Abstract
In 2010, the Clinical and Laboratory Standards Institute (CLSI) lowered the MIC breakpoints for many beta-lactam antibiotics to enhance detection of known resistance among Enterobacteriaceae. The decision to implement these new breakpoints, including the changes announced in both 2010 and 2014, can have a significant impact on both microbiology laboratories and antimicrobial stewardship programs. In this commentary, we discuss the changes and how implementation of these updated CLSI breakpoints requires partnership between antimicrobial stewardship programs and the microbiology laboratory, including data on the impact that the changes had on antibiotic usage at our own institution.
TEXT
In 2010, the Clinical and Laboratory Standards Institute (CLSI) lowered the MIC breakpoints for many beta-lactam antibiotics, including extended-spectrum cephalosporins, to enhance the detection of known resistance among Enterobacteriaceae (Table 1). At that time, breakpoints for cefepime were not changed. The CLSI Antibiotic Subcommittee asserted that routine extended-spectrum-beta-lactamase (ESBL) testing was no longer necessary and that treatment decisions can be made solely on MICs (1). Subsequently, a reassessment of cefepime breakpoints identified clinical failures for isolates with cefepime MICs of 4 and 8 μg/ml when lower doses were used, leading to the reduction of the susceptible cefepime breakpoint for Enterobacteriaceae from 8 to 2 μg/ml in the CLSI 2014 guidelines (2). The “intermediate” category (4 to 8 μg/ml) was replaced by the “susceptible-dose-dependent” (SDD) category (Table 2) (2). It should be noted that the European Committee on Antimicrobial Susceptibility Testing (EUCAST) also suggested avoidance of routine ESBL testing but implemented a lower breakpoint of 1 μg/ml for cefepime (3). The decision to implement the new CLSI breakpoints of both 2010 and 2014 can have a significant impact on both microbiology laboratories and antimicrobial stewardship programs.
TABLE 1.
CLSI revised breakpoints, 2009 to 2010a
| Agent | MIC breakpoint for isolates in indicated category by: |
|||||
|---|---|---|---|---|---|---|
| CLSI M100-S19 (2009) |
CLSI M100-S20 (2010) |
|||||
| Susc | Int | Res | Sus | Int | Res | |
| Cefazolin | ≤8 | 16 | ≥32 | ≤1 | 2 | ≥4 |
| Cefotaxime | ≤8 | 16–32 | ≥64 | ≤1 | 2 | ≥4 |
| Ceftriaxone | ≤8 | 16–32 | ≥64 | ≤1 | 2 | ≥4 |
| Ceftazidime | ≤8 | 16 | ≥32 | ≤4 | 8 | ≥16 |
| Aztreonam | ≤8 | 16 | ≥32 | ≤4 | 8 | ≥16 |
TABLE 2.
