Abstract
Fifty-five hospitals in the United States have been designated Ebola treatment centers (ETCs) by their state and local health authorities. Designated ETCs must have appropriate plans to manage a patient with confirmed Ebola virus disease (EVD) for the full duration of illness and must have these plans assessed through a CDC site visit conducted by an interdisciplinary team of subject matter experts. This study determined the clinical laboratory capabilities of these ETCs. ETCs were electronically surveyed on clinical laboratory characteristics. Survey responses were returned from 47 ETCs (85%). Forty-one (87%) of the ETCs planned to provide some laboratory support (e.g., point-of-care [POC] testing) within the room of the isolated patient. Forty-four (94%) ETCs indicated that their hospital would also provide clinical laboratory support for patient care. Twenty-two (50%) of these ETC clinical laboratories had biosafety level 3 (BSL-3) containment. Of all respondents, 34 (72%) were supported by their jurisdictional public health laboratory (PHL), all of which had available BSL-3 laboratories. Overall, 40 of 44 (91%) ETCs reported BSL-3 laboratory support via their clinical laboratory and/or PHL. This survey provided a snapshot of the laboratory support for designated U.S. ETCs. ETCs have approached high-level isolation critical care with laboratory support in close proximity to the patient room and by distributing laboratory support among laboratory resources. Experts might review safety considerations for these laboratory testing/diagnostic activities that are novel in the context of biocontainment care.
INTRODUCTION
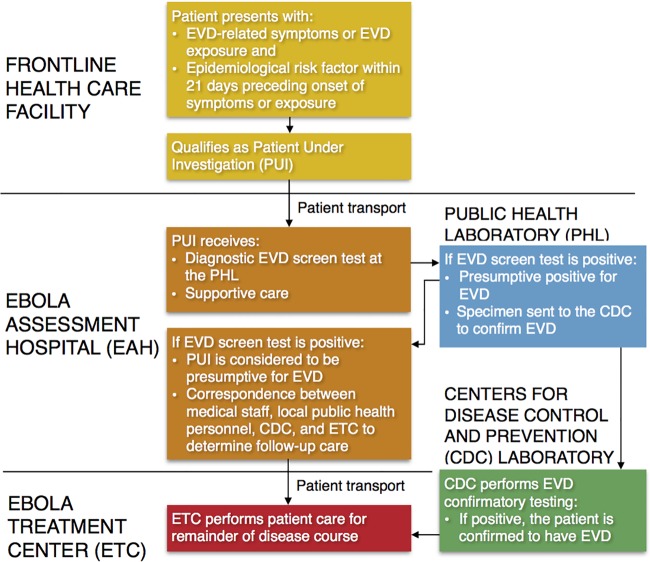
The ongoing West African Ebola virus disease (EVD) epidemic, and the occurrence of three domestic EVD cases in the United States, has prompted national revision of strategies to combat EVD and other highly infectious diseases (1, 2). The U.S. Department of Health and Human Services (HHS), through the Centers for Disease Control and Prevention (CDC) in coordination with the Office of the Assistant Secretary for Preparedness and Response (ASPR), has created interim guidance for hospitals and health departments intended to assist them in developing preparedness plans for evaluating patients under investigation for EVD and for patients with confirmed EVD (3). HHS also recommends that hospitals work to develop a coordinated, networked approach by designating medical facilities frontline health care facilities, Ebola assessment hospitals (EAHs), or Ebola treatment centers (ETCs) (4). Personnel in frontline facilities (e.g., hospital-based emergency departments, critical-access hospitals, and urgent-care clinics) should be trained to quickly detect and isolate patients and notify local and state public health departments when patients present with EVD-related symptoms in combination with an Ebola virus exposure history (4). Patients who meet the criteria for a patient under investigation (PUI) are recommended to be transported to an EAH for supportive care and for diagnostic testing by the jurisdictional public health laboratory (PHL) to evaluate patients for the presence of EVD (Fig. 1) (5). Patients identified with a presumptive positive test for EVD would subsequently have specimens sent to the CDC for confirmatory testing (5). If EVD is confirmed, patients would then be transferred to an ETC, where the patient with EVD is cared for in an isolated patient room for the remainder of the disease course.
