Abstract
Accurate identification of drug-resistant Mycobacterium tuberculosis is imperative for effective treatment and subsequent reduction in disease transmission. Line probe assays rapidly detect mutations associated with resistance and wild-type sequences associated with susceptibility. Examination of molecular-level performance is necessary for improved assay result interpretation and for continued diagnostic development. Using data collected from a large, multisite diagnostic study, probe hybridization results from line probe assays, MTBDRplus and MTBDRsl, were compared to those of sequencing, and the diagnostic performance of each individual mutation and wild-type probe was assessed. Line probe assay results classified as resistant due to the absence of wild-type probe hybridization were compared to those of sequencing to determine if novel mutations were inhibiting wild-type probe hybridization. The contribution of absent wild-type probe hybridization to the detection of drug resistance was assessed via comparison to a phenotypic reference standard. In our study, mutation probes demonstrated significantly higher specificities than wild-type probes and wild-type probes demonstrated marginally higher sensitivities than mutation probes, an ideal combination for detecting the presence of resistance conferring mutations while yielding the fewest number of false-positive results. The absence of wild-type probe hybridization without mutation probe hybridization was determined to be primarily the result of failure of mutation probe hybridization and not the result of novel or rare mutations. Compared to phenotypic culture-based drug susceptibility testing, the absence of wild-type probe hybridization without mutation probe hybridization significantly contributed to the detection of phenotypic rifampin and fluoroquinolone resistance with negligible increases in false-positive results.
INTRODUCTION
While the number of incident cases of tuberculosis (TB) has been steadily declining over the past decade, the prevalence of drug-resistant disease threatens to reverse these declines (1, 2). The World Health Organization (WHO) estimated that, in 2014, 3.3% of new and 20% of previously treated TB cases were multidrug-resistant (MDR) or were resistant to isoniazid (INH) and rifampin (RIF) (3). Furthermore, 9.7% of these MDR cases were extensively drug resistant (XDR) or were resistant to at least one fluoroquinolone (FQ) in addition to a second-line injectable drug (SLID), amikacin (AMK), kanamycin (KAN), or capreomycin (CAP). Although detection of M/XDR-TB is imperative for effective disease management, less than half of all MDR-TB cases were detected in 2014 (2–4). This detection gap is due, in part, to the lack of low-cost, rapid, and accurate diagnostic technologies for M/XDR-TB (4–6).
Conventional culture-based phenotypic drug susceptibility testing (DST) methods can take months to yield results (7). Molecular-based tests, however, can produce results in <1 day (5, 8–12). The WHO has endorsed line probe hybridization and molecular beacon assays for the detection of drug resistance in high-incidence settings (13). While these molecular-based tests have significantly increased M/XDR-TB detection, no test has yet replaced phenotypic DST as the gold standard for M/XDR-TB diagnosis. The approved molecular beacon assay, Xpert MTB/RIF (Cepheid, Sunnyvale, CA), detects only RIF resistance and fails to provide information regarding INH, FQ, or SLID resistance. The line probe assay, MTBDRplus version 2 (v2) (Hain Lifescience, Nehren, Germany), detects INH and RIF resistance, and the MTBDRsl (Hain Lifescience, Nehren, Germany) is designed to detect FQ, SLID, and ethambutol resistance. However, the reported sensitivities of these assays remain less than ideal for INH and SLID (14–16).
Line probe assays utilize a combination of two methods for classifying specimens as resistant. (i) If a probe containing a resistance conferring mutation (MUT probe) hybridizes on the test strip and at least one probe containing a wild-type sequence (WT probe) does not hybridize, the presence of the known mutation can be assumed, and the specimen is classified as resistant. (ii) If no MUT probes hybridize on the test strip and one or more WT probes also do not hybridize, the presence of an unknown mutation in that region is inferred, and the specimen is classified as resistant. Two recent studies assessing the performance of the MTBDRsl assay detected an unusually high proportion of FQ-resistant specimens that were eventually determined to be falsely positive after comparison to sequencing and/or conventional culture results (17, 18). False-positive (FP) FQ resistance results from the two studies were determined to be the result of absent WT probe hybridization (interpreted to mean the presence of a non-wild-type or unknown mutation sequence) rather than direct detection of a known mutation (17, 18). Given the significant impact of FQ resistance on MDR-TB treatment and the potential for the sole absence of WT probe hybridization to increase the number of false-positive test results, we analyzed individual probe performance to further understand the underlying subtleties of the MTBDRplus v2 and MTBDRsl assay results (19).