Changes to cefepime breakpointsa
| Agent | MIC breakpoint for isolates in indicated category by: |
|||||
|---|---|---|---|---|---|---|
| CLSI M100-S23 (2013) |
CLSI M100-S24 (2014) |
|||||
| Susc | Int | Res | Susc | SDD | Res | |
| Cefepime | ≤8 | 16 | ≥32 | ≤2 | 4–8 | ≥16 |
CONSIDERATIONS FOR IMPLEMENTATION IN THE MICROBIOLOGY LABORATORY
Microbiologists in the laboratory, in conjunction with the antimicrobial stewardship leadership, need to decide to implement the new breakpoints or to keep the old breakpoints with resistance testing. There are many things to consider when deciding to change the breakpoints and to confront the barriers that can make implementation difficult. The main barrier to implementation in the laboratory is the use of commercial Food and Drug Administration (FDA)-approved automated susceptibility testing systems. These instruments and any changes to their systems, including software, require clearance from the FDA. For some of these commercial systems, the antimicrobial susceptibility testing (AST) panels may or may not contain the beta-lactam antibiotics at the lower concentrations that encompass the new lower breakpoints. Even for the instruments that contain the lower concentrations that encompass the new breakpoints, the performance of the instruments at these concentrations has not been established; therefore, the instruments are not FDA approved. Implementation of the lower breakpoints in these commercial systems would result in “off-label” testing, and appropriate verification and validation and documentation are needed before using the systems for clinical care. Therefore, until the companies change the concentrations of drugs within their cards and obtain approval from the FDA, the lower concentrations will not be available to the laboratory. For a list of available companies and the availability of lower concentrations of antibiotics, see http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/Guideline_Methodology_and_Other_Resources/Educational_Resources/AppendixB.pdf. In these situations, it is difficult to implement these new breakpoints but not impossible. An appropriate in-house validation study can be performed. The Infectious Diseases Society of America has posted guidance for a verification protocol on its website (http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/Guideline_Methodology_and_Other_Resources/Educational_Resources/Appendix%20A%20Brief%20Validation%20Protocol%20FINAL.pdf). The College of Pathology also has specific isolates available for such verifications that are included in a breakpoint implementation tool (BIT) found at www.cap.org. The BIT includes 31 well-characterized Enterobacteriaceae species with reference MIC results as well as instructions and a worksheet to aid in data analysis.
Laboratories that make their own antimicrobial panels or perform disk diffusion susceptibility testing methods can easily begin to use the new lower breakpoints. The methods for disk diffusion are the same, and a verification study is therefore not necessary.
In the current health care environment, many institutions are consolidating microbiology laboratories and focusing on workflow efficiency. With the implementation of the new breakpoints, resistance testing is no longer recommended; therefore, faster reporting of MICs is available and less technologist time is needed for the setup and reading of additional testing.
Additionally, some hospitals no longer have microbiology laboratories on site. In these cases, laboratorians and antimicrobial stewardship leadership must talk with the laboratorians at off-site microbiology laboratories to understand if and when they might implement the newer breakpoints and to understand how this would affect therapeutic options at their institution. Many laboratories in the United States have not implemented the new breakpoints due to the costs of a verification of the new breakpoints, the use of automated systems, and the use of the confirmation assays that are still needed for epidemiological purposes.
CLINICAL IMPACT OF BREAKPOINT CHANGES ON ANTIMICROBIAL STEWARDSHIP
The implementation of the CLSI breakpoint changes can have a major impact from the perspective of an antimicrobial stewardship program. Antimicrobial stewardship programs consist of coordinated, multidisciplinary teams dedicated to improving antibiotic use by optimizing the treatment of infections while reducing rates of adverse events associated with antibiotics. A major goal of stewardship teams is to ensure appropriate use of broad-spectrum antibiotics to preserve their use for the treatment of multidrug-resistant infections. Stewardship program teams may anticipate increased use of broad-spectrum antibiotics, with increased reporting of resistance, particularly with respect to extended-spectrum cephalosporins, after implementation of the new breakpoints. Coordination between the microbiology laboratory and the antimicrobial stewardship team is essential to the successful implementation of the updated CLSI recommendations.
One of the biggest controversies concerning the changes results from the fact that antibiotic treatment choices can be predicted based only on an MIC value regardless of the resistance mechanism. Organisms harboring extended-spectrum beta-lactamases (ESBLs) can have drug MICs that fall below the susceptibility threshold in the new CLSI guidelines (4). McWilliams and colleagues analyzed 638 ESBL-producing Escherichia coli isolates and 229 ESBL-producing Klebsiella pneumoniae isolates using the reduced breakpoints for cephalosporins and aztreonam from the 2010 guidelines (5). A large proportion (89.2%) of ESBL-producing E. coli isolates and 67.7% of ESBL-producing K. pneumoniae isolates would be reported to be susceptible or intermediate to at least one extended-spectrum cephalosporin or aztreonam. Kristo and colleagues reported similar findings: around half of their isolates tested susceptible to one of the cephalosporins tested (cefotaxime, cefepime, and ceftazidime), although they harbored an ESBL (6).
