Abstract
Chronic wasting disease (CWD), a transmissible spongiform encephalopathy of cervids, was first documented nearly 50 years ago in Colorado and Wyoming and has since been detected across North America and the Republic of Korea. The expansion of this disease makes the development of sensitive diagnostic assays and antemortem sampling techniques crucial for the mitigation of its spread; this is especially true in cases of relocation/reintroduction or prevalence studies of large or protected herds, where depopulation may be contraindicated. This study evaluated the sensitivity of the real-time quaking-induced conversion (RT-QuIC) assay of recto-anal mucosa-associated lymphoid tissue (RAMALT) biopsy specimens and nasal brushings collected antemortem. These findings were compared to results of immunohistochemistry (IHC) analysis of ante- and postmortem samples. RAMALT samples were collected from populations of farmed and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni; n = 323), and nasal brush samples were collected from a subpopulation of these animals (n = 205). We hypothesized that the sensitivity of RT-QuIC would be comparable to that of IHC analysis of RAMALT and would correspond to that of IHC analysis of postmortem tissues. We found RAMALT sensitivity (77.3%) to be highly correlative between RT-QuIC and IHC analysis. Sensitivity was lower when testing nasal brushings (34%), though both RAMALT and nasal brush test sensitivities were dependent on both the PRNP genotype and disease progression determined by the obex score. These data suggest that RT-QuIC, like IHC analysis, is a relatively sensitive assay for detection of CWD prions in RAMALT biopsy specimens and, with further investigation, has potential for large-scale and rapid automated testing of antemortem samples for CWD.
INTRODUCTION
Transmissible spongiform encephalopathies are a group of progressively fatal neurodegenerative diseases caused by infectious proteins known as prions (1). The pathogenesis of prion diseases involves conversion of the endogenous cellular prion protein (PrPC) present within specific tissues to the abnormal, protease-resistant form (PrPres) following exposure to an infectious dose of PrPres (1). Chronic wasting disease (CWD), a naturally occurring prion disease of white-tailed deer (Odocoileus virginianus), mule deer (Odocoileus hemionus), Rocky Mountain elk (Cervus elaphus nelsoni), and moose (Alces alces), is the only known prion disease affecting free-ranging, nondomestic animals (2, 3). CWD was first described nearly 50 years ago as a fatal, wasting, spongiform encephalopathy of cervids in Colorado and Wyoming (4). The disease has since been documented in 23 U.S. states, 2 Canadian provinces, and, via exportation of farmed cervids, the Republic of Korea (5–8). Four of the 23 states (Texas, Iowa, Pennsylvania, and Ohio) were considered CWD free prior to 2012, with primary cases in three of these states reportedly arising in farmed cervids (9–11). With the movement of cervids across state and national borders, these new epidemic foci illustrate the increased need for highly sensitive surveillance methods and appropriate antemortem tissue collection in order to potentially mitigate both natural and anthropogenic spread and more accurately estimate prevalence.
Presently, there is significant variation in the prevalence of CWD throughout North America, with levels ranging from 0 to 30% in wild populations and approaching 80% in specific captive populations (12, 13). Current prevalence rates are dependent on the use of conventional diagnostic assays, including enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry (IHC) analysis—two assays similar in sensitivity and specificity that utilize a proteolytic pretreatment step to abolish PrPC cross-reactivity (14). Despite specificities nearing 100% with these assays (14, 15), it is generally acknowledged that these pretreatments may lead to underestimation of the level of PrPres in a given sample (16–19). This shortcoming has led to the development of assays that utilize amplification of PrPres (e.g., serial protein misfolding cyclic amplification [18, 20]), fluorometric quantitation of seeding activity (e.g., real-time quaking-induced conversion [RT-QuIC] assay [21–23]), or other methods devoid of harsh proteolytic treatments (e.g., the conformation-dependent immunoassay [24]). While the specificities of these assays also approach 100%, studies to specifically identify sensitivity are difficult without costly bioassay studies. As a result, the true sensitivities of conventional IHC analysis and ELISA, as well as experimental detection assays, are difficult to estimate. To date, experimental detection assays have not been reported in conventional surveillance for CWD, such assays have the potential for increased sensitivity and earlier detection of CWD-positive animals (17, 18), an important component of surveillance and detection protocols.
Aside from the selection of a sensitive diagnostic assay for disease detection, definitive diagnosis also requires appropriate tissue collection (25–27). In most species, the obex, a region of the caudal brainstem containing the dorsal motor nucleus of the vagus nerve, is generally considered the most sensitive region of the central nervous system for detection of PrPres, 100% given the above caveats (28–30). However, several studies utilizing IHC analysis of the medial retropharyngeal lymph nodes (RLN) have demonstrated a species-dependent improvement in sensitivity over the brainstem/obex for detection of the infectious prion protein of CWD (PrPCWD) in cervids (25, 27). In white-tailed deer, RLN tissues appear to offer nearly 100% sensitivity for the detection of CWD infection (12, 26, 31), while in elk, upwards of 12% of positive animals may have PrPres deposition limited to the brainstem at the time of necropsy (25). Unfortunately, the brainstem and RLN, the two tissues of choice for sensitivity, are currently available only as postmortem samples. This limitation makes these tissues problematic for understanding epidemiology through population surveillance and individual screening in areas without hunting or culling practices. For this reason, major efforts have been undertaken to identify peripheral lymphoid tissues for antemortem collection and diagnosis which may exhibit sensitivities comparable to those of the brainstem/RLN, including third-eyelid, tonsil, and recto-anal mucosa-associated lymphoid tissue (RAMALT) samples (27, 32–37). Previous studies have additionally demonstrated high levels of PrPres in olfactory epithelium and nasal secretions in several prion diseases (38–48), though this prospect has not been assessed with CWD. Both RAMALT biopsy specimens and nasal brush samples collected from the olfactory epithelium are easily and efficiently collected and processed, making these tissues promising additions in the area of antemortem detection of prion diseases and the samples of choice for our study.
In the present study, we applied a standardized RT-QuIC assay to blindly examine RAMALT biopsy specimens collected from 316 Rocky Mountain elk (Cervus elaphus nelsoni) and nasal brush samples from a subpopulation of 205 of these elk. RT-QuIC has previously been shown to efficiently amplify and detect PrPres/CWD in a number of tissues and bodily fluids, including cerebrospinal fluid (CSF), urine, saliva, blood, brain tissue, lymph node tissue, and nasal lavage fluid/swabs (21, 38, 39, 47, 49–54), but to our knowledge, this is the first study to utilize RAMALT biopsy specimen tissue homogenates and nasal brush preparations for amplification and detection of PrPCWD by RT-QuIC. RT-QuIC results were subsequently correlated with ante- and postmortem IHC analysis results obtained with RAMALT, RLN, and brainstem samples at the level of the obex (including obex scoring) and the PRNP genotype (27, 55). We hypothesized that the sensitivity of RT-QuIC in antemortem samples would correlate with postmortem IHC analysis of these animals, with our findings demonstrating a relatively rapid and sensitive detection of PrPCWD in both RAMALT and nasal epithelial brush samples collected.
MATERIALS AND METHODS
Study populations.
