorly based or superiorly based design [1, 13]. We agree with Biglioli and Autelitano [16] that for very large and deep defects free vascularized adipo fasial tissues should be used as the subsequent muscle atrophy due to muscle denervation is inevitable. Alternatively, other muscle flaps with larger bulk such as gracilis with 20–30 % overcorrection to compensate for the future decrease in volume can be also utilized. Rhee et al. mentioned that whether superiorly based or inferiorly based partial thickness sternocleidomastoid muscle flap was used, optimal correction of moderate-sized contour defects was achieved, and that the choice between the two techniques was based on surgeon’s preference and can’t be justified on strong scientific evidence. In the current study, we have chosen the anteriorly based muscle flap as we believe that the dissection was technically easier. The sternocleidomastoid flap’s role in minimizing Frey’s syndrome has been controversial. Some authors have questioned this role [5, 13, 17], while others reports have proven its benefit [1, 4, 10, 18]. In the current study, despite that none of our patients reported complaints suggestive of Frey’s syndrome, neither the duration of follow up in this study nor the performance of an objective testing are sufficient to prove the flap’s potential benefit in this regard.
Conclusion
Parotidectomy with immediate reconstruction of the surgical defect with anteriorly based partial thickness superficial sternocleidomastoid muscle flap allows adequate and safe resection of most benign parotid tumors with a good aesthetic outcome with minimal donor site morbidity and decreased chance of Frey syndrome.
References
- 1.Sanabria A, Kowalski LP, Bradley PJ, Hartl DM, Bradford CR, et al. Sternocleidomastoid muscle flap in preventing Frey’s syndrome after parotidectomy: a systemic review. Head Neck. 2012;34(4):589–598. doi: 10.1002/hed.21722. [DOI] [PubMed] [Google Scholar]
- 2.Appiani E, Delfino MC. Plastic incisions for facial and neck tumors. Ann Plast Surg. 1984;13:335–352. doi: 10.1097/00000637-198410000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Witt RL, Pribtikin EA. How can Frey’s syndrome be prevented or treated following parotid surgery? Laryngoscope. 2013;123(7):1573–1574. doi: 10.1002/lary.23779. [DOI] [PubMed] [Google Scholar]
- 4.Cesteleyn L, Helman J, King S, Van de Vyvere G. Temporoparietal fascia flaps and superficial musculoaponeurotic system plication in parotid surgery reduces Frey’s syndrome. J Oral Maxillofac Surg. 2002;60:1284–1297. doi: 10.1053/joms.2002.35725. [DOI] [PubMed] [Google Scholar]
- 5.Fee WE, Jr, Tran LE. Functional outcome after total parotidectomy reconstruction. Laryngoscope. 2004;114:223–226. doi: 10.1097/00005537-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Nouraei SA, Al-Yaghchi C, Ahmed J, Kirkpatrick N, MansuriS Singh A, et al. An anatomical comparison of Blair and facelift incisions for parotid surgery. Clin Otolaryngol. 2006;31:531–534. doi: 10.1111/j.1365-2273.2006.01334.x. [DOI] [PubMed] [Google Scholar]
- 7.Hui Y, Wong DS, Wong LY, Ho WK, Wei WI. A prospective controlled double-blind trial of great auricular nerve preservation at parotidectomy. Am J Surg. 2003;185:574–579. doi: 10.1016/S0002-9610(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 8.Meningaud JP, Bertolus C, Bertrand JC. Parotidectomy; assessment of a surgical technique including facelift incision and SMAS advancement. J Craniomaxillofac Surg. 2006;34:34–37. doi: 10.1016/j.jcms.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Amin A, Moustafa A, Rihata M. Parotidectomy for benign parotid tumors: an aesthetic approach. J Egypt Natl Cancer Inst. 2011;23:67–72. doi: 10.1016/j.jnci.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed OA, Kolhe PS. Prevention of Frey’s syndrome and volume deficit after parotidectomy using the superficial temporal artery fascial flap. Br J Plast Surg. 1999;52:256–260. doi: 10.1054/bjps.1998.0137. [DOI] [PubMed] [Google Scholar]
- 11.Bugis SP, Young JE, Archibald SD. Sternocleidomastoid flap following parotidectomy. Head Neck. 1990;12:430–435. doi: 10.1002/hed.2880120511. [DOI] [PubMed] [Google Scholar]
- 12.Chow TL, Lam CY, Chiu PW, Lim BH, Kwok SP. Sternomastoid- muscle transposition improves the cosmetic outcome of superficial parotidectomy. Br J Plast Surg. 2001;54:409–411. doi: 10.1054/bjps.2001.3586. [DOI] [PubMed] [Google Scholar]
- 13.Gooden EA, Gullane PJ, Irish J, Katz M, Carroll C. Role of the sternocleidomastoid muscle flap preventing Frey’s syndrome and maintaining facial contour following superficial parotidectomy. J Otolaryngol. 2001;30:98–101. doi: 10.2310/7070.2001.19876. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton J, Avitia S, Osborne RF. Designing a bipedicled sternocleidomastoid muscle flap for parotidectomy contour deformities. Ear Nose Throat J. 2006;85:20–21. [PubMed] [Google Scholar]
- 15.Kim SY, Mathog RH. Platysma muscle-cervical fascia sternocleidomastoid muscle (PCS) flap for parotidectomy. Head Neck. 1999;21:428–433. doi: 10.1002/(SICI)1097-0347(199908)21:5<428::AID-HED8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Biglioli F, Autelitano L. Reconstruction after total parotidectomy using a de-epithelialized free flap. J Craniomaxillofacial Surg. 2007;35:364–368. doi: 10.1016/j.jcms.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Kornblut AD, Westphal P, Miehlke A. The effectiveness of a sterno-mastoid muscle flap in preventing postparotidectomy occurrence of the Frey syndrome. Acta Otolaryngol. 1974;77:368–373. doi: 10.3109/00016487409124638. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Yang X, Pan J, Shi Z, Li L. Graft for prevention of Frey syndrome after parotidectomy: a systemic review and meta-analysis of randomised controlled trials. J Oral Maxillofacial Surg. 2013;71(2):419–427. doi: 10.1016/j.joms.2012.06.007. [DOI] [PubMed] [Google Scholar]