Using data collected from a large, multisite diagnostic study, probe hybridization results from the MTBDRplus v2 and MTBDRsl assays were compared to those of sequencing (as determined by pyrosequencing [PSQ]), and the diagnostic performance (sensitivity and specificity) of each individual MUT and WT probe was assessed. Additionally, MTBDRplus v2 and MTBDRsl results classified as resistant due to the sole absence of WT probe hybridization were compared to those of sequencing to determine if novel mutations were inhibiting WT probe hybridization. Finally, the overall contribution of the sole absence of WT probe hybridization to the detection of drug resistance was assessed via comparison to a phenotypic reference standard.
MATERIALS AND METHODS
Study design.
Specimens were collected as part of a prospective cohort study (ClinicalTrials registration number NCT02170441) conducted by the Global Consortium for Drug-resistant TB Diagnostics (GCDD), a research collaboration with study sites in India, South Africa, and Moldova. This study was approved by the institutional review board at the University of California, San Diego and by participating institutions at their respective study sites. Detailed descriptions of the study protocol and major findings have been published elsewhere (20, 21). In brief, patients presenting with suspected M/XDR-TB were invited to participate at the respective clinic sites. Sputum specimens were tested using two line probe assays, MTBDRplus v2 and MTBDRsl; a commercially available pyrosequencing platform, the PyroMark Q96 platform (Qiagen, Valencia, CA, USA), which was repurposed for Mycobacterium tuberculosis resistance detection; and a phenotypic reference standard, mycobacterial growth indicator tube (MGIT) 960 (BD Biosciences, Sparks, MD, USA). All culture-positive specimens were tested for drug susceptibility to INH, RIF, moxifloxacin (MXF), ofloxacin (OFX), KAN, CAP, and AMK using WHO recommended critical concentrations of 0.1, 1.0, 0.25, 2.0, 2.5, 2.0, and 1.0 μg/ml, respectively. All phenotypic DST was performed according to the manufacturer's instructions or as per standard procedures.
Line probe assays.
MTBDRplus v2 and MTBDRsl utilize PCR amplification followed by reverse hybridization to specific, immobilized oligonucleotide probes to detect either M. tuberculosis wild-type sequences or mutations associated with RIF, INH, FQ, and/or SLID resistance (22, 23). MTBDRplus v2 relies on a series of probes that hybridize DNA from codons 505 to 534 of the rpoB gene, codon 315 of the katG gene, and positions −1 to −22 on the inhA regulatory region. Specific mutations associated with RIF resistance are detected by the mutation probe rpoBMUT1, which detects the D516V amino acid change, as well as by rpoBMUT2A(H526Y), rpoBMUT2B(H526D), and rpoBMUT3(S531L). Mutations associated with INH resistance are detected by mutation probes katGMUT1(AGC315ACC), katGMUT2(AGC315ACA), inhAMUT1(C15T), inhAMUT2(A16G), inhAMUT3A(T8C), and inhAMUT3B(T8A) (22, 24). Although MTBDRsl detects resistance to FQ, SLID, and ethambutol, phenotypic resistance to ethambutol was not performed as part of this study, and consequently only resistance to FQ and SLID were assessed. MTBDRsl probes detect mutations in codons 85 to 97 of the gyrA gene positions 1401 to 1402 and position 1484 of the rrs gene (17, 23). Mutations associated with FQ resistance are detected by mutation probes gyrAMUT1(A90V), gyrAMUT2(S91P), gyrAMUT3A(D94A), gyrAMUT3B(D94N/Y), gyrAMUT3C(D94G), and gyrAMUT3D(D94H); mutations associated with resistance to SLID are detected by mutation probes rrsMUT1(A1401G) and rrsMUT2(G1484T).
Line probe assay interpretation.
The MTBDRplus v2 and MTBDRsl assays each contain a series of control probes. The conjugate and amplification probes document successful DNA extraction and amplification. The M. tuberculosis identification probe and specific gene loci probes confirm the presence of M. tuberculosis and each gene region being assessed. If either the conjugate or the amplification probe fails to hybridize (denoting test failure), the result is classified as indeterminate. If the M. tuberculosis probe is absent, results for all gene regions being assessed are classified as indeterminate; however, if a gene loci probe is absent, the results are classified as indeterminate only for the gene region associated with the absent loci probe. If all wild-type (WT) probes are present and all mutation (MUT) probes are absent for a specific gene region, the result is classified as susceptible to the drugs for which resistance is being assessed. Results are classified as resistant if one or more MUT probes hybridize and one or more WT probes fail to hybridize on the test strip or if one or more WT probes fail to hybridize (without MUT probe hybridization). These two different methods of resistance detection are unique to line probe assays, as they provide an indirect method through which to identify novel or rare mutations located in those regions covered by the WT probes. If all WT probes and at least one MUT probe are present, interpretation is difficult and a repeat test or confirmation by sequencing is recommended. For this study, however, the assay was not repeated and such results were classified as indeterminate (25).