FIG 1.
Communication flow for diagnosing and transferring a PUI for EVD and a patient with EVD after confirmatory testing.
Patients with EVD become critically ill several days into their illness, requiring high levels of supportive care, including aggressive intravenous fluid resuscitation and management of electrolytes due to the high rates of fluid loss in the fulminant stages of the disease (6, 7). The CDC recommends that hospitals caring for a PUI and/or a patient with confirmed EVD be able to perform a variety of laboratory tests, including a complete blood cell count, measurement of basic electrolyte levels, liver function tests, coagulation studies, blood cultures, urinalysis, as well as tests for the presence of other infectious diseases such as malaria and influenza (8). Hospital planning to provide aggressive intensive care therapies for a patient with fulminant EVD has been complicated. The highly infective nature of the patients' body fluids has prompted many laboratorians to be concerned about their ability to safely provide support for the care of EVD patients using standard hospital laboratory equipment (9). Indeed, perspectives from West African ETCs during the 2014-2015 outbreak emphasized the laboratories' vital role in monitoring pathophysiology in patients with EVD (10).
As of August 2015, 55 U.S. hospitals were designated ETCs by state and local health authorities. To validate EVD care capabilities, these hospitals volunteered for assessments by the Rapid Ebola Preparedness (REP) teams of CDC personnel and subject matter experts (5, 8, 11). As part of this designation, ETC-qualifying medical facilities had arranged to have “laboratory procedures/protocols, dedicated space, [and] if possible, point-of-care testing, equipment, staffing, reagents, training, and specimen transport” capabilities available (11). The CDC has offered additional guidance on personal protective equipment (PPE), risk assessment and mitigation, laboratory instruments, point-of-care (POC) testing, transportation of specimens with Ebola virus, decontamination, and waste management (8). In addition, Emory University and Nebraska Medicine, as part of their treatment protocols, reported lists of essential and supplemental laboratory equipment and tests for high-risk patient care (9, 12, 13).
Although limited standards for laboratory support have been identified for the 55 ETCs, no documentation on their current capabilities has been reported. This report discloses the laboratory support for participating U.S. ETCs as they prepared to care for patients with EVD in their hospital biocontainment setting.
MATERIALS AND METHODS
Referencing European Network of Infectious Diseases (EUNID) checklists (14), a survey was developed to determine current structural and operational features of U.S. ETCs, including laboratory characteristics, infection control infrastructure, laboratory location, costs of establishment and operation, and patient capacity. These checklists were derived from EUNID consensus agreements on the structural aspects of highly-infectious-disease patient care units in Europe (http://www.eunid.eu/) (14). Survey questions related to laboratories are listed in Table 1.
TABLE 1.
Laboratory capability survey questions distributed to U.S. Ebola treatment centers
| Survey question |
|---|
| Location of nearest laboratory support (check all that apply) |
| Patient care room, yes or no |
| Isolation unit, yes or no |
| Same campus, yes or no |
| Same city, yes or no |
| No information/other (please specify) |
| Classification of laboratory support (check all that apply) |
| Bedside point-of-care testing, yes or no |
| Clinical laboratory, yes or no |
| Public health laboratory, yes or no |
| No information/other (please specify) |
| Biosafety designation of accessible clinical laboratory |
| BSL-2 |
| BSL-3 |
| BSL-4 |
| No information/other (please specify) |
| Biosafety designation of accessible public health laboratory |
| BSL-2 |
| BSL-3 |
| BSL-4 |
| No information/other (please specify) |
The location of ETC laboratory support in relation to the isolation unit was defined as a location within the patient care room, within the unit, on the same campus as the unit, or within the same city as the unit or a combination of these locations. A patient care room was defined as the room in which the ETC planned to contain the patient within the isolation unit. The isolation unit was defined as the patient care area separated from other patient care wards, with access restricted to personnel entering under appropriate isolation precautions.