The first group of animals consisted of a population of farmed elk with a recent history of CWD that was identified in Saskatchewan (n = 120), in an area with a history of endemic CWD. This population included 40 calves, 38 adult bulls, and 42 adult cows. The second group of animals consisted of a population of elk from a study area described previously (27) and consisted of adult female free-ranging elk in Rocky Mountain National Park (RMNP) that were initially captured and sampled (n = 136) or recaptured in later study years for supplemental sample collection (n = 39) and released with radio collars during the winters of 2012 to 2014. Two additional females from RMNP showing clinical signs suggestive of CWD were sampled perimortem. A third and separate free-ranging study population in an area of North Dakota (Theodore Roosevelt National Park [TRNP]) where CWD is not known to occur provided for negative control RAMALT biopsy specimens (n = 28) and nasal brushings (n = 16) collected perimortem (Table 1). All samples from each group were collected with single-use instruments and included RAMALT (n = 323) and nasal brush samples (n = 205), in accordance with IACUC protocols and state/federal permits (IACUC protocols KSU3503 and IMR_ROMO_Monello_Elk_11/21/2011, National Park Service permits ROMO-2012-SCI-0064 and THRO-2012-SCI-0008, and Colorado Parks and Wildlife permit 13TR2088). Blood collected by cephalic or jugular venipuncture was used to determine the elk PRNP genotype (specifically, PRNP position 132 methionine [M] or leucine [L]) as described by O'Rourke et al. (56, 57). Elk were ultimately assessed for CWD via IHC analysis of RAMALT tissue (antemortem) or RLN and brainstem samples at the level of the obex (postmortem). Free-ranging animals determined to be CWD positive were monitored until death or humane euthanasia when exhibiting end-stage clinical signs of CWD. Brainstem and RLN samples were collected from these animals for confirmatory IHC analysis. Farmed animals were humanely euthanized immediately following sample collection.
TABLE 1.
Summary of study populations, including sex, samples collected, and postmortem CWD status as determined by obex and retropharyngeal lymph node IHC analysisa
| Group | No. of males | No. of females | No. of RAMALT samples | No. of nasal brush samples | No. CWD positive (postmortem) | No. CWD negative (postmortem) |
|---|---|---|---|---|---|---|
| Canada | 59 | 61 | 120 | 120 | 44 | 76 |
| RMNP | 0 | 136 (39)b | 136 (39) | 66 (3) | 5 | 170 |
| TRNP | 0 | 28 | 28 | 16 | 0 | 28 |
The number of RAMALT samples collected from females at RMNP included 136 initial and 39 follow-up biopsy specimens; nasal brush samples included 66 initial collections and 3 follow-up collections.
Values in parentheses represent the number of repeat samples collected in subsequent years.
Tissue collection and processing.
Elk in both Canada and RMNP were immobilized with a combination of carfentanil and xylazine as previously described (27). Samples from TRNP elk were collected perimortem in the course of a herd management initiative. RAMALT biopsy specimens were collected by removing a 1.5-by-0.75-cm strip of mucosal tissue from the wall of the rectum approximately 1.0 cm anterior to the mucocutaneous junction of the anus and perpendicular to the cranial/caudal axis of the rectum (27). The sample was divided into two pieces, an approximately 0.5-by-0.5-cm section was frozen and maintained at −80°C, and the remainder was placed in 10% neutral buffered formalin prior to IHC analysis. Frozen RAMALT biopsy specimens were later prepared as an ∼2% homogenate in RT-QuIC dilution buffer (phosphate-buffered saline [PBS] with 0.05% SDS) with a TissueLyser II (Qiagen) with a single 5-mm stainless steel bead and 2-ml conical snap cap tubes with two 2-min cycles of homogenization at a power setting of 20. Homogenates were then maintained at −80°C until analysis by RT-QuIC.
Nasal brush samples were cleanly collected from the right nasal cavity contemporaneously with RAMALT biopsy specimens as follows. A sterile uterine single-sheathed cytology brush (Jorgenson Laboratories no. J0273C) was gently inserted into the right nasal vestibule, directed dorso-caudally through the dorsal nasal meatus, and fed in approximately 6 to 7 in. until located directly rostral to the ethmoid turbinate (Fig. 1A and B). At that time, the sampling brush was fed into the sheath and advanced until obstructed by the ethmoid turbinates. The brush was spun gently to collect turbinate epithelial tissue and retracted into the sheath, and the entire unit was removed from the nasal cavity. The brush tip was then placed in PBS and refrigerated at 4°C between collection and processing. The sample was processed by vortexing vigorously in PBS to remove and suspend cellular matter present on the brush. The cellular suspension was then centrifuged at 3,000 × g for 10 min at 4°C. The supernatant from the cellular suspensions was poured off, and the cellular pellet was resuspended in 0.5 ml of PBS and homogenized as described above. Homogenates were then maintained at −80°C until analysis by RT-QuIC.
FIG 1.

In vivo (A) and ex vivo (B) demonstrations of nasal brush collection on Rocky Mountain elk.
RAMALT biopsy specimen, RLN tissue, and brainstem/obex tissue IHC analysis.
Reference tissues were assayed for PrPCWD by IHC analysis as previously described (37, 58). Briefly, tissue was preserved in 10% neutral buffered formalin and then embedded in paraffin blocks. Cross sections 5 μm thick were mounted on glass slides and deparaffinized before treatment with 99% formic acid for chemical denaturation of PrPC. IHC staining for PrPCWD was performed with the primary antibody Anti-prion 99 (Ventana Medical Systems, Tucson, AZ) and then counterstained with hematoxylin. Biopsy specimens were considered positive if at least one follicle exhibited PrPCWD-specific staining (58). The numbers of staining and nonstaining follicles in each RAMALT biopsy specimen were documented. Samples not demonstrating IHC staining were considered CWD “not detected.” The same protocol was used for postmortem brainstem/obex and RLN analysis, with obex sections scored on a PrPCWD deposition scale of 0 (no PrPCWD staining) to 4 (heavy accumulation of PrPCWD) as previously described (59).
RT-QuIC preparation and procedure.
RT-QuIC assays were performed with a truncated form of recombinant Syrian hamster PrP (SHrPrP; residues 90 to 231) in pET41b and expressed and purified as previously described (47). In brief, 1-liter cultures of lysogeny broth (LB) containing autoinduction supplements (EMD Biosciences) were inoculated with SHrPrP-expressing Rosetta strain Escherichia coli, which was grown overnight and harvested when an optical density at 600 nm of ∼3 was reached. Cells were lysed with BugBuster reagent with supplemented Lysonase (EMD Biosciences), and inclusion bodies (IB) were harvested by centrifugation of the lysate at 15,000 × g. IB pellets were washed twice and solubilized overnight in 8 M guanidine hydrochloride (GuHCl) in 100 mM NaPO4 and 10 mM Tris (pH 8.0), clarified by centrifugation at 15,000 × g for 15 min, and added to Superflow nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen) preequilibrated with denaturing buffer (6.0 M GuHCl, 100 mM NaPO4, 10 mM Tris, pH 8.0). Denatured SHrPrP and Ni-NTA resin were incubated by rotation at room temperature for 1 h and then added to an XK fast protein liquid chromatography column (GE Healthcare). Refolding was achieved on column with a linear refolding gradient of denaturing buffer (6 M GuHCl, 100 mM NaPO4, 10 mM Tris, pH 8.0) to refolding buffer (100 mM NaPO4, 10 mM Tris, pH 8.0) over 3 h at 1.5 ml/min. SHrPrP was eluted with a linear gradient of refold buffer to elution buffer (100 mM NaPO4, 10 mM Tris [pH 8.0], 500 mM imidazole [pH 5.8]) over 40 min at 2.0 ml/min. Peak UV 280-nm fractions were pooled and dialyzed overnight against two changes of 4.0 liters of dialysis buffer (20 mM NaPO4, pH 5.8). Recovered SHrPrP was adjusted to a final concentration of ∼0.5 mg/ml and stored at 4°C for up to 45 days. Purity was evaluated through analyses of fast protein liquid chromatography spectroscopy and Western blotting profiles but most importantly through functionality in the RT-QuIC assay. Seeded amplification with a positive control consisting of pooled CWD-positive brain tissue from six experimentally infected white-tailed deer (cervid brain pool 6 [CBP6]) was evaluated in each experimental run to confirm the consistency and repeatability of the amplification rate, reproducibly amplifying in triplicate between cycles 20 and 24 (data not shown).