Sequencing.
Pyrosequencing (PSQ), the reference sequencing standard used for this study, has previously been described in detail (20, 26). In brief, PSQ methods include DNA extraction, amplification by PCR, and real-time sequencing. Primers were used to synthesize nucleotides into growing DNA chains of no larger than 30 bp (5). The gene regions sequenced included codons 507 to 521 and 522 to 533 of the rpoB gene, codons 312 to 316 of katG, the inhA promoter region −4 to −20, gyrA codons 88 to 95, and the rrs gene region from nucleotides 1397 to 1406 (20, 26). Each gene target was initially sequenced once. However, if the sequencing result did not generate a 100% match with a library sequence, then sequencing was repeated for the sample in duplicate for the specific gene target.
Statistical analysis.
Standard sensitivity and specificity calculations were used to compare each individual MUT and WT probe of the MTBDRplus v2 and the MTBDRsl assays with sequencing as the reference standard in every instance where PSQ generated a valid sequencing read at the target location. Ninety-five percent confidence intervals (95% CI) were calculated using the Wilson score method (27). MTBDRplus v2 and MTBDRsl results classified as resistant for RIF, INH, FQ, and SLID were dichotomized as either resistant by MUT probe hybridization and the absence of WT probe hybridization or as resistant by the sole absence of WT probe hybridization. Specimens identified as resistant by the sole absence of WT probe hybridization were compared with PSQ results to confirm if a novel, rare, or expected mutation was present. The final analysis compared MTBDRplus v2 and MTBDRsl results to phenotypic DST results for all culture-positive specimens. Resistance based on the presence of MUT probe hybridization and on the absence of WT probe hybridization was compared to resistance based both on the presence of MUT probe hybridization and the absence of WT probe hybridization and on the sole absence of WT probe hybridization by assessing the area under the receiver operating characteristic curve for equality (AUC). Analysis was performed using Stata 13 (StataCorp, College Station, TX). Statistical significance was established at an alpha level of 0.05.
RESULTS
Resistance detection by method.
A total of 1,128 specimens were assessed by line probe assay (LPA), PSQ, and MGIT 960. The numbers of specimens classified as resistant, susceptible, indeterminate, or error/not done/culture negative for each drug or drug class tested are presented in Table 1. Indeterminate results varied for the two line probe assays (ranging from 24% for RIF to 38% for SLIDs) and for PSQ (ranging from 20% for SLIDs to 37% for RIF). A total of 214 specimens were culture negative for M. tuberculosis and therefore did not undergo phenotypic DST.
TABLE 1.
Final result classification of line probe assay (MTBDRplus and MTBDRsl), pyrosequencing (PyroMark Q96 ID), and phenotypic (MGIT 960) drug susceptibility testing by drug category
| Testing method and drug category | No. (%) by result category |
|||
|---|---|---|---|---|
| Resistant | Susceptible | Indeterminate | Error/not done/culture negative | |
| Line probe assay | ||||
| RIF | 478 (42) | 375 (33) | 275 (24) | 0 |
| INH | 484 (43) | 347 (31) | 297 (26) | 0 |
| FQ | 237 (21) | 541 (48) | 350 (31) | 0 |
| SLID | 46 (4) | 656 (58) | 426 (38) | 0 |
| Pyrosequencing | ||||
| RIF | 448 (40) | 264 (23) | 414 (37) | 2 (<1) |
| INH | 608 (54) | 282 (25) | 232 (21) | 6 (<1) |
| FQ | 277 (25) | 528 (47) | 322 (29) | 1 (<1) |
| SLID | 74 (7) | 825 (73) | 227 (20) | 2 (<1) |
| Phenotypic | ||||
| RIF | 540 (48) | 368 (33) | 0 | 220 (20) |
| INH | 592 (52) | 316 (28) | 0 | 220 (20) |
| MXF | 310 (27) | 597 (53) | 0 | 221 (20) |
| OFX | 314 (28) | 594 (53) | 0 | 220 (20) |
| AMK | 82 (7) | 826 (73) | 0 | 220 (20) |
| CAP | 79 (7) | 829 (73) | 0 | 220 (20) |
| KAN | 145 (13) | 763 (68) | 0 | 220 (20) |
Individual probe performance.