Types of laboratory support available for the ETCs were classified as bedside POC testing, clinical laboratory support, PHL support, or a combination of these types of support. Laboratory containment was defined as biosafety level 2 (BSL-2), BSL-3, or BSL-4 (15).
Surveys were distributed electronically in April 2015 for self-completion to the directors and/or assistant directors of the 55 U.S. ETCs. Survey responses were collected via e-mail. Any discrepancies were followed up by email, phone call, or referencing of information available online. Responses were coded and analyzed for the number and percentage of ETCs indicating their specific location of laboratory support, classification of laboratory support, BSL containment of accessible hospital laboratories, and BSL containment of PHLs by using Microsoft Excel (Microsoft Corporation, Redmond, WA).
RESULTS
Survey responses were obtained from 47 of the 55 ETCs (85%). Of these ETCs, 41 (87%) reported that the patient room was the nearest location of some laboratory support relative to the location of the patient (Table 2). Of the six ETCs without laboratory support in the patient room, three each had laboratory support within the unit and on the same campus. Each of the ETCs with laboratory support limited to the same campus as their isolation unit indicated support from a clinical laboratory and/or their jurisdictional PHL.
TABLE 2.
Reported location closest to the patient room and classification of laboratory support in caring for patients with Ebola virus disease from U.S. Ebola treatment centersa
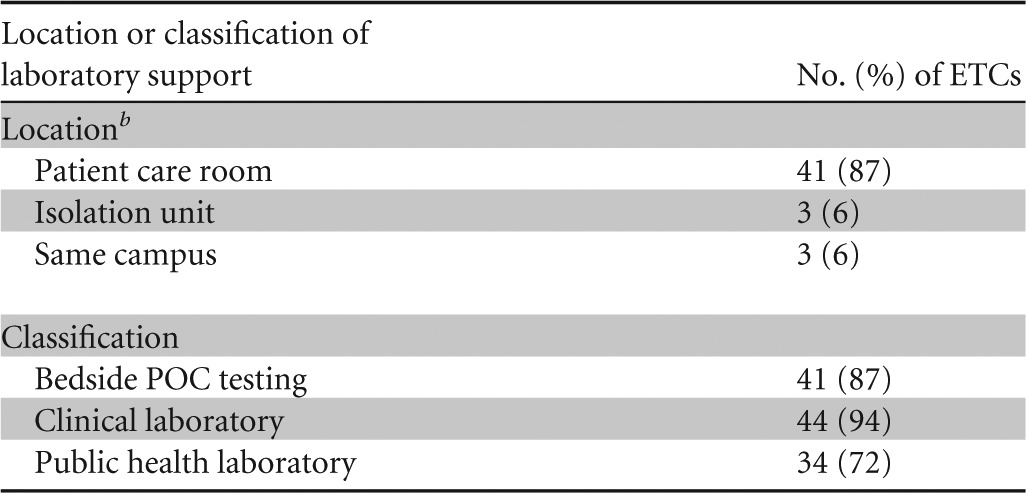
The number of responders was 47.
The laboratory support location is defined as follows: patient care room indicates a location within the patient's room, isolation unit is located in a designated space contained within the isolation unit, and same campus is located within the same medical facility.
All ETCs provided at least one type of laboratory support (i.e., POC testing within the patient room, clinical laboratory, or PHL). In classifying the type of laboratory support, 41 (87%) of the respondents indicated that bedside POC testing was available (Table 2). Forty-four (94%) ETCs indicated that they were supported by a clinical laboratory, and 34 (72%) indicated that they were supported by their jurisdictional PHL. Overall, 30 (64%) of the ETCs offered a combination of bedside POC testing and assistance from a clinical laboratory and their PHLs, with all but 1 of these ETCs having access to a BSL-3 laboratory.