Nasal brush preparations were diluted 1:10 in RT-QuIC dilution buffer, while RAMALT homogenates were diluted 1:100 in RT-QuIC dilution buffer. Five microliters of this 10−1 or 10−2 dilution was added to 95 μl of RT-QuIC reaction buffer consisting of 50 mM NaPO4, 350 mM NaCl, 1.0 mM EDTA tetrasodium salt, 10 μM thioflavin T (ThT), and 0.1 mg/ml truncated SHrPrPC to yield a final volume of 100 μl. Each sample was tested in triplicate on a single plate in two separate experiments. Nasal brushings were repeated at two different institutions (Kansas State University [KSU], Rocky Mountain Laboratories [RML]). Positive controls consisting of 5 μl of a 10−3 dilution of CBP6 spiked into 95 μl of RT-QuIC reaction buffer were included in triplicate in each experiment. Negative controls, also prepared in triplicate, consisted of RAMALT biopsy specimens or nasal brush samples collected from elk known to be negative (confirmed by IHC analysis of brainstem or RLN tissue) from an area where CWD has not been reported (TRNP), as well as untreated RT-QuIC reaction buffer spiked with 5 μl of RT-QuIC dilution buffer. Reactions were prepared in a black 96-well optical-bottom plate that was then sealed and incubated in a BMG Labtech Polarstar fluorimeter at 42°C for 24 h (96 15-min cycles) with intermittent shaking cycles; specifically, 1-min shaking periods (700 rpm, double orbital pattern) alternating with 1-min rest periods. ThT fluorescence measurements (450-nm excitation and 480-nm emission wavelengths) were taken every 15 min with the gain set at 1,200. The relative fluorescence (in relative fluorescence units) of each triplicate sample was progressively monitored against time with orbital averaging and 20 flashes/well at the 4-mm setting.
A replicate well was considered positive when the relative fluorescence crossed a predefined positive threshold, calculated as 10 standard deviations above the mean fluorescence of all of the sample wells from cycles 2 to 8. Positive samples were considered those crossing the threshold in ≥2/6 replicates for both RAMALT and nasal brush analyses.
Correlation of RT-QuIC results with PrPCWD IHC analysis, obex scoring, and the PRNP genotype.
Considering only our findings from farmed elk, we sought to examine if RT-QuIC results from RAMALT and collected nasal brush samples could be associated with a number of predictor variables, including RAMALT, RLN, and brainstem/obex IHC analysis results; the obex score; and the PRNP genotype. We used Spearman correlations to assess the relationship and direction of relationship between RT-QuIC results obtained with RAMALT and nasal brush samples to conventional postmortem methods of detecting CWD infection and to compare results of assays performed at different institutions.
RESULTS
CWD-positive population data and IHC analyses.
In the herd of 120 farmed elk, 25 (41%) of the 61 elk cows examined were positive by RLN or brainstem/obex IHC analysis or both, while 19 (32.2%) of 59 bulls were considered positive. Forty (43.5%) of 92 animals that had a PrP 132MM genotype were CWD positive in the obex or RLN by IHC analysis, and 33/40 (82.5%) were RAMALT positive by IHC analysis. Four (14.2%) of 28 132ML elk were CWD positive by postmortem obex/RLN analysis, and just 1/4 was identified by positive follicular staining of RAMALT in IHC analysis. Elk identified as positive postmortem had obex scores ranging from 0 to 4 (Tables 1 and 2).
TABLE 2.
Summary of testing data for farmed and free-ranging elka
| Group | No. negative |
No. positive |
No. with IHC analysis of: |
No. with obex score of: |
No. tested byRT-QuIC |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | RAMALT | RLN | Obex | RLN+ Obex− | RLN− Obex+ | 0 | 1 | 2 | 3 | 4 | RAMALT | NB |
||
| KSU | RML | ||||||||||||||||
| Canada | |||||||||||||||||
| 132MM | 26 | 26 | 18 | 22 | 33 | 37 | 35 | 5 | 3 | 5 | 3 | 2 | 10 | 20 | 33 | 15 | 14 |
| 132ML | 14 | 10 | 1 | 3 | 1 | 4 | 1 | 3 | 0 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| RMNP | |||||||||||||||||
| 132MM | NAc | NA | 4 | 4 | 4 | 4 | 0 | 0 | NA | 4 | 2b | 2b | |||||
| 132ML | NA | NA | 1 | 1 | 1 | 1 | 0 | 0 | NA | 1 | 0 | 0 | |||||
Ante- and postmortem RT-QuIC and IHC test results were highly correlative. Testing of RAMALT and nasal brushing (NB) samples also correlated highly with both the obex score and the PRNP genotype at position 132. All animals positive by RAMALT testing were also positive by RLN IHC analysis, while some obex-positive animals were RLN negative and vice versa. Neg, chronic wasting disease negative; Pos, chronic wasting disease positive; M, male; F, female.
These positive samples were from a subpopulation of the overall sample population of free-ranging elk, and represented the only two positive elk for which nasal brushings were acquired.
NA, not available.
Of the animals sampled at RMNP, five were identified as CWD positive by RAMALT IHC analysis and eventually confirmed through postmortem IHC analysis of the brainstem and RLN. All brainstem/obex samples of CWD-positive free-ranging cow elk were considered highly positive, indicating disseminated infection and central nervous system accumulation of PrPCWD at the time of euthanasia or death (Fig. 2A and B). Four of these CWD-positive animals were homozygous for methionine at PRNP codon 132 (132MM), while a fifth was heterozygous at this position (132ML).
FIG 2.
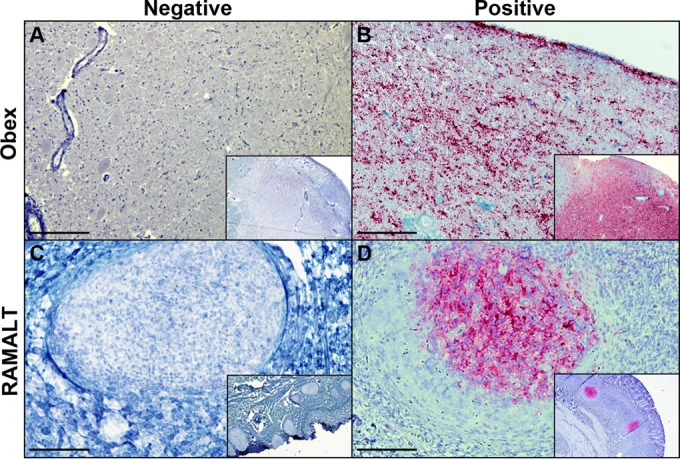
IHC detection of PrPCWD in brainstem (obex) and RAMALT samples by previously described protocols. Panels: A, CWD-negative obex section of an elk from RMNP; B, obex section of an elk from RMNP showing heavy accumulation of material staining positive for PrPCWD; C, RAMALT biopsy specimen from an elk from RMNP showing negative staining for PrPCWD; D, CWD-positive RAMALT biopsy specimen from an elk from RMNP showing heavy accumulation of material staining positive for PrPCWD. IHC analysis was performed with anti-prion 99 antibody (Ventana Medical Systems, Tucson, AZ). Bars = 250 μm.
RT-QuIC analysis of RAMALT biopsy specimens.
Biopsy specimens from 34/120 farmed elk were positive by RT-QuIC in 3/3 replicates in two separate experiments. Of these RT-QuIC-positive biopsy specimens, 33 were also positive by IHC analysis, though there was an additional specimen positive by IHC analysis that was RT-QuIC negative; RAMALT RT-QuIC correlated 96% with RAMALT IHC analysis (Tables 2 and 3) in this group.
TABLE 3.
Spearman correlation between CWD-positive animals and clinical variablesa
| Correlation | Obex | RLN | RAMALT |
NB RT-QuIC |
132MM | 132ML | Obex score of: |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHC analysis | RT-QuIC | KSU | RML | 0 | 1 | 2 | 3 | 4 | |||||
| Positiveb | 0.86 (<0.001)c | 0.95 (<0.001) | 0.83 (<0.001) | 0.83 (<0.001) | 0.50 (<0.001) | 0.48 (<0.001) | 0.26 (0.19) | −0.26 (0.19) | −0.86 (<0.001) | 0.24 (0.25) | 0.17 (1.00) | 0.40 (<0.001) | 0.59 (<0.001) |
| RAMALT IHC analysis | 0.92 (<0.001) | 0.87 (<0.001) | 1.0 (<0.001) | 0.96 (<0.001) | 0.60 (<0.001) | 0.58 (<0.001) | 0.30 (0.04) | −0.30 (0.04) | −0.92 (<0.001) | −0.01 (1.0) | 0.21 (0.69) | 0.48 (<0.001) | 0.71 (<0.001) |
| 132MM | 0.32 (<0.001) | 0.23 (0.01) | 0.30 (<0.001) | 0.30 (<0.001) | 0.21 (0.02) | 0.20 (0.03) | 1.0 (<0.001) | −1.0 (<0.001) | −0.32 (0.02) | −0.01 (1.0) | 0.07 (1.0) | 0.17 (1.0) | 0.25 (0.24) |
| 132ML | −0.32 (<0.001) | −0.23 (0.01) | −0.30 (<0.001) | −0.30 (<0.001) | −0.21 (0.02) | −0.20 (0.03) | −1.0 (<0.001) | 1.0 (<0.001) | 0.32 (0.02) | 0.01 (1.0) | −0.07 (1.0) | −0.17 (1.0) | −0.25 (0.24) |
Including postmortem IHC results, antemortem IHC and RT-QuIC analyses of RAMALT and nasal brushings (NB), the PRNP 132 genotype, and the stage of clinical disease as assessed by obex score.
Correlation applies to tissue correlated with either an obex or an RLN sample that was positive for CWD by IHC analysis.
Shown are correlations (P values).
Initial biopsy specimens from 5/136 elk from RMNP (each positive by IHC analysis) showed evidence of prion amplification in 3/3 replicates in two separate experiments (Table 2). None of the 39 animals sampled on recapture or of the 28 sampled from TRNP were considered positive by RT-QuIC or IHC analysis.
RT-QuIC analysis of nasal brush samples.
Nasal brush samples collected from 120 farmed elk in Canada, 69 elk in RMNP, and 16 in TRNP were analyzed by a modified RT-QuIC assay as described above. Brush samples collected from farmed elk were positive in 15/120 (12.5%, KSU) or 14/120 (11.7%, RML) cases. Spearman correlation of the results from the two institutions was significant, with a coefficient of 0.883 (P < 0.001). Initial brush samples collected from 2/66 elk from RMNP—animals whose RAMALT samples were positive by IHC analysis and whose brainstems were positive by RT-QuIC and IHC analysis—produced amplification in 3/3 replicates, in two separate experiments. In this subgroup of the larger group of RMNP elk, no other CWD-positive animals were identified through analyses of ante- or postmortem tissues (i.e., these two positive nasal brushings represented the only CWD-positive animals in this subgroup). Likewise, no positive elk that represented RMNP recaptures (0/3) or those sampled from TRNP (0/16) were identified (Fig. 3; Table 2).
FIG 3.
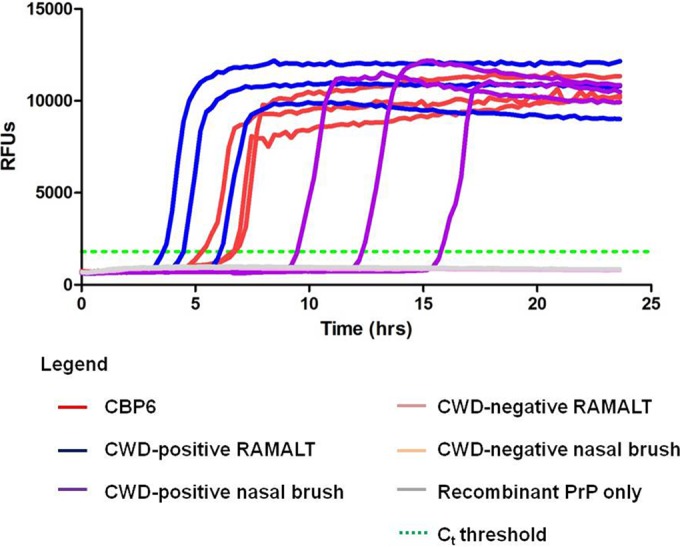
Prion-seeded RT-QuIC amplification of RAMALT and nasal brush samples. CBP6 acted as the positive control. The data are from elk 819 from RMNP, which was ante- and postmortem IHC analysis positive for CWD. Recombinant PrP, SHrPrP. Ct threshold, threshold cycle calculated as 10 standard deviations above the mean fluorescence of all of the samples through cycles 2 to 8.
Correlation of RT-QuIC results with RAMALT, RLN, and obex IHC analysis results; obex scores; and PRNP genotypes.
There was a positive correlation (96%) between RAMALT RT-QuIC and IHC analysis results for farmed elk in Canada, an RT-QuIC result obtained with RAMALT from a single animal that failed to detect PrPCWD that was identified by RAMALT IHC analysis and vice versa. RT-QuIC results obtained with RAMALT were negatively correlated with an obex score of 0 (−92%) but positively correlated with obex scores of 3 (48%) and 4 (71%). RT-QuIC results obtained with RAMALT were positively correlated with the 132MM genotype (30%) but negatively correlated with the 132ML genotype (−30%). RT-QuIC results obtained with nasal brush samples were not as reliable in detecting PrPCWD as RT-QuIC results obtained with RAMALT in comparison with RLN and brainstem/obex IHC analyses at 52 and 58%, respectively. However, RT-QuIC results obtained with nasal brush samples were negatively correlated (−58%) with an obex score of 0 but positively correlated (64%) with an obex score of 4 (Fig. 4 and 5 Table 3).
FIG 4.
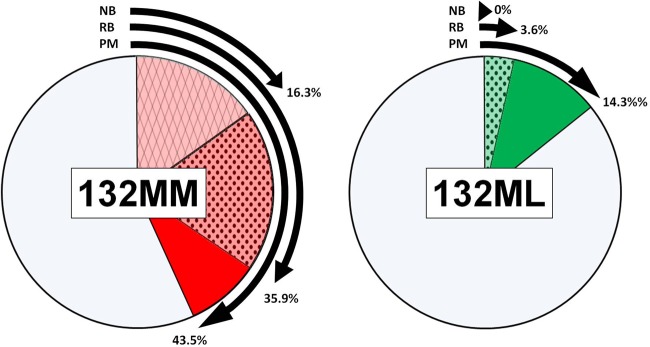
Prevalence of CWD in farmed elk PRNP 132 alleles based on RT-QuIC amplification of nasal brush (NB) samples, RAMALT biopsy (RB) specimens, or postmortem (PM) IHC analysis. CWD was more prevalent in 132MM elk; higher sensitivities in RAMALT biopsy specimens and nasal brush analyses were also observed in this genotype.
FIG 5.
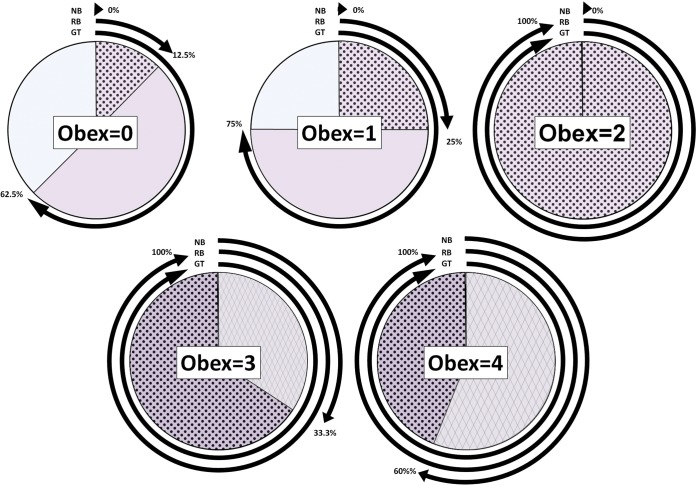
Associations of obex scores with antemortem testing and the genetic background of farmed elk. As obex scores increased, a greater proportion of positive 132MM animals was observed, along with a higher sensitivity observed through both nasal brush and RAMALT biopsy specimen analyses. NB, nasal brush analysis by RT-QuIC; RB, RAMALT biopsy specimen analysis by RT-QuIC; GT, 132MM allele proportion among animals identified as CWD positive.
DISCUSSION
The geographic distribution and/or detection of CWD has been progressively expanding in captive and free-ranging populations since its initial documentation in Colorado and Wyoming nearly 50 years ago (4, 60). Increased surveillance efforts during the past several years have led to the detection of new cases in U.S. states previously thought to be outside the area where CWD is endemic (e.g., Texas, Iowa, and Pennsylvania in 2012 and Ohio in 2014) (9–11). As this devastatingly fatal disease spreads across the United States and beyond, the importance of highly sensitive antemortem detection becomes increasingly evident. This study sought to evaluate the use of RT-QuIC as a fast, efficient, and highly sensitive PrPCWD detection assay, with the incorporation of RAMALT and nasal brush samples as useful antemortem target samples.
The results of this study support the hypothesis that RAMALT RT-QuIC exhibits a sensitivity comparable to that of RAMALT IHC analysis for the antemortem detection of CWD infection in elk. Of the 49 animals identified postmortem as CWD positive in the present study, RT-QuIC found seeded amplification in 39 RAMALT biopsy specimens collected antemortem—revealing a sensitivity of 79.6% compared to postmortem testing. No animal considered negative through postmortem testing was positive by antemortem RT-QuIC, indicating a high specificity for CWD infection. With further development, it seems possible that RT-QuIC could have the potential for continued improvement in sensitivity over conventional methods, and while it is seemingly approaching the limits of sensitivity with RAMALT samples, it may prove useful for the identification of CWD prions in other antemortem samples. This is highlighted by the significant progress made in the field of RT-QuIC analysis within the last several years, demonstrating its utility for the identification of prion seeding activity in a multitude of tissues, including CSF, urine, saliva, blood, brain, lymph node tissue, and nasal lavage fluid/swabs (21, 38, 39, 47, 49–54). Additionally, intraassay variability has proven to be low (23, 61), and with the ability to run a large number of samples simultaneously, generating rapid (<24 h), quantitative results, RT-QuIC is a fast, easy, and user-friendly assay with potential for widespread application in CWD research and monitoring.
While confirming its limitations, this study offers additional support for the use of RAMALT as diagnostic tissue. There have been a number of previous studies of elk demonstrating the sensitivity of RAMALT compared to postmortem evaluation (27, 37, 58), making this a potentially useful antemortem sample for understanding the epizootiology of the disease and for management of captive herds in areas where CWD is endemic. While lymphoid follicle counts in RAMALT biopsy specimens have been shown to decline with age (62) and the sensitivity of RAMALT is decreased in cases of early infection (25, 27, 58) and in animals with specific PRNP alleles, these limitations should not preclude the continued evaluation of RAMALT as an antemortem testing tissue. Ultimately, shortcomings in sensitivity may be overcome through continued development of the RT-QuIC or similar assays—specifically, as progress is made on amplification substrates that may enhance diagnostic sensitivity. However, it should be acknowledged that current and past studies indicate that detectable prions may not accumulate in currently employed peripheral tissues from some proportion of animals or until very late in the course of clinical disease, and as a result, either IHC analysis or RT-QuIC may perpetually fall short of a perfect sensitivity critical for use in a screening assay prior to animal movement.
In the present study, the sensitivity of nasal brush sample analysis was quite low compared to that of other antemortem and postmortem sample analyses and to the apparently high sensitivity reported with human Creutzfeldt-Jakob disease (CJD) cases (48), which indicates that it is unsuitable for use in CWD surveillance. Although the anatomic target of nasal brush sampling—the rostral ethmoid turbinates—is a reported site of olfactory epithelium in ruminants (63, 64), it is possible that our sampling technique failed to appropriately collect from this area without rhinoscopic assistance. Alternatively, there may be a delay in the appearance of amplifiable PrPres in olfactory epithelium, as has been suggested for RAMALT. Ongoing studies may help further assess the quality of the olfactory epithelium of cervids and define the kinetics of prion accumulation in nasal tissues. Despite the low sensitivity, the correlation to the obex score (and thus the clinical stage of disease) (31) should not be overlooked. We found the highest sensitivity, 60%, in advanced cases of CWD in elk, with a steady decline toward earlier, preclinical stages of disease. This likely translates to the potential utility of nasal brush samples collected for the diagnosis of CJD—in that preclinical screening of individuals with a genetic predisposition for, or a history of iatrogenic exposure to, prion diseases may not be as fruitful as examination of individuals showing overt clinical symptoms.
Recently, additional large- and small-scale depopulation efforts have been undertaken to reduce the impact of CWD in captive and free-ranging cervid herds. In some cases, where the incidence of CWD is likely to have been low, these efforts have proven successful (65, 66). In many cases, however, depopulation efforts were unable to control the spread of CWD in susceptible populations (67, 68). With the demonstrated link between PRNP alleles, susceptibility, and antemortem test sensitivity, a combination of antemortem testing, genetic screening, and selective breeding in farmed herds may help reduce the dependence on depopulation regimes. Future antemortem test developments would prove critical in cases of cervid trade, relocation, or reintroduction, in which case euthanasia and postmortem testing are not an option. The unintentional transfer of CWD between Canada and the Republic of Korea, for example, might have been prevented if a perfectly sensitive antemortem test had been available (7). CWD control in free-ranging cervid herds presents a more complex problem because of animal inaccessibility and seasonal migration. The incorporation of antemortem testing strategies could be beneficial, however, when prevalence rates are high or depopulation efforts are contraindicated, as with protected herds. Despite the lower sensitivity of antemortem samples compared to postmortem tissue collection, antemortem tests remain an important tool for monitoring prevalence, mitigating spread of the disease, and developing an expanded understanding of CWD resistance.
In summary, we report the antemortem detection of prion seeding activity by RT-QuIC in RAMALT and nasal brush samples collected from CWD-positive elk. Seeded amplification results from antemortem samples were comparable to those arrived at by IHC analysis with common samples, though both were less sensitive than postmortem testing. As has been reported previously, the stage of clinical disease and the PRNP genotype can have a strong influence on antemortem test sensitivity—a finding that could directly translate to efforts to identify preclinical patients at risk of CJD. Although significantly less sensitive than RAMALT biopsy specimen testing, nasal brushing offers the benefits of ease of sample collection, reduced trauma, and simplicity in its use of disposable equipment and sample processing. The employment of antemortem sample collection and testing would be beneficial in better understanding of CWD in cervids across North America, especially as diagnostic techniques—including the RT-QuIC assay—improve.
ACKNOWLEDGMENTS
We sincerely thank the elk farmers and both Harvey Petracek and Travis Lowe with the North American Elk Breeders Association and Elk Research Council for their cooperation in completing this study. Although a finding of CWD on a farm is trying on many levels, emotionally, socially, politically, and economically, without their assistance this work could not have been performed. Alex McIsaac with the Canadian Food Inspection Agency was instrumental in sample collections in Canada. The assistance of Candace Mathiason with Colorado State University was also invaluable for the procurement of samples. We also thank the staff at RMNP and TRNP who supported and enabled sample collection at those locations, especially Elizabeth Wheeler, as well as Terry Spraker with the Colorado State University Diagnostic Laboratory for his assistance with IHC evaluation of National Park Service samples.
This work is supported in part by NIH NCRR K01OD010994, the North American Elk Breeders Association and the Elk Research Council, Merial, and the Intramural Research Program of the NIAID.
The content of this report is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
REFERENCES
- 1.Johnson RT. 2005. Prion diseases. Lancet Neurol 4:635–642. doi: 10.1016/S1474-4422(05)70192-7. [DOI] [PubMed] [Google Scholar]
- 2.Miller MW, Williams ES, McCarty CW, Spraker TR, Kreeger TJ, Larsen CT, Thorne ET. 2000. Epizootiology of chronic wasting disease in free-ranging cervids in Colorado and Wyoming. J Wildl Dis 36:676–690. doi: 10.7589/0090-3558-36.4.676. [DOI] [PubMed] [Google Scholar]
- 3.Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG, Spowart RA, O'Rourke KI, Miller JM, Merz PA. 1997. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis 33:1–6. doi: 10.7589/0090-3558-33.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Sigurdson CJ. 2008. A prion disease of cervids: chronic wasting disease. Vet Res 39:41. doi: 10.1051/vetres:2008018. [DOI] [PubMed] [Google Scholar]
- 6.Argue CK, Ribble C, Lees VW, McLane J, Balachandran A. 2007. Epidemiology of an outbreak of chronic wasting disease on elk farms in Saskatchewan. Can Vet J 48:1241–1248. [PMC free article] [PubMed] [Google Scholar]
- 7.Kim TY, Shon HJ, Joo YS, Mun UK, Kang KS, Lee YS. 2005. Additional cases of chronic wasting disease in imported deer in Korea. J Vet Med Sci 67:753–759. doi: 10.1292/jvms.67.753. [DOI] [PubMed] [Google Scholar]
- 8.Walsh DP, Miller MW. 2010. A weighted surveillance approach for detecting chronic wasting disease foci. J Wildl Dis 46:118–135. doi: 10.7589/0090-3558-46.1.118. [DOI] [PubMed] [Google Scholar]
- 9.Thorne L, Holder T, Ramsay A, Edwards J, Taema MM, Windl O, Maddison BC, Gough KC, Terry LA. 2012. In vitro amplification of ovine prions from scrapie-infected sheep from Great Britain reveals distinct patterns of propagation. BMC Vet Res 8:223. doi: 10.1186/1746-6148-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith D. 20 July 2012. CWD found for 1st time in Iowa. Star Tribune Media Co., Minneapolis, MN. [Google Scholar]
- 11.Romeo T. 10 October 2012. Pennsylvania officials have announced what they say is the state's 1st confirmed case of a deer found to be suffering from a disorder similar to “mad cow” disease. CBS Local Media, Philadelphia, PA. [Google Scholar]
- 12.Keane DP, Barr DJ, Bochsler PN, Hall SM, Gidlewski T, O'Rourke KI, Spraker TR, Samuel MD. 2008. Chronic wasting disease in a Wisconsin white-tailed deer farm. J Vet Diagn Invest 20:698–703. doi: 10.1177/104063870802000534. [DOI] [PubMed] [Google Scholar]
- 13.Miller MW, Swanson HM, Wolfe LL, Quartarone FG, Huwer SL, Southwick CH, Lukacs PM. 2008. Lions and prions and deer demise. PLoS One 3:e4019. doi: 10.1371/journal.pone.0004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hibler CP, Wilson KL, Spraker TR, Miller MW, Zink RR, DeBuse LL, Andersen E, Schweitzer D, Kennedy JA, Baeten LA, Smeltzer JF, Salman MD, Powers BE. 2003. Field validation and assessment of an enzyme-linked immunosorbent assay for detecting chronic wasting disease in mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), and Rocky Mountain elk (Cervus elaphus nelsoni). J Vet Diagn Invest 15:311–319. doi: 10.1177/104063870301500402. [DOI] [PubMed] [Google Scholar]
- 15.Spraker TR, O'Rourke KI, Balachandran A, Zink RR, Cummings BA, Miller MW, Powers BE. 2002. Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest 14:3–7. doi: 10.1177/104063870201400102. [DOI] [PubMed] [Google Scholar]
- 16.Safar JG, Geschwind MD, Deering C, Didorenko S, Sattavat M, Sanchez H, Serban A, Vey M, Baron H, Giles K, Miller BL, Dearmond SJ, Prusiner SB. 2005. Diagnosis of human prion disease. Proc Natl Acad Sci U S A 102:3501–3506. doi: 10.1073/pnas.0409651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. 2009. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One 4:e7990. doi: 10.1371/journal.pone.0007990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haley NJ, Mathiason C, Carver S, Telling GC, Zabel MC, Hoover EA. 2012. Sensitivity of protein misfolding cyclic amplification versus immunohistochemistry in antemortem detection of CWD infection. J Gen Virol 93:1141–1150. doi: 10.1099/vir.0.039073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selariu A, Powers JG, Nalls A, Brandhuber M, Mayfield A, Fullaway S, Wyckoff CA, Goldmann W, Zabel MM, Wild MA, Hoover EA, Mathiason CK. 2015. In utero transmission and tissue distribution of chronic wasting disease-associated prions in free-ranging Rocky Mountain elk. J Gen Virol 96:3444–3455. doi: 10.1099/jgv.0.000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saborio GP, Permanne B, Soto C. 2001. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 21.Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S, Yamada M, Mizusawa H, Kitamoto T, Klug G, McGlade A, Collins SJ, Nishida N. 2011. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 17:175–178. doi: 10.1038/nm.2294. [DOI] [PubMed] [Google Scholar]
- 22.Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, Onwubiko HA, Priola SA, Caughey B. 2008. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods 5:211–212. doi: 10.1038/nmeth0308-211. [DOI] [PubMed] [Google Scholar]
- 23.Haley NJ, Carver S, Hoon-Hanks LL, Henderson DM, Davenport KA, Bunting E, Gray S, Trindle B, Galeota J, LeVan I, Dubovos T, Shelton P, Hoover EA. 2014. Detection of chronic wasting disease in the lymph nodes of free-ranging cervids by real-time quaking-induced conversion. J Clin Microbiol 52:3237–3243. doi: 10.1128/JCM.01258-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thackray AM, Hopkins L, Bujdoso R. 2007. Proteinase K-sensitive disease-associated ovine prion protein revealed by conformation-dependent immunoassay. Biochem J 401:475–483. doi: 10.1042/BJ20061264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spraker TR, Balachandran A, Zhuang D, O'Rourke KI. 2004. Variable patterns of distribution of PrP(CWD) in the obex and cranial lymphoid tissues of Rocky Mountain elk (Cervus elaphus nelsoni) with subclinical chronic wasting disease. Vet Rec 155:295–302. doi: 10.1136/vr.155.10.295. [DOI] [PubMed] [Google Scholar]
- 26.Keane DP, Barr DJ, Keller JE, Hall SM, Langenberg JA, Bochsler PN. 2008. Comparison of retropharyngeal lymph node and obex region of the brainstem in detection of chronic wasting disease in white-tailed deer (Odocoileus virginianus). J Vet Diagn Invest 20:58–60. doi: 10.1177/104063870802000110. [DOI] [PubMed] [Google Scholar]
- 27.Monello RJ, Powers JG, Hobbs NT, Spraker TR, O'Rourke KI, Wild MA. 2013. Efficacy of antemortem rectal biopsies to diagnose and estimate prevalence of chronic wasting disease in free-ranging cow elk (Cervus elaphus nelsoni). J Wildl Dis 49:270–278. doi: 10.7589/2011-12-362. [DOI] [PubMed] [Google Scholar]
- 28.Wells GA, Hancock RD, Cooley WA, Richards MS, Higgins RJ, David GP. 1989. Bovine spongiform encephalopathy: diagnostic significance of vacuolar changes in selected nuclei of the medulla oblongata. Vet Rec 125:521–524. doi: 10.1136/vr.125.21.521. [DOI] [PubMed] [Google Scholar]
- 29.Debeer SO, Baron TG, Bencsik AA. 2001. Immunohistochemistry of PrPsc within bovine spongiform encephalopathy brain samples with graded autolysis. J Histochem Cytochem 49:1519–1524. doi: 10.1177/002215540104901205. [DOI] [PubMed] [Google Scholar]
- 30.Chaplin MJ, Barlow N, Ryder S, Simmons MM, Spencer Y, Hughes R, Stack MJ. 2002. Evaluation of the effects of controlled autolysis on the immunodetection of PrP(Sc) by immunoblotting and immunohistochemistry from natural cases of scrapie and BSE. Res Vet Sci 72:37–43. doi: 10.1053/rvsc.2001.0518. [DOI] [PubMed] [Google Scholar]
- 31.Fox KA, Jewell JE, Williams ES, Miller MW. 2006. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol 87:3451–3461. doi: 10.1099/vir.0.81999-0. [DOI] [PubMed] [Google Scholar]
- 32.O'Rourke KI, Baszler TV, Besser TE, Miller JM, Cutlip RC, Wells GA, Ryder SJ, Parish SM, Hamir AN, Cockett NE, Jenny A, Knowles DP. 2000. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J Clin Microbiol 38:3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Keulen LJ, Schreuder BE, Meloen RH, Mooij-Harkes G, Vromans ME, Langeveld JP. 1996. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. J Clin Microbiol 34:1228–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill AF, Zeidler M, Ironside J, Collinge J. 1997. Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet 349:99–100. doi: 10.1016/S0140-6736(97)24002-X. [DOI] [PubMed] [Google Scholar]
- 35.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI, Hoover EA. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol 80(Pt 10):2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 36.González L, Dagleish MP, Bellworthy SJ, Siso S, Stack MJ, Chaplin MJ, Davis LA, Hawkins SA, Hughes J, Jeffrey M. 2006. Postmortem diagnosis of preclinical and clinical scrapie in sheep by the detection of disease-associated PrP in their rectal mucosa. Vet Rec 158:325–331. doi: 10.1136/vr.158.10.325. [DOI] [PubMed] [Google Scholar]
- 37.Spraker TR, Gidlewski TL, Balachandran A, VerCauteren KC, Creekmore L, Munger RD. 2006. Detection of PrP(CWD) in postmortem rectal lymphoid tissues in Rocky Mountain elk (Cervus elaphus nelsoni) infected with chronic wasting disease. J Vet Diagn Invest 18:553–557. doi: 10.1177/104063870601800605. [DOI] [PubMed] [Google Scholar]
- 38.Bessen RA, Shearin H, Martinka S, Boharski R, Lowe D, Wilham JM, Caughey B, Wiley JA. 2010. Prion shedding from olfactory neurons into nasal secretions. PLoS Pathog 6:e1000837. doi: 10.1371/journal.ppat.1000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bessen RA, Wilham JM, Lowe D, Watschke CP, Shearin H, Martinka S, Caughey B, Wiley JA. 2012. Accelerated shedding of prions following damage to the olfactory epithelium. J Virol 86:1777–1788. doi: 10.1128/JVI.06626-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corona C, Porcario C, Martucci F, Iulini B, Manea B, Gallo M, Palmitessa C, Maurella C, Mazza M, Pezzolato M, Acutis P, Casalone C. 2009. Olfactory system involvement in natural scrapie disease. J Virol 83:3657–3667. doi: 10.1128/JVI.01966-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeJoia C, Moreaux B, O'Connell K, Bessen RA. 2006. Prion infection of oral and nasal mucosa. J Virol 80:4546–4556. doi: 10.1128/JVI.80.9.4546-4556.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford MJ, Burton LJ, Morris RJ, Hall SM. 2002. Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience 113:177–192. doi: 10.1016/S0306-4522(02)00155-0. [DOI] [PubMed] [Google Scholar]
- 43.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 44.Perrott MR, Sigurdson CJ, Mason GL, Hoover EA. 2013. Mucosal transmission and pathogenesis of chronic wasting disease in ferrets. J Gen Virol 94:432–442. doi: 10.1099/vir.0.046110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabaton M, Monaco S, Cordone MP, Colucci M, Giaccone G, Tagliavini F, Zanusso G. 2004. Prion deposition in olfactory biopsy of sporadic Creutzfeldt-Jakob disease. Ann Neurol 55:294–296. doi: 10.1002/ana.20038. [DOI] [PubMed] [Google Scholar]
- 46.Zanusso G, Ferrari S, Cardone F, Zampieri P, Gelati M, Fiorini M, Farinazzo A, Gardiman M, Cavallaro T, Bentivoglio M, Righetti PG, Pocchiari M, Rizzuto N, Monaco S. 2003. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jakob disease. N Engl J Med 348:711–719. doi: 10.1056/NEJMoa022043. [DOI] [PubMed] [Google Scholar]
- 47.Wilham JM, Orrú CD, Bessen RA, Atarashi R, Sano K, Race B, Meade-White KD, Taubner LM, Timmes A, Caughey B. 2010. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog 6:e1001217. doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orrú CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, Fiorini M, Pocchiari M, Monaco S, Caughey B, Zanusso G. 2014. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med 371:519–529. doi: 10.1056/NEJMoa1315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atarashi R, Sano K, Satoh K, Nishida N. 2011. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion 5:150–153. doi: 10.4161/pri.5.3.16893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanco RA, De Wolf C, Tan B, Agarwal S, Orrú C, Caughey B, Raeber A, Gill A, Manson J, McCutcheon S. 2012. Analysis of BSE-infected sheep tissues and plasma using the real-time quaking induced conversion (RT-QuIC) assay. Prion 6:94. [Google Scholar]
- 51.John TR, Schatzl HM, Gilch S. 2013. Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion 7:253–258. doi: 10.4161/pri.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peden AH, McGuire LI, Appleford NEJ, Mallinson G, Wilham JM, Orrú CD, Caughey B, Ironside JW, Knight RS, Will RG, Green AJE, Head MW. 2012. Sensitive and specific detection of sporadic Creutzfeldt-Jakob disease brain prion protein using real-time quaking-induced conversion. J Gen Virol 93:438–449. doi: 10.1099/vir.0.033365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takatsuki H, Atarashi R, Sano K, Satoh K, Nishida N. 2012. Quantitation of seeding activity of human prion using real-time quaking induced conversion assay. Prion 6:130–131. [Google Scholar]
- 54.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, Caughey B, Hoover EA. 2013. Rapid antemortem detection of CWD prions in deer saliva. PLoS One 8:e74377. doi: 10.1371/journal.pone.0074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomsen BV, Schneider DA, O'Rourke KI, Gidlewski T, McLane J, Allen RW, McIsaac AA, Mitchell GB, Keane DP, Spraker TR, Balachandran A. 2012. Diagnostic accuracy of rectal mucosa biopsy testing for chronic wasting disease within white-tailed deer (Odocoileus virginianus) herds in North America: effects of age, sex, polymorphism at PRNP codon 96, and disease progression. J Vet Diagn Invest 24:878–887. doi: 10.1177/1040638712453582. [DOI] [PubMed] [Google Scholar]
- 56.O'Rourke KI, Besser TE, Miller MW, Cline TF, Spraker TR, Jenny AL, Wild MA, Zebarth GL, Williams ES. 1999. PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol 80(Pt 10):2765–2769. doi: 10.1099/0022-1317-80-10-2765. [DOI] [PubMed] [Google Scholar]
- 57.O'Rourke KI, Spraker TR, Zhuang D, Greenlee JJ, Gidlewski TE, Hamir AN. 2007. Elk with a long incubation prion disease phenotype have a unique PrPd profile. Neuroreport 18:1935–1938. doi: 10.1097/WNR.0b013e3282f1ca2f. [DOI] [PubMed] [Google Scholar]
- 58.Spraker TR, VerCauteren KC, Gidlewski T, Schneider DA, Munger R, Balachandran A, O'Rourke KI. 2009. Antemortem detection of PrPCWD in preclinical, ranch-raised Rocky Mountain elk (Cervus elaphus nelsoni) by biopsy of the rectal mucosa. J Vet Diagn Invest 21:15–24. doi: 10.1177/104063870902100103. [DOI] [PubMed] [Google Scholar]
- 59.Spraker TR, O'Rourke KI, Gidlewski T, Powers JG, Greenlee JJ, Wild MA. 2010. Detection of the abnormal isoform of the prion protein associated with chronic wasting disease in the optic pathways of the brain and retina of Rocky Mountain elk (Cervus elaphus nelsoni). Vet Pathol 47:536–546. doi: 10.1177/0300985810363702. [DOI] [PubMed] [Google Scholar]
- 60.Williams ES, Young S. 1982. Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis 18:465–471. doi: 10.7589/0090-3558-18.4.465. [DOI] [PubMed] [Google Scholar]
- 61.Cramm M, Schmitz M, Karch A, Mitrova E, Kuhn F, Schroeder B, Raeber A, Varges D, Kim YS, Satoh K, Collins S, Zerr I. 1 April 2015. Stability and reproducibility underscore utility of RT-QuIC for diagnosis of Creutzfeldt-Jakob disease. Mol Neurobiol doi: 10.1007/s12035-015-9133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spraker TR, VerCauteren KC, Gidlewski TL, Munger RD, Walter WD, Balachandran A. 2009. Impact of age and sex of Rocky Mountain elk (Cervus elaphus nelsoni) on follicle counts from rectal mucosal biopsies for preclinical detection of chronic wasting disease. J Vet Diagn Invest 21:868–870. doi: 10.1177/104063870902100618. [DOI] [PubMed] [Google Scholar]
- 63.Budras KD, Habel RE, Mulling CKW, Greenough PR, Wunsche A, Buda S. 2011. Bovine anatomy, 2nd ed Schlütersche Verlagsgesellschaft mbH & Co., KG, Hannover, Germany. [Google Scholar]
- 64.Reece WO, Erickson H, Goff JP (ed). 2015. Dukes' physiology of domestic animals, 13th ed Wiley-Blackwell, Hoboken, NJ. [Google Scholar]
- 65.Saunders SE, Bartelt-Hunt SL, Bartz JC. 2012. Occurrence, transmission, and zoonotic potential of chronic wasting disease. Emerg Infect Dis 18:369–376. doi: 10.3201/eid1803.110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.New York State Department of Environmental Conservation. 2013. Surveillance plan for chronic wasting disease in New York State 2013–2014. New York State Department of Environmental Conservation Cornell University Animal Health Diagnostic Center, Ithaca, NY. [Google Scholar]
- 67.Bartelt G, Pardee J, Thiede K. 2003. Environmental impact statement on rules to eradicate chronic wasting disease from Wisconsin's free-ranging white-tailed deer herd. Wisconsin Department of Natural Resources, Madison, WI: http://dnr.wi.gov/files/PDF/pubs/ea/EA0063.pdf. [Google Scholar]
- 68.Wasserberg G, Osnas EE, Rolley RE, Samuel MD. 2009. Host culling as an adaptive management tool for chronic wasting disease in white-tailed deer: a modelling study. J Appl Ecol 46:457–466. doi: 10.1111/j.1365-2664.2008.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]