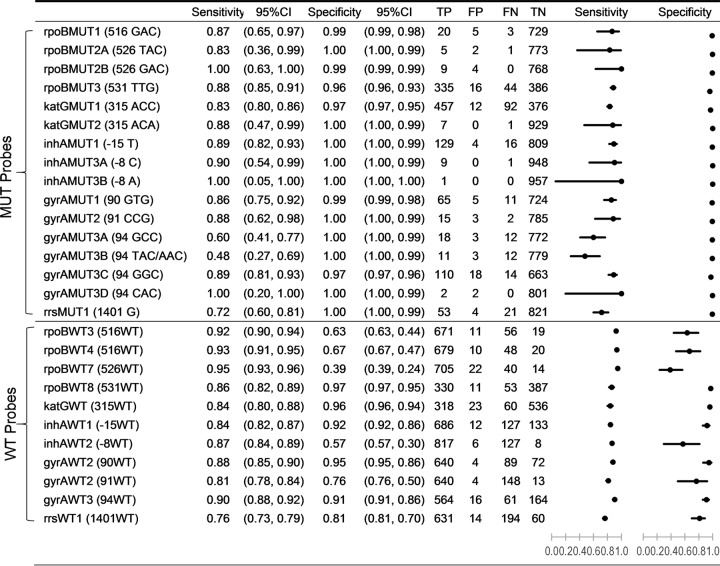
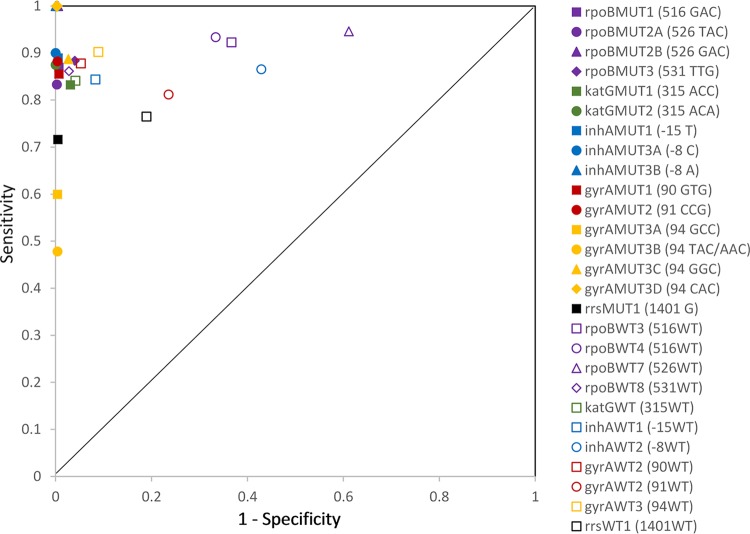
Sixteen specific MTBDRplus v2 and MTBDRsl MUT probes and 11 corresponding WT probes interrogated 10 gene regions with potential resistance-conferring mutations. The sequence of each of these regions was examined independently in each isolate using targeted PSQ as the reference sequencing method (Fig. 1). The sensitivity of the MUT probes when compared to reference sequence results ranged from 0.48 to 1.00, and the specificity ranged from 0.96 to 1.00. MUT probes for gyrA codon 94 (gyrAMUT3A and gyrAMUT3B) were the least sensitive. WT probe sensitivity ranged from 0.76 to 0.95, and WT specificity ranged from 0.39 to 0.97. WT probes rpoBWT7 (codon 526) and inhAWT2 (nucleotide position −8) demonstrated the lowest specificity. Figure 2 shows the point estimates for sensitivity by 1 − specificity for WT and MUT probes.
FIG 1.
Estimates and 95% confidence intervals for sensitivity and specificity in addition to true-positive, false-positive, false-negative, and true-negative (TP, FP, FN, and TN) values for the comparison of individual MTBDRplus and MTBDRsl mutation and wild-type probe results to PyroMark Q96 ID pyrosequencing results.
FIG 2.
Sensitivity versus 1 − specificity scatter plot of MTBDRplus and MTBDRsl mutation and wild-type probe results compared to PyroMark Q96 ID pyrosequencing results.
MTBDRplus v2 and MTBDRsl resistance determination versus sequencing.
The majority of MTBDRplus v2 and MTBDRsl assay results were classified as resistant due to the direct detection of a resistance-conferring mutation by hybridization of genomic M. tuberculosis DNA to an MUT probe. However, as Table 2 demonstrates, 13% (60) of RIF-resistant, 12% (19) of inhA-associated INH-resistant, 6% (15) of FQ-resistant, and 2% (1) of SLID-resistant results were due to the absence of WT probe hybridization without MUT probe hybridization, indicating the absence of a wild-type sequence, which was interpreted as the presence of an unknown mutation in that region. Of these 95 resistant isolates that were classified as resistant based only on absent WT probe hybridization, 24 could not be compared to sequencing, as PSQ results were also indeterminate for the corresponding sequence region. Of the 71 with comparable sequence data, 11 were discordant with sequencing (no hybridization occurred on an rpoB WT probe while PSQ reported a wild-type sequence in the corresponding region), 56 were concordant with sequencing results (no hybridization occurred on an MTBDRplus v2 or MTBDRsl WT probe, and PSQ detected a mutation in the corresponding region), and 4 were mixed (no hybridization occurred on multiple MTBDRplus v2 or MTBDRsl WT probes, and PSQ reported a wild-type sequence in one region and a mutation sequence in another region). Among the 56 specimens concordant with sequencing, the mutation identified by sequencing was identical to an MUT probe in 28 (50%) cases, indicating that the corresponding MUT probe failed to hybridize even though the mutation was present. The most frequent MUT probe to fail to hybridize was rpoBMUT3 (531TTG), which occurred in 17 instances despite the presence of that mutation.
TABLE 2.
Specimens classified as resistant by MTBDRplus or MTBDRsl due to failure of wild-type probe hybridization only and corresponding pyrosequencing results
| No. by drug resistance category and gene loci | Resistance classification |
|
|---|---|---|
| Absent wild-type probe(s)a | Pyrosequencing result | |
| RIF (rpoB) | ||
| 19 | WT1, WT2, WT3, WT4, WT6, WT7, and/or WT8 | Indeterminate |
| 7 | WT8 | No mutations |
| 1 | WT3 and WT4 | No mutations |
| 1 | WT2 and WT3 | 511CGG and 516TAC |
| 1 | WT1, WT2, and WT8 | 515ATA |
| 1 | WT1 and WT7 | 515ATA and 526AAC |
| 1 | WT3 | 516TAC |
| 1 | WT3 and WT4 | 516TAC |
| 2 | WT4 | 516TAC |
| 1 | WT7 | 526AAC |
| 1 | WT7 | 526TGC |
| 1 | WT8 | 526CTC |
| 1 | WT8 | 526TGC |
| 4 | WT8 | 531TCG/TGG |
| 17 | WT8b | 531TTG |
| 1 | WT8 | 533CCG |
| INH (inhA) | ||
| 4 | WT1 | Indeterminate |
| 13 | WT1 | −17 T |
| 2 | WT1c and WT2 | −15 T |
| FQ (gyrA) | ||
| 1 | WT2 | Indeterminate |
| 1 | WT1 and WT3 | 88GCC |
| 1 | WT1 | 88GCC and 95ACC |
| 1 | WT1 | 88TGC and 95ACC |
| 1 | WT1, WT2, and WT3 | 90GTG and 95ACC |
| 2 | WT3d | 94GGC and 95ACC |
| 8 | WT3e | 94TAC and 95ACC |
| SLID (rrs) | ||
| 1 | WT1f | 1401G |
Discordant pairs are italicized and concordant pairs are underlined.
rpoBWT8 and rpoBMUT3 probes were absent.
inhAWT1 and inhAMUT1 probes were absent.
gyrAWT3 and gyrAMUT3C probes were absent.
gyrAWT3 and gyrAMUT3B probes were absent.
rrsWT1 and rrsMUT1 probes were absent.
Contribution of absent WT probe hybridization to phenotypic resistance classification.
In order to determine if the inclusion of resistant results due to the sole absence of WT probe hybridization contributes significantly to the molecular detection of drug resistance, resistance classification based on the presence of MUT probe hybridization and the absence of WT probe hybridization was compared to resistance classification based on both the presence of MUT probe hybridization and the absence of WT probe hybridization and on the absence of WT probe hybridization only (Table 3). For RIF and FQs, the inclusion of the two resistance classification methods significantly improved the detection of phenotypic resistance when the areas under the curve were compared. This was also true for inhA-associated INH resistance; the inclusion of resistance classification based on the sole absence of WT probe hybridization significantly improved the detection of phenotypic INH resistance. However, when assessed in conjunction with katG-associated INH resistance, the added value was not significant, as the majority of inhA-associated INH resistance co-occurred with a katG315 mutation and would have been detected based on katG-associated INH resistance.
TABLE 3.
Comparison of line probe assay resistance classification (either by mutation probe hybridization plus failure of wild-type probe hybridization or by mutation probe hybridization plus failure of wild-type probe hybridization and failure of wild-type probe hybridization alone) to MGIT results by area under the receiver operating characteristic curve
| Drug resistance category (no. of specimens)a | LPA resistance classification | No. TP | No. FP | No. FN | No. TN | AUC | P valueb |
|---|---|---|---|---|---|---|---|
| RIF (809) | MUT present + absent WT | 415 | 2 | 63 | 329 | 0.9311 | <0.001 |
| MUT present + absent WT or absent WT only | 462 | 7 | 16 | 324 | 0.9727 | ||
| INH katG and inhA (790) | MUT present + absent WT | 472 | 0 | 37 | 281 | 0.9637 | 0.17 |
| MUT present + absent WT or absent WT only | 478 | 1 | 31 | 280 | 0.9678 | ||
| INH katG | MUT present + absent WT | 450 | 0 | 59 | 281 | 0.9420 | n/ac |
| MUT present + absent WT or absent WT only | 450 | 0 | 59 | 281 | 0.9420 | ||
| INH inhA | MUT present + absent WT | 139 | 0 | 370 | 281 | 0.6365 | <0.001 |
| MUT present + absent WT or absent WT only | 156 | 1 | 353 | 280 | 0.6515 | ||
| MXF (742) | MUT present + absent WT | 218 | 4 | 25 | 495 | 0.9446 | <0.001 |
| MUT present + absent WT or absent WT only | 232 | 5 | 11 | 494 | 0.9724 | ||
| OFX (742) | MUT present + absent WT | 221 | 1 | 23 | 497 | 0.9519 | <0.001 |
| MUT present + absent WT or absent WT only | 234 | 3 | 10 | 495 | 0.9765 | ||
| AMK (672) | MUT present + absent WT | 45 | 0 | 8 | 619 | 0.9245 | 0.32 |
| MUT present + absent WT or absent WT only | 46 | 0 | 7 | 619 | 0.9340 | ||
| CAP (672) | MUT present + absent WT | 43 | 2 | 8 | 619 | 0.9200 | 0.32 |
| MUT present + absent WT or absent WT only | 44 | 2 | 7 | 619 | 0.9298 | ||
| KAN (672) | MUT present + absent WT | 45 | 0 | 51 | 576 | 0.7344 | 0.32 |
| MUT present + absent WT or absent WT only | 46 | 0 | 50 | 576 | 0.7396 |
Only specimens with valid (resistant or susceptible) results for LPA and MGIT were included in the analysis.
Significant P values for change in AUC identified in bold.
n/a, not available.
DISCUSSION
The MUT probes included on the MTBDRplus v2 and MTBDRsl tests demonstrated significantly higher specificity than that of the WT probes. Conversely, WT probes demonstrated marginally higher sensitivity than that of MUT probes. Together, these diagnostic differences are ideal for determining drug resistance profiles, as they yield the fewest number of false-positive results as determined by sequencing. Plausible explanations for resistance classification based solely on the absence of WT hybridization (without MUT probe hybridization) include technical obstacles to hybridization or the presence of a mutation for which no MUT probes were designed to detect. In our study, for any gene region, the absence of the WT probe hybridization most often indicated a failure of MUT probe hybridization rather than the presence of novel or rare mutations. PSQ confirmed the presence of expected mutations (for which there were MUT probes) in approximately 50% of instances where the MUT and WT probes were absent.
Significant variability, however, was evident when assessing the sensitivity of the various MUT probes, including probes covering the same codons. For example, two different gyrA94 mutations (GCC and TAC/AAC) had low sensitivities of 0.60 and 0.48 while two others (GGC and CAC) had high to very high sensitivities of 0.89 and 1.00 when results were compared to the results of the reference sequencing method. Initially, we thought this poor gyrA sensitivity was caused by differential co-occurrence of a nonresistance conferring mutation at gyrA95. However, upon closer inspection, of the 180 specimens with gyrA94 mutations, more than 97% harbored a co-occurring mutation at gyrA95, indicating that the presence of the gyrA95 mutation did not inhibit binding to the gyrA94 MUT probe. The poor sensitivity seen for the gyrA94 GCC and TAC/AAC MUT probes may be due instead to poor mutant template binding. The low sensitivity of the TAC/AAC probe, in particular, may be a result of the MUT probe having to recognize two different mutations, which ultimately resulted in poor sensitivity. Specificity was also found to be low (less than 0.67) for rpoB WT probes at codons 516, 526, and 531. These low specificities may also be attributable to differences in strand accessibility between wild-type and mutant sequences, making the WT probes less likely to bind to the template DNA versus their corresponding MUT probes (28).
Among those specimens classified as resistant by MTBDRplus v2, only 13% of RIF resistance was due to the sole absence of WT probe hybridization for rpoB. This is lower than the 21% to 33% contribution of resistance due to the sole absence of WT probe hybridization reported in India, France, and Vietnam (29–31). Interestingly, in the current study, no katG-associated INH resistance was due the sole absence of WT probe hybridization at katG as opposed to 6% of results previously reported in India and 9% in Vietnam (29, 32). Approximately 12% of inhA-associated INH resistance in the current study was attributable to the sole absence of WT probe hybridization at the inhA promoter, which is higher than the 9% reported in India and 0% reported in Vietnam. Six percent of FQ resistance, as classified by MTBDRsl, was due to the sole absence of WT probe hybridization. Our results contrast significantly with the results of two studies conducted in the Congo and the Democratic Republic of Congo, where over 60% of FQ resistance was attributable to the sole absence of WT probe hybridization (17, 18). Our results more closely resemble those reported by Brossier et al. (33) and Huang et al. (34), in which less than 20% of FQ resistance was attributable to the sole absence of WT probe hybridization. Given the regional proximity of the two studies, this may indicate the local presence of a mutation that inhibits hybridization of MTBDRsl probes. This underscores the need for continued evaluation of regional mutation frequencies and for a greater understanding of oligonucleotide binding mechanisms to continue to ensure optimal MTBDRplus v2 and MTBDRsl performance.
Rare or novel mutations, as detected by sequencing, did not account for the majority of resistance determination based on the absence of WT probe hybridization in this study. Rather, half of the specimens contained mutations that should have hybridized to the corresponding MUT probes but failed to do so. The MUT probes that most commonly failed to hybridize were rpoBMUT3 531TTG (17 times) and gyrAMUT3B 94TAC (8 times). This may indicate the need for continued assay optimization over the inclusion of novel MUT probes.
Compared to phenotypic DST, resistance determination based on the sole absence of WT probe hybridization added an additional 10% sensitivity to the detection of phenotypically RIF-resistant isolates and 6% to phenotypically FQ-resistant isolates, with less than 1% false-positive results. These differences in performance were significant by AUC analysis. The absence of WT probe hybridization resistance classification provided a unique fail-safe method for indirect detection of novel or rare mutations in this study.
Limitations.
MTBDRplus v2 and MTBDRsl results were not included in the sequencing comparison analysis if specimens yielded uninterpretable PSQ results. Inherent errors in the PSQ system were most likely the cause of uninterpretable PSQ results; however, the possibility of the presence of a novel mutation cannot be excluded.
In conclusion, resistance determination based on the sole absence of WT probe hybridization is critical for detecting drug resistance by MTBDRplus v2 and MTBDRsl and other fixed probe hybridization systems. The inclusion of these results significantly improved phenotypic resistance detection for RIF and FQs in our study, with negligible increases in false-positive results.
ACKNOWLEDGMENTS
T.C.V. acknowledges support from the South African Medical Research Council (SA-MRC) Centre for Tuberculosis Research, Department of Science and Technology/National Research Foundation Centre of Excellence for Biomedical Tuberculosis Research, Division of Molecular Biology and Human Genetics, University of Stellenbosch.
Funding Statement
T.C.R. receives salary support from the Foundation for Innovative New Diagnostics (FIND), a nonprofit organization. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies.
REFERENCES
- 1.World Health Organization. 2014. Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2014. Drug-resistant TB surveillance and response. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.World Health Organization. 2015. Global tuberculosis report 2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Hoek KG, Van Rie A, van Helden PD, Warren RM, Victor TC. 2011. Detecting drug-resistant tuberculosis: the importance of rapid testing. Mol Diagn Ther 15:189–194. doi: 10.1007/BF03256410. [DOI] [PubMed] [Google Scholar]
- 5.Kalokhe AS, Shafiq M, Lee JC, Ray SM, Wang YF, Metchock B, Anderson AM, Nguyen ML. 2013. Multidrug-resistant tuberculosis drug susceptibility and molecular diagnostic testing. Am J Med Sci 345:143–148. doi: 10.1097/MAJ.0b013e31825d32c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme CC, Saacks S, O'Brien RJ. 2013. The changing landscape of diagnostic services for tuberculosis. Semin Respir Crit Care Med 34:17–31. doi: 10.1055/s-0032-1333468. [DOI] [PubMed] [Google Scholar]
- 7.Bwanga F, Hoffner S, Haile M, Joloba ML. 2009. Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis. BMC Infect Dis 9:67. doi: 10.1186/1471-2334-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drobniewski F, Nikolayevskyy V, Maxeiner H, Balabanova Y, Casali N, Kontsevaya I, Ignatyeva O. 2013. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med 11:190. doi: 10.1186/1741-7015-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad S, Mokaddas E. 2009. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. Respir Med 103:1777–1790. doi: 10.1016/j.rmed.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Alcaide F, Coll P. 2011. Advances in rapid diagnosis of tuberculosis disease and anti-tuberculous drug resistance. Enferm Infecc Microbiol Clin 29(Suppl):S34–S40. [DOI] [PubMed] [Google Scholar]
- 11.Naidoo P, du Toit E, Dunbar R, Lombard C, Caldwell J, Detjen A, Squire SB, Enarson DA, Beyers N. 2014. A comparison of multidrug-resistant tuberculosis treatment commencement times in MDRTBplus line probe assay and Xpert MTB/RIF-based algorithms in a routine operational setting in Cape Town. PLoS One 9(7):e103328. doi: 10.1371/journal.pone.0103328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Grady J, Maeurer M, Mwaba P, Kapata N, Bates M, Hoelscher M, Zumla A. 2011. New and improved diagnostics for detection of drug-resistant pulmonary tuberculosis. Curr Opin Pulm Med 17:134–141. doi: 10.1097/MCP.0b013e3283452346. [DOI] [PubMed] [Google Scholar]
- 13.Pai M, Schito M. 2015. Tuberculosis diagnostics in 2015: landscape, priorities, needs, and prospects. J Infect Dis 211:S21–S28. doi: 10.1093/infdis/jiu803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Liu S, Wang Q, Wang L, Tang S, Wang J, Lu W. 2013. Rapid diagnosis of drug resistance to fluoroquinolones, amikacin, capreomycin, kanamycin and ethambutol using genotype MTBDRsl assay: a meta-analysis. PLoS One 8(2):e55292. doi: 10.1371/journal.pone.0055292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theron G, Peter J, Richardson M, Barnard M, Donegan S, Warren R, Steingart KR, Dheda K. 2014. The diagnostic accuracy of the GenoType MTBDRsl assay for the detection of resistance to second-line anti-tuberculosis drugs. Cochrane Database Syst Rev 10:CD010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacoma A, Garcia-Sierra N, Prat C, Maldonado J, Ruiz-Manzano J, Haba L, Gavin P, Samper S, Ausina V, Dominguez J. 2012. GenoType MTBDRsl for molecular detection of second-line-drug and ethambutol resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol 50:30–36. doi: 10.1128/JCM.05274-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaswa MK, Aloni M, Nkuku L, Bakoko B, Lebeke R, Nzita A, Muyembe JJ, de Jong BC, de Rijk P, Verhaegen J, Boelaert M, Ieven M, Van Deun A. 2014. Pseudo-outbreak of pre-extensively drug-resistant (Pre-XDR) tuberculosis in Kinshasa: collateral damage caused by false detection of fluoroquinolone resistance by GenoType MTBDRsl. J Clin Microbiol 52:2876–2880. doi: 10.1128/JCM.00398-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aubry A, Sougakoff W, Bodzongo P, Delcroix G, Armand S, Millot G, Jarlier V, Courcol R, Lemaitre N. 2014. First evaluation of drug-resistant Mycobacterium tuberculosis clinical isolates from Congo revealed misdetection of fluoroquinolone resistance by line probe assay due to a double substitution T80A-A90G in GyrA. PLoS One 9(4):e95083. doi: 10.1371/journal.pone.0095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurenzo D, Mousa SA. 2011. Mechanisms of drug resistance in Mycobacterium tuberculosis and current status of rapid molecular diagnostic testing. Acta Trop 119:5–10. doi: 10.1016/j.actatropica.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Hillery N, Groessl EJ, Trollip A, Catanzaro D, Jackson L, Rodwell TC, Garfein RS, Lin SY, Eisenach K, Ganiats TG, Park D, Valafar F, Rodrigues C, Crudu V, Victor TC, Catanzaro A. 2014. The Global Consortium for Drug-resistant Tuberculosis Diagnostics (GCDD): design of a multi-site, head-to-head study of three rapid tests to detect extensively drug-resistant tuberculosis. Trials 15:434. doi: 10.1186/1745-6215-15-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catanzaro A, Rodwell TC, Catanzaro DG, Garfein RS, Jackson RL, Seifert M, Georghiou SB, Trollip A, Groessl E, Hillery N, Crudu V, Victor TC, Rodrigues C, Lin GS, Valafar F, Desmond E, Eisenach K. