Abstract
Four HIV rapid tests were subjected to field validation in Panama and compared to an enzyme-linked immunosorbent assay/Multispot-based testing algorithm. The sensitivities of Determine, Uni-Gold, SD Bioline, and INSTI were 99.8%. The specificities of Determine, SD-Bioline, and Uni-Gold were 100%, and the specificity of INSTI was 99.8%. On the basis of these data, we determined that these rapid tests can be used in an appropriate algorithm to diagnose HIV infection and are suitable for use in testing and counseling settings in Panama.
TEXT
Early diagnosis of HIV infection is important for prevention and patient management; delays in diagnosis of HIV infection represent a loss of opportunities for improving individual health and public health. The risk of transmitting the virus is higher if the patient does not know his/her status, does not reduce risk behaviors, and has a high viral load. The late start of treatment adversely affects the patient's prognosis, increasing morbidity and mortality (1). Several different assays are available for the detection of specific antibodies to diagnose HIV infection (2). Although the enzyme immunoassay (EIA) is most commonly used for diagnosis, the disadvantages of EIA are the need for well-trained technicians, appropriate equipment, laboratory infrastructure, and batch testing (3). In developing countries, such as those in Central America, technical support is not available in most of the peripheral primary care units (4–6). The number of samples screened per day is usually small, and the infrastructure and facilities for performing the EIA are not ideal or cost-effective. There is also a need to establish voluntary counseling and testing (VCT) facilities as part of the HIV infection prevention strategy. In these situations, tests need to be simple and rapid, reducing the time between infection and diagnosis (7, 8). The development of HIV rapid tests has facilitated the massive scale-up of HIV testing and counseling at thousands of testing venues, allowing millions of individuals to receive their HIV diagnosis outside of a primary care facility (9, 10).
The purpose of this field validation was to evaluate the performance of four rapid diagnostic tests: Determine HIV-1/2 (Alere Medical Co., Ltd., Japan), SD Bioline HIV-1/2 (Standard Diagnostics, Inc., South Korea), INSTI HIV antibody test (Biolytical Laboratories, Canada), and Uni-Gold HIV-1/2 (Trinity Biotech, Ireland), to accurately diagnose HIV infection.
Venous whole-blood specimens were collected into EDTA tubes from consenting patients who tested for HIV infection at eight selected health facilities, representing five regions of Panama (Western Panama, Colón, Gnobe Bugle, Chiriquí, and Guna Yala) during May through August 2014. Eligible patients included men and nonpregnant women ≥18 years of age and pregnant women of any age recruited at these selected sites, which represented ∼20% of the participants. All samples were tested onsite by the four rapid tests listed above. Kit protocols were strictly followed in carrying out the tests, and the technicians who performed these tests were well trained in the testing procedures and quality assurance principles, including the use of standardized registers (6). Proficiency panels of dried tube samples were provided twice during the study as external quality assessments for testers performing the rapid testing (11). In addition, sites were provided with positive and negative controls to be used as quality control specimens, which were routinely used to ensure kit performance. Additional oversight included regular supervisory visits every 3 weeks. Blood samples were subsequently processed into plasma and transported to a central laboratory (Gorgas Memorial Institute) for further testing (see below).
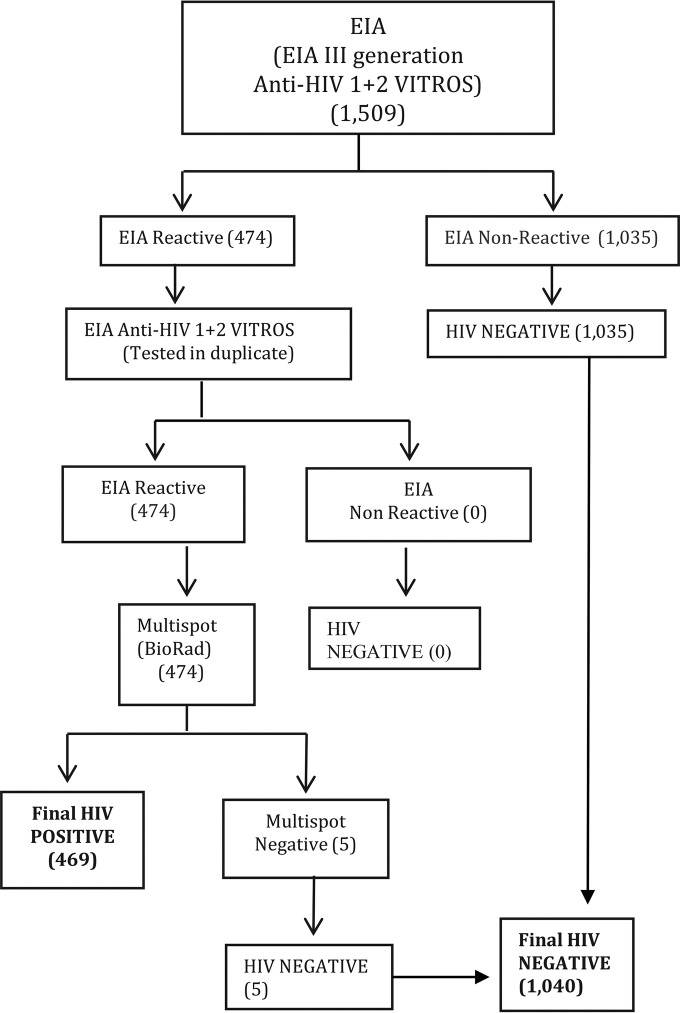
Reference testing was performed at the Gorgas Memorial Institute using the third-generation anti-HIV-1/2 Vitros EIA, followed by the Multispot HIV-1/2 rapid test (Bio-Rad) to confirm EIA positive results (Fig. 1). The EIA plus Multispot algorithm is recommended by the U.S. CDC and was used as a reference standard and to confirm HIV status. All specimens with EIA-nonreactive results were classified negative, and all reactive specimens were repeated in duplicate by the same EIA. EIA-reactive specimens were further tested by Multispot to confirm HIV status. The sensitivity and specificity of individual rapid tests were calculated based on reference testing results with the EIA plus Multispot algorithm. Ninety-five percent confidence intervals (95% CI) were calculated for sensitivity and specificity with the Clopper-Pearson method based on the exact binomial distribution.
FIG 1.
Reference standard algorithm for HIV testing, Panama. EIA, enzyme-linked immunosorbent assay.
Of 1,527 persons approached, 1,509 (98.8%) individuals consented to be tested for HIV infection. The median age of the participants was 31 years (interquartile range, 24 to 43 years), 66% were women, and 31% were pregnant. Whole-blood samples were tested by using Determine HIV-1/2, SD Bioline HIV-1/2, INSTI HIV antibody, and Uni-Gold HIV-1/2; of these, 1,503 specimens for Determine HIV-1/2, 1,508 specimens for Uni-Gold HIV-1/2, 1,509 for SD Bioline HIV-1/2, and 1,505 for INSTI HIV antibody with complete data were analyzed (Table 1). Two specimens with likely mix-ups and specimens giving invalid rapid test results were excluded from analysis for a given test. The performances of the four rapid tests compared to the reference standard (EIA plus Multispot) and EIA alone are summarized in Table 1. Overall, all four rapid tests exhibited high sensitivities of 99.8%; only one specimen showed a false negative, possibly being a recent HIV infection. Low titer/low avidity antibody present in recent infections may be missed occasionally by the short antigen-antibody interaction required by rapid tests. The specificities of three rapid tests (Determine, SD Bioline, and Uni-Gold) were 100%, and that of INSTI was 99.8% (kappa, ≥0.995). In comparison, EIA alone had a sensitivity of 100%, but the specificity was 99.5%, with five specimens falsely reactive. All five specimens had low signal/cutoff ratios on EIA (<5.5), and Multispot confirmed that they were negative. Some level of false positivity with EIAs is common as these sensitive tests are developed for screening purposes, including blood safety, and a positive result requires confirmation with more specific tests for HIV diagnosis (12).
TABLE 1.
Summary of sensitivities, specificities, positive predictive values, and negative predictive values of HIV rapid tests in comparison to those of EIA plus Multispot and EIA alone in Panama
| Test | Totala | Result (n)b |
Sensitivity, (% [95% CI]) | Specificity (% [95% CI]) | Predictive value (%)c |
||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | Positive | Negative | ||||
| Determine | 1,503 | 465 | 0 | 1,037 | 1 | 99.8 (98.81–99.99) | 100.0 (99.64–100) | 100.0 | 99.9 |
| Uni-Gold | 1,508 | 467 | 0 | 1,039 | 1 | 99.8 (98.47–99.95) | 100.0 (99.65–100) | 100.0 | 99.8 |
| SD Bioline | 1,509 | 468 | 0 | 1,040 | 1 | 99.8 (98.82–99.99) | 100.0 (99.65–100) | 100.0 | 99.9 |
| INSTI | 1,505 | 467 | 2 | 1,035 | 1 | 99.8 (98.82–99.99) | 99.8 (98.30–99.98) | 99.6 | 99.9 |
Total numbers vary due to an inadequate volume of specimen or invalid results on a given rapid test.
TP, true positive; FP, false positive; TN, true negative; FN, false negative.
Predictive values are a function of HIV prevalence, so 95% CI are not included.
The operational characteristics (e.g., number of steps, reagent preparation, and reading time) among the four rapid tests are somewhat different, but the accuracy indices of these four rapid tests were found to be satisfactory in comparison to reference test results. In fact, three of the tests had specificities of 100%, which is important when used in testing and counseling settings in rural areas away from the central laboratory. In addition, the rapid tests have advantages, such as ease of use, ease of interpretation, room temperature storage, and a long shelf life. These tests can be done with a short turnaround time, avoiding the delay incurred in batching. Thus, rapid tests can be used as alternatives to EIAs in small peripheral primary care units, and VCT centers, which lack facilities and skilled laboratory technicians. None of the results from the four rapid tests were given to the participants, since this was a field validation exercise. There are several reports of evaluations of rapid assays, often with a small panel of samples; only a few are field studies. The accuracy indices of all those kits were close to 100% (5, 13, 14). However, only a few reports were on real-time evaluations with primary care unit-based samples (10). No single false-positive result was observed with three rapid tests (Determine, Uni-Gold, and SD Bioline). One rapid test (INSTI) did identify one specimen as falsely reactive. Although not known, the cause of this falsely reactive result could be due to tester error, poor adherence to standard operating procedure, or other factors. Note that the INSTI test has a different format (flowthrough device) than the other three tests evaluated here (lateral-flow devices).
These results emphasize the importance of using two different HIV rapid tests in order to minimize or eliminate the number of false-positive or false-negative results. In conclusion, our results demonstrate that HIV rapid tests can be used to provide reliable HIV diagnoses and thus can increase patient access to testing and counseling services in resource-limited settings, especially when implemented with good training, quality assurance, and a monitoring program (15). HIV rapid tests should play an important role as part of a national testing strategy and can facilitate early diagnosis, prevention efforts, and linkage to care and treatment of HIV-positive individuals.
ACKNOWLEDGMENTS
We thank the Ministry of Health of Panama for their political commitment and all the laboratory staff from the eight health facilities for their hard work.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Funding Statement
This study was supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through a cooperative agreement with SECOMISCA under grant number SG/SICA/COMISCA/CDC 5U19GH00064 and the guidance and technical expertise provided by the Centers for Disease Control and Prevention (CDC).
REFERENCES
- 1.Firestone R, Rivas J, Lungo S, Cabrera A, Ruether S, Wheeler J, Vu L. 2014. Effectiveness of a combination prevention strategy for HIV risk reduction with men who have sex with men in Central America: a mid-term evaluation. BMC Public Health 14:1244. doi: 10.1186/1471-2458-14-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syed Iqbal H, Balakrishnan P, Murugavel KG, Suniti S. 2008. Performance characteristics of a new rapid immunochromatographic test for the detection of antibodies to human immunodeficiency virus (HIV) types 1 and 2. J Clin Lab Anal 22:178–185. doi: 10.1002/jcla.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berhanu H, Burke E, Gershy-Damet GM, Feluzi H, Howard S, Kakonkanya B, Lyamuya E, Marshall T, Mururi L, Mutingwende M, Mwangi C, Osewe P, Rayfield M, Ridderhof J, Rugimbanya P, St. Louis M, Stakteas S, Ziyambi Z, Wethers J. 2000. Guidelines for appropriate evaluations of HIV testing technologies in Africa. Department of Health and Human Services Centers for Disease Control and Prevention, Washington, DC. [Google Scholar]
- 4.Manak M, Njoku O, Shutt A, Malia J, Jagodzinski L, Milazzo M, Suleiman A, Ogundeji A, Nelson R, Ayemoba O, O'Connell R, Singer D, Michael N, Peel S. 2015. Evaluation of performance of two rapid tests for detection of HIV-1 and -2 in high- and low-prevalence populations in Nigeria. J Clin Microbiol 53:3501–3506. doi: 10.1128/JCM.01432-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer C, Dubon J, Koenig E, Perez E, Ager A, Jayaweera D, Cuadrado R, Rivera A, Rubido A, Palmer D. 1999. Field evaluation of the Determine rapid human immunodeficiency virus diagnostic test in Honduras and the Dominican Republic. J Clin Microbiol 37:3698–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekh BS, Kalou MB, Alemnji G, Ou CY, Gershy-Damet GM, Nkengasong JN. 2010. Scaling up HIV rapid testing in developing countries: comprehensive approach for implementing quality assurance. Am J Clin Pathol 134:573–584. doi: 10.1309/AJCPTDIMFR00IKYX. [DOI] [PubMed] [Google Scholar]
- 7.Lyamuya EF, Aboud S, Urassa WK, Sufi J, Mbwana J, Ndugulile F, Massambu C. 2009. Evaluation of simple rapid HIV assays and development of national rapid HIV test algorithms in Dar es Salaam, Tanzania. BMC Infect Dis 9:19. doi: 10.1186/1471-2334-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannangai R, Ramalingam S, Pradeepkumar S, Damodharan K, Sridharan G. 2000. Hospital-based evaluation of two rapid human immunodeficiency virus antibody screening tests. J Clin Microbiol 38:3445–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marum E, Taegtmeyer M, Parekh B, Mugo N, Lembariti S, Phiri M, Moore J, Cheng AS. 2012. “What took you so long?” The impact of PEPFAR on the expansion of HIV testing and counseling services in Africa. J Acquir Immune Defic Syndr 60(Suppl 3):S63–S69. doi: 10.1097/QAI.0b013e31825f313b. [DOI] [PubMed] [Google Scholar]
- 10.Duong Y, Mavengere Y, Patel H, Moore C, Manjengwa J, Sibandze D, Rasberry Mlambo C, Li Z, Emel L, Bock N, Moore J, Nkambule R, Justman J, Reed J, Bicego G, Ellenberger D, Nkengasong JN, Parekh BS. 2014. Poor performance of the Determine HIV-1/2 Ag/Ab combo fourth-generation rapid test for detection of acute infections in a national household survey in Swaziland. J Clin Microbiol 52:3743–3748. doi: 10.1128/JCM.01989-14 Epub 2014 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parekh B, Anyanwu J, Patel H, Downer M, Kalow M, Gichimu C, Ou C, Nkengasong J. 2010. Dried tube specimens: a simple cost-effective method for preparation of HIV proficiency testing panels and quality control materials for use in resource-limited settings. J Virol Methods 163:295–300. doi: 10.1016/j.jviromet.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance. 2015. Monitoring the impact of the HIV epidemic using population-based surveys. http://www.unaids.org/en/resources/documents/2015/population_based_surveys.
- 13.Giles RE, Perry KR, Parry JV. 1999. Simple/rapid devices for anti-HIV screening: do they come up to the mark? J Med Virol 59:104–109. [PubMed] [Google Scholar]
- 14.Piwowar-Manning E, Tustin N, Sikateyo P, Kamwendo D, Chipungu C, Maharaj R, Mushanyu J, Richardson BA, Hillier S, Jackson JB. 2010. Validation of rapid HIV antibody tests in 5 African countries. J Int Assoc Physicians AIDS Care (Chic). 9:170–172. doi: 10.1177/1545109710368151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO/CDC. 2015. Improving the quality of HIV-related point-of-care testing: ensuring the reliability and accuracy of test results. http://apps.who.int/iris/bitstream/10665/199799/1/9789241508179_eng.pdf?ua=1.