Of 42 ETCs responding regarding clinical laboratory containment, 20/42 (43%) reported BSL-2 containment, and 22/42 (52%) reported BSL-3 containment. Thirty-four of 47 (72%) ETCs reported that they had access to their jurisdictional PHL, all of which (34/34) have available BSL-3 containment laboratories.
In total, 40 of the 44 ETCs (91%) reported that they had access to a BSL-3 laboratory facility (a clinical laboratory and/or PHL), while 4 (9%) reported that they had access to BSL-2 laboratory facilities. Of the four latter facilities, three were supported by POC tests in the patient room. The remaining facility was supported by a laboratory within the isolation unit.
DISCUSSION
This survey investigated laboratory support for the 55 CDC-designated ETCs. A majority of these ETCs offered laboratory testing in close proximity to the patient room while simultaneously dispersing support through their clinical laboratory and PHL. Given the critical nature of EVD and the potential need to assess patients for other diagnoses, laboratory support is well recognized as a crucial aspect of the optimum clinical care of patients with or without EVD (6).
The location of the laboratory to support a patient with EVD was defined as the location nearest the patient room. Previously reported laboratory support located within an isolation area was found to reduce specimen processing times, provide personnel improved safety assurance for handling of specimens, and decrease exposure risks (9, 13, 16). In this study, we noted that many ETCs have adopted at least some portion of a contained laboratory care model in which the location of the laboratory is located in close proximity to the patient care area to allow rapid laboratory processing and enhanced laboratory safety and patient supportive care.
Conversely, laboratory support within the patient room or isolation unit may be disadvantageous. For instance, laboratory technologists may be required to enter the isolation unit where space may be limited to minimize the risks of occupational exposures (8). Exclusion of laboratory personnel from the isolation unit requires that clinicians with less familiarity with POC technologies complete testing while simultaneously performing other care activities. Additionally, the isolation unit may not have a contained area large enough for the placement of a biosafety cabinet for the safe processing of specimens.
The Nebraska Biocontainment Unit (NBU) described the safe utilization of multiple laboratories to care for patients with EVD to include an in-unit BSL-3 laboratory, a BSL-3 laboratory at the on-campus PHL, and a core hospital laboratory (9). In contrast, Emory University contained nearly all laboratory testing (excluding specimens sent to the CDC or other government agencies for testing) within the patient care isolation unit (13). Both models have proven to be safe and effective in providing laboratory care for patients with EVD (9, 13). ETCs have equally approached providing laboratory support within the patient room and/or isolation unit (44/47 [94%]) as well as from their hospital clinical laboratory (44/47 [94%]). Of the 47 surveyed ETCs, 30 (64%) have laboratory support including bedside POC testing, a clinical laboratory, and assistance from their PHLs, likely sharing responsibilities among resources. Distributing laboratory tasks among various locations may, however, also introduce exposure risks for additional laboratory personnel in each setting. A laboratory risk assessment at each location can help to reduce these risks (8, 12).
Of the hospitals in the United States that have cared for patients with EVD, both BSL-2 and BSL-3 containment laboratories have been used for clinical laboratory testing, with BSL-3 containment being available in 40/44 (91%) ETCs (8).
In comparison, a survey of European high-level isolation units (HLIUs) showed that only 17 and 27% of these units performed microbiological and routine tests, respectively, within the isolation patient care area (Table 3) (17). Overall, 32/47 (68%) and 15/48 (31%) of these HLIUs sent specimens for microbiological testing and routine clinical testing, respectively, to a reference BSL-3 laboratory, while 24/47 (51%) and 41/48 (85%) HLIUs sent specimens for microbiological testing and routine testing, respectively, to a central hospital laboratory (with and without closed-type automated analyzers) (17). A total of 39/48 (81%) European HLIUs had access to BSL-3 containment laboratories for diagnosis within the same city/facility as the unit, and 11/48 (23%) had access to BSL-4 containment laboratories (with overlap in access to BSL-3 and BSL-4 containment laboratories).
TABLE 3.
Comparison of U.S. ETC and European HLIU laboratory supporta
| Laboratory support | No. of centers with support/total no. of centers surveyed (%) |
|||
|---|---|---|---|---|
| U.S. ETCs overall | European HLIUs |
|||
| Microbiological test | Routine test | Overall | ||
| Testing within isolation unitb | 44/47 (94) | 8/47(17) | 13/48 (27) | |
| Testing in hospital laboratoryc | 44/47 (94) | 24/47 (51) | 41/48 (85) | |
| Testing in reference BSL-3 laboratoryd | 34/47 (72) | 32/47 (68) | 15/48 (31) | |
| Access to BSL-3 containment laboratorye | 40/44 (91) | 39/48 (81) | ||
| Access to BSL-4 containment laboratorye | 0/47 (0) | 11/48 (23) | ||
See reference 17.
Within isolation unit was defined as a location within the patient room or other room contained in the isolation area.
Hospital laboratory testing was defined as access to a clinical laboratory for U.S. ETCs and tests performed in a central hospital laboratory (general laboratory with or without closed-type automated analyzers) for European high-level isolation units.
Reference laboratory for U.S. ETCs was defined as access to a public health laboratory.
European high-level isolation units reported access to BSL-3 and BSL-4 containment facilities for diagnosis within the same facility/city, while U.S. ETCs reported BSL-3 and BSL-4 containment facilities within accessible clinical and public health laboratories.
Limitations of this study included that the survey did not differentiate which tests, diagnostic or routine, were performed within the isolation unit, clinical laboratory, or PHL. Some ETCs responded to the survey question on the location of laboratory support by making only a single selection rather than checking all that applied, so answers were interpreted as laboratory support in closest proximity to the patient room. Thus, the cross-sectional design of this survey provided a limited snapshot of the current laboratory capabilities of U.S. ETCs. One ETC also indicated simultaneous construction of a BSL-3 laboratory within their isolation unit during completion of the survey.
In general, U.S. ETCs were rapidly created in response to the Ebola epidemic of 2014 to 2015, and the care and laboratory capabilities of these facilities will continue to transform as plans for sustainability and the national role in responses to highly infectious diseases are refined.
Further details need to be considered regarding specific recommendations for the types of tests that need to be available to care for a patient with a highly infectious pathogen, the locations that are optimal for laboratory testing, the types of PPE utilized and training available, and qualified staff to perform laboratory testing. An expanded future survey to demonstrate the evolution of ETC facilities and to gain a more complete picture of national capabilities within this area is planned.
ACKNOWLEDGMENTS
We thank the U.S. Highly Infectious Disease Network and the dedicated Nebraska Biocontainment Unit and Emory Serious Communicable Diseases Unit for their unwavering commitment to patient care, quality improvement, and safety.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Gostin LO, Hodge JG Jr, Burris S. 2014. Is the United States prepared for Ebola? JAMA 312:2497–2498. doi: 10.1001/jama.2014.15041. [DOI] [PubMed] [Google Scholar]
- 2.Santora M. 24 October 2014. New York doctor, back from Africa, is sick with Ebola, p A1. New York Times, New York, NY. [Google Scholar]
- 3.HHS. 2015. HHS invests in enhancing domestic preparedness efforts for Ebola. HHS, Washington, DC. [Google Scholar]
- 4.CDC. 2015. Interim guidance for U.S. hospital preparedness for patients under investigation (PUIs) or with confirmed Ebola virus disease (EVD): a framework for a tiered approach. CDC, Atlanta, GA. [Google Scholar]
- 5.CDC. 2015. Guidance for collection, transport and submission of specimens for Ebola virus testing. CDC, Atlanta, GA. [Google Scholar]
- 6.Lyon GM, Mehta AK, Varkey JB, Brantly K, Plyler L, McElroy AK, Kraft CS, Towner JS, Spiropoulou C, Ströher U, Uyeki TM, Ribner BS. 2014. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med 371:2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DW, Sullivan JN, Piquette CA, Hewlett AL, Bailey KL, Smith PW, Kalil AC, Lisco SJ. 2015. Lessons learned: critical care management of patients with Ebola in the United States. Crit Care Med 43:1157–1164. doi: 10.1097/CCM.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 8.CDC. 2015. Guidance for U.S. laboratories for managing and testing routine clinical specimens when there is a concern about Ebola virus disease. CDC, Atlanta, GA. [Google Scholar]
- 9.Iwen PC, Garrett JL, Gibbs SG, Lowe JJ, Herrera VL, Sambol AR, Stiles K, Wisecarver JL, Salerno KJ, Pirruccello SJ, Hinrichs SH. 2014. An integrated approach to laboratory testing for patients with Ebola virus disease. Lab Med 45:e146–e151. doi: 10.1309/LMTULFM62W3RKMYI. [DOI] [PubMed] [Google Scholar]
- 10.Fowler RA, Fletcher T, Fischer WA II, Lamontagne F, Jacob S, Brett-Major D, Lawler JV, Jacquerioz FA, Houlihan C, O'Dempsey T, Ferri M, Adachi T, Lamah MC, Bah EI, Mayet T, Schieffelin J, McLellan SL, Senga M, Kato Y, Clement C, Mardel S, Vallenas Bejar De Villar RC, Shindo N, Bausch D. 2014. Caring for critically ill patients with Ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med 190:733–737. doi: 10.1164/rccm.201408-1514CP. [DOI] [PubMed] [Google Scholar]
- 11.CDC. 2014. Interim guidance for preparing Ebola treatment centers. CDC, Atlanta, GA. [Google Scholar]
- 12.Iwen PC, Smith PW, Hewlett AL, Kratochvil CJ, Lisco SJ, Sullivan JN, Gibbs SG, Lowe JJ, Fey PD, Herrera VL, Sambol AR, Wisecarver JL, Hinrichs SH. 2015. Safety considerations in the laboratory testing of specimens suspected or known to contain Ebola virus. Am J Clin Pathol 143:4–5. doi: 10.1309/AJCP26MIFUIETBPL. [DOI] [PubMed] [Google Scholar]
- 13.Hill CE, Burd EM, Kraft CS, Ryan EL, Duncan A, Winkler AM, Cardella JC, Ritchie JC, Parslow TG. 2014. Laboratory test support for Ebola patients within a high-containment facility. Lab Med 45:e109–e111. doi: 10.1309/LMTMW3VVN20HIFS. [DOI] [PubMed] [Google Scholar]
- 14.Fusco F, Schilling S, Puro V, Brodt H, Follin P, Jarhall B, Bannister B, Maltezou H, Thomsen G, Brouqui P. 2009. EuroNID checklists for the assessment of high-level isolation units and referral centres for highly infectious diseases: results from the pilot phase of a European survey. Clin Microbiol Infect 15:711–719. doi: 10.1111/j.1469-0691.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- 15.HHS. 2009. Section IV—laboratory biosafety level criteria, p 30–59. In Chosewood LC, Wilson DE (ed), Biosafety in microbiological and biomedical laboratories, 5th ed US Department of Health and Human Services, Washington, DC: http://www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf. [Google Scholar]
- 16.Kortepeter MG, Martin JW, Rusnak JM, Cieslak TJ, Warfield KL, Anderson EL, Ranadive MV. 2008. Managing potential laboratory exposure to Ebola virus by using a patient biocontainment care unit. Emerg Infect Dis 14:881–887. doi: 10.3201/eid1406.071489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiberville SD, Schilling S, De Iaco G, Fusco FM, Thomson G, Maltezou HC, Gottschalk R, Brodt RH, Bannister B, Puro V, Ippolito G, Brouqui P, EuroNHID Working Group. 2012. Diagnostic issues and capabilities in 48 isolation facilities in 16 European countries: data from EuroNHID surveys. BMC Res Notes 5:527. doi: 10.1186/1756-0500-5-527. [DOI] [PMC free article] [PubMed] [Google Scholar]