Despite their being reported to be susceptible in vitro, the concern is that these antibiotics may not be effective in vivo. However, the thought is that the ESBL is irrelevant as long as the drug is still able to attain its pharmacodynamics targets (7). Inoculum effect can dilute out “resistant” populations and give false susceptible results, and MIC results can vary by more than 3 dilutions (8). For example, a study of 99 CTX-M-producing E. coli isolates found that 34 (34.3%) of all of the isolates and 32 (76.2%) of the CTX-M-14-producing isolates tested as susceptible when the revised CLSI ceftazidime breakpoint of 4 μg/ml was used. The MIC50 increased from 16 μg/ml with standard-inoculum tests to >512 μg/ml in high-inoculum tests. For the CTX-M-14 producers, the percentage of susceptible isolates was 82.1% with the standard inoculum compared to 0% with the high inoculum (9). This is of clinical importance because ceftazidime is associated with poor outcomes when used to treat ESBL-producing Enterobacteriaceae (10–11). While there are increasing data indicating that noncarbapenem beta-lactams such as piperacillin-tazobactam and cefepime, at certain MICs, may provide adequate treatment for severe infections by ESBL-producing strains, carbapenems remain the mainstay for treatment of high-ESBL-inoculum infections (12–14).
In the absence of specified mechanisms of resistance, many treating physicians may not be able to recognize the potential for ESBL production on the basis of a susceptibility profile alone. A survey of 569 infectious diseases physicians in the Emerging Infectious Disease Network (of 1,332 members; 44% response rate) regarding the use of the new CLSI breakpoints found that the majority of respondents favored using the lower cephalosporin breakpoints in combination with ESBL resistance testing to make treatment recommendations. Presented with different clinical scenarios, most respondents indicated that they would not treat based on the lower breakpoints alone without ESBL testing and would not use an extended-spectrum cephalosporin for treatment of an infection by an Enterobacteriaceae strain that was susceptible by the new breakpoints but was ESBL positive (15). Additionally, the consequences from an epidemiologic standpoint of no longer testing for specific mechanisms of resistance are unknown.
With the implementation of the new breakpoints, it is anticipated that microbiology laboratories will be reporting more isolates as either intermediate or resistant to the tested antibiotics, potentially leading to increased prescribing of broad-spectrum antibiotics. An assessment of 2,076 nonduplicate clinical Enterobacteriaceae isolates using disk diffusion to compare the 2009 breakpoint to the 2010 breakpoint saw resistance to the third-generation cephalosporin cefotaxime jump from 13.1% to 23.6% (16). Another assessment of 3,713 nonduplicate Gram-negative bacillus isolates with susceptibility determinations performed by disk diffusion using the CLSI 2009, CLSI 2010, CLSI 2011, and EUCAST 2011 guidelines found that, in aggregate, rates of resistance to cefepime, ceftriaxone, and ertapenem increased from 1.6%, 9.5%, and 1.0% (CLSI 2009) to 6.3%, 14.2%, and 1.6%/2.2% (CLSI 2010/2011 and EUCAST 2011, respectively) (17). This increase in reporting of resistance, specifically, in the reporting of resistance to third-generation cephalosporins, could shift antibiotic use to more broad-spectrum agents and increase the selective pressure on other antimicrobials. This is of particular concern for antimicrobial stewardship programs, which are responsible for providing oversight and regulation of antibiotic use with a goal of minimizing unnecessary broad-spectrum antibiotic use.
At our institution, based on discussions between the microbiology laboratory leadership and the antimicrobial stewardship team, the decision was made to implement the updated CLSI breakpoints, with the exception of those for cefepime and aztreonam, for which we elected to use the EUCAST breakpoints, and to discontinue ESBL confirmatory testing. After an internal verification was performed, the new breakpoints were officially implemented in December of 2012. After implementation, we analyzed antibiotic susceptibility data from 2013 using the CLSI M100-S19 breakpoints compared to the CLSI M100-S20 breakpoints (Fig. 1). We also compared aggregate levels of antibiotic use before and after implementation of the new breakpoint. Chart review was performed for patients who had an infecting organism reported as ceftriaxone resistant in 2013 that would have been reported as ceftriaxone susceptible in 2012 to identify the antibiotics used.
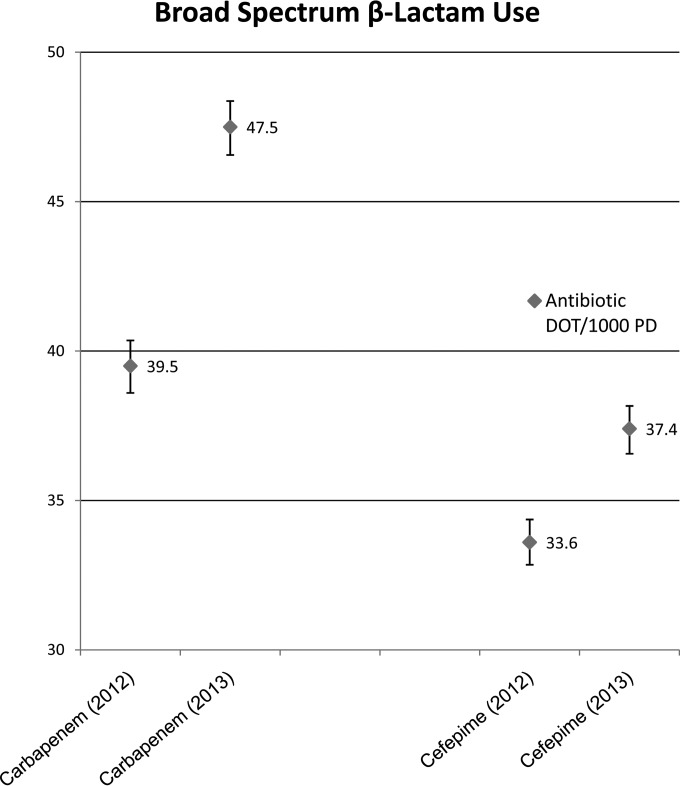
FIG 1.
Changes in broad-spectrum-antibiotic use quantified as days of therapy (DOT) per 1,000 patient-days (PD) from 2012 (preimplementation of 2010 CLSI breakpoints) to 2013 (postimplementation of 2010 CLSI breakpoints) at the University of Maryland Medical Center (UMMC).
Our analysis included a total of 3,785 nonduplicate clinical isolates of Enterobacteriaceae collected from 1 January 2013 to 31 December 2013. The proportion of isolates reported as ceftriaxone resistant based on the new breakpoint (≥4 μg/ml) was 18% greater what would have been reported based on the old breakpoint (≥64 μg/ml). Aggregate levels of antimicrobial use before and after implementation of the new breakpoint recommendations were compared by calculating Poisson 95% confidence intervals (CIs) on the basis of the number of antimicrobial days of therapy (DOT)/1,000 standard-inoculum s; nonoverlapping CIs were considered statistically significant. Carbapenem use increased significantly from 39.5 days of therapy (DOT)s/1,000 patient-days (PD) (95% CI, 38.7 to 40.4) in 2012 to 47.5 (95% CI, 46.6 to 48.4) in 2013, as did cefepime use, which increased from 33.6 DOT/1,000 PD (95% CI, 32.8 to 34.4) to 37.4 (95% CI, 36.6 to 38.2). Piperacillin-tazobactam use did not change significantly. Of the patients in 2013 with an organism reported as ceftriaxone resistant that would have been reported to be susceptible in 2012, 37% received a carbapenem and 31% received cefepime.
We also analyzed the data utilizing the comparison of the 2013 breakpoints to the 2014 breakpoints for cefepime. Reporting of resistance to cefepime increased from 1.7% to 2.4% with the 2014 breakpoints. In ceftriaxone-resistant isolates, there was a 13% decrease in cefepime susceptibility overall. This difference was statistically significant for Escherichia coli and Klebsiella pneumoniae but not for Enterobacter species. Our internal analysis provides evidence that implementation of the lower breakpoints can impact not only reported rates of resistance but also broad-spectrum-antibiotic usage, although this finding needs to be confirmed in larger studies. It must be noted that antibiotic use was assessed in aggregate and that changes cannot be attributed solely to the changes in breakpoints. However, there were no changes in antimicrobial stewardship practices in the year preimplementation and the year postimplementation of the breakpoint changes, which would have been a confounding variable(s).
With the 2014 update, the susceptible-dose-dependent (SDD) category was introduced for cefepime. While this category has been applied to antifungal susceptibility test results for years, its use for antibacterial susceptibility testing may be unfamiliar to practitioners. The term “susceptible dose dependent” implies that it is necessary to use a higher dose or more-frequent doses of an antibiotic than are used to establish the susceptible breakpoint in order to achieve levels that will be clinically effective for the SDD result. The concept of SDD has been included within the “intermediate” category, but clinicians may not have fully understood the “intermediate” concept. An informal survey of internal medicine house personnel at our institution revealed that 0% (0/30) of respondents would use cefepime to treat an organism reported as “intermediate” on a microbiology report but 50% (15/30) would use cefepime to treat an organism reported as “susceptible dose dependent” (unpublished data). Of interest, the EUCAST guidelines have largely eliminated the intermediate category (3). Education provided to medical providers on the definition of “susceptible dose dependent” and on the corresponding doses to meet those pharmacodynamics targets is essential, and providing such education is an important task for antimicrobial stewardship teams. Consideration should be given to providing commentary in the reporting of laboratory results that explain the SDD terminology. The recommended wording from CLSI is as follows. “The interpretive criterion for susceptible is based on a dosage regimen of 1 g every 12 h. The interpretive criterion for susceptible-dose dependent is based on dosing regimens that result in higher cefepime exposure, either higher doses or more frequent doses or both, up to approved maximum dosing regimens” (2).
A retrospective cohort study of patients who were treated with cefepime for Gram-negative bloodstream infections (including those by Enterobacteriaceae and nonfermentative organisms) was conducted to define a risk-adjusted mortality breakpoint for cefepime MICs based on the 2014 CLSI recommendations. A cefepime MIC of greater than or equal to 4 μg/ml was independently predicted to correspond to increased mortality (adjusted odds ratio, 3.21; 95% confidence interval, 1.02 to 10.2), and there was no significant effect of pathogen type on multivariate mortality estimates. Since most patients received aggressive doses of cefepime (the renal equivalent of 2 g every 8 h), the study results support the clinical breakpoint of an MIC of 2 μg/ml but raise concerns with respect to the ability of aggressive dosing to provide acceptable clinical success at the SDD MIC range of 4 to 8 μg/ml (18). At our institution, the microbiology laboratorians, after discussion with the antimicrobial stewardship team and infectious diseases division, implemented the EUCAST MIC breakpoint of 1 μg/ml for cefepime and Enterobacteriaceae, as the high dose of cefepime was already used as a standard in our clinical practice, and this was felt to be the most conservative approach.
In general, there is much opportunity to maximize training in the principles of antimicrobial resistance in medical education. A survey of 311 fourth-year medical students found that 79% of students agreed that they would like more education on antimicrobial resistance (19). With many institutions having a limited number of infectious diseases consultants, medical education and postgraduate training on antimicrobial susceptibility testing and resistance must be improved. All health care providers should have a basic understanding of the information presented to them in a microbiology susceptibility report and should be able to recognize when infectious diseases consultation is needed. Antimicrobial stewardship teams can fill the gaps in these education needs. At our institution, the antimicrobial stewardship team members provide routine formal and informal teaching for the house personnel.
Partnership between a microbiology laboratory and an antimicrobial stewardship program is essential for successful rollout of the new recommendations to ensure that patients receive optimal antibiotic therapy. At our institution, representatives from the laboratory and the stewardship program worked together to develop educational comments to be reported with susceptibility results for organisms that would have been tested for ESBL or carbapenemase production based on previous guidelines. These comments also include a suggestion to seek out infectious diseases consultation in certain scenarios. Additionally, the stewardship team reviews culture data from the laboratory to provide individual education and therapeutic recommendations with respect to patient-specific cultures as needed.
Two major potential challenges are that many institutions do not have an onsite microbiology laboratory and that antimicrobial stewardship programs are not yet present nationwide. For institutions without an antimicrobial stewardship program, microbiology laboratories should engage consultants from infectious diseases when making breakpoint implementation decisions. Extra effort should be made in these situations to include education within the result reports to help guide therapeutic decisions in conjunction with infectious diseases.
CONCLUSIONS
The updated CLSI breakpoints obviate ESBL screening, resulting in less work for clinical microbiology laboratories and quicker turnaround time of susceptibility results, and also provide recommendations that are more consistent with international recommendations from EUCAST. However, controversy remains surrounding the importance of continued testing and confirmation of ESBL production for Enterobacteriaceae in guiding therapeutic decisions. Therefore, the decision to implement the updated CLSI breakpoints and the subsequent rollout require partnership between antimicrobial stewardship programs and the microbiology laboratory. We believe that implementation of the updated breakpoints is important for consistency in susceptibility reporting across institutions; however, we still have concerns about the lack of resistance screening. We would like to see consistency between the recommendations of CLSI and EUCAST to bring standardization to reporting internationally. Additionally, given the lack of resistance testing recommended by both guidelines, we recommend the use of the more conservative EUCAST breakpoints for cefepime, ceftazidime, and aztreonam. Education on the changes in reporting, specifically with regard to changes in reporting of mechanisms of resistance, is essential. Changes in the rates of reported resistance and broad-spectrum antibiotic use may be expected. Not all institutions have on-site microbiology laboratories or antimicrobial stewardship programs, posing challenges to successful implementation.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Funding Statement
No funding was received from any funding agency in the public, commercial, or not-for-profit sector.
REFERENCES
- 1.CLSI. 2010. Performance standards for antimicrobial susceptibility testing: 20th informational supplement, CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 2.CLSI. 2014. Performance standards for antimicrobial susceptibility testing: 24th informational supplement, CLSI document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 3.The European Committee on Antimicrobial Susceptibility Testing. 2015. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0, 2015 http://www.eucast.org.
- 4.Marchaim D, Sunkara B, Lephart PR, Gudur UM, Bhargava A, Mynatt RP, Zhao JJ, Bheemreddy S, Hayakawa K, Chopra T, Dhar S, Kaye KS. 2012. Extended-spectrum beta-lactamase producers reported susceptible to piperacillin-tazobactam, cefepime and cefuroxime in the era of lowered breakpoints and no confirmatory test. Infect Control Hosp Epidemiol 33:853–855. doi: 10.1086/666632. [DOI] [PubMed] [Google Scholar]
- 5.McWilliams CS, Condon S, Schwartz RM, Ginocchio CC. 2014. Incidence of extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates that test susceptible to cephalosporins and aztreonam by the revised CLSI breakpoints. J Clin Microbiol 52:2653–2655. doi: 10.1128/JCM.03613-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristo I, Pitiriga V, Poulou A, Zarkotou O, Kimouli M, Poumaras S, Tsakris A. 2013. Susceptibility patterns to extended-spectrum cephalosporins among Enterobacteriaceae harbouring extended-spectrum beta-lactamases using the updated Clinical and Laboratory Standards Institute interpretive criteria. Int J Antimicrob Agents 41:383–387. doi: 10.1016/j.ijantimicag.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Dudley MN, Ambrose PG, Bhavnani SM. 2013. Background and rationale for revised Clinical and Laboratory Standards Institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. Cephalosporins and aztreonam. Clin Infect Dis 56:1301–1309. doi: 10.1093/cid/cit017. [DOI] [PubMed] [Google Scholar]
- 8.Thomson KS, Moland ES. 2001. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 45:3548–3554. doi: 10.1128/AAC.45.12.3548-3554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang C, Cha MK, Kim SO, Wi YM, Chung DR, Peck KR, Lee NY, Song JH. 2014. Extended-spectrum cephalosporins and the inoculum effect in tests with CTX-M-type extended-spectrum beta-lactamase-producing Escherichia coli: potential clinical implications of the revised CLSI interpretive criteria. Int J Antimicrob Agents 43:456–459. doi: 10.1016/j.ijantimicag.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2004. Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother 48:4574=81. doi: 10.1128/AAC.48.12.4574-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterson DL, Ko WC, Von Gottberg A, Casellas JM, Mulazimoglu L, Klugman KP, Bonomo RA, Rice LB, McCormack JG, Yu VL. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 39:2206–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen HM, Shier KL, Graber CJ. 2014. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 69:871–880. doi: 10.1093/jac/dkt450. [DOI] [PubMed] [Google Scholar]
- 14.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. 2013. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis 56:488–495. doi: 10.1093/cid/cis916. [DOI] [PubMed] [Google Scholar]
- 15.Kallen AJ, Beekmann SE, Limbago B, Lentnek AL, Polgreen PM, Patel J, Srinivasan A. 2011. Prevalence of beta-lactam nonsusceptible Gram-negative bacilli and use and interpretation of current susceptibility breakpoints: a survey of infectious disease physicians. Diagn Microbiol Infect Dis 71:316–319. doi: 10.1016/j.diagmicrobio.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Liu PY, Shi ZY, Tung KC, Shyu CL, Chan KW, Liu JW, Wu ZY, Kao CC, Huang YC, Lin CF. 2014. Antimicrobial resistance to cefotaxime and ertapenem in Enterobacteriaceae: the effects of altering clinical breakpoints. J Infect Dev Ctries 8:289–296. doi: 10.3855/jidc.3335 24619258. [DOI] [PubMed] [Google Scholar]
- 17.Hombach M, Bloemberg GV, Bottger EC. 2012. Effects of clinical breakpoint changes in CLSI guidelines 2010/2011 and EUCAST guidelines 2011 on antibiotic susceptibility test reporting of gram-negative bacilli. J Antimicrob Chemother 67:622–632. doi: 10.1093/jac/dkr524. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes NJ, Liu J, McLaughlin MM, Qi C, Scheetz MH. 2015. Evaluation of clinical outcomes in patients with Gram-negative bloodstream infections according to cefepime MIC. Diagn Microbiol Infect Dis 82:165–171. doi: 10.1016/j.diagmicrobio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Abbo LM, Cosgrove SE, Pottinger PS, Pereyra M, Sinkowitz-Cochran R, Srinivasan A, Webb DJ, Hooton TM. 2013. Medical students' perceptions and knowledge about antimicrobial stewardship: how are we educating our future prescribers? Clin Infect Dis 57:631–638. doi: 10.1093/cid/cit370. [DOI] [PubMed] [Google Scholar]
- 20.CLSI. 2009. Performance standards for antimicrobial susceptibility testing: 20th informational supplement, CLSI document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.CLSI. 2013. Performance standards for antimicrobial susceptibility testing: 20th informational supplement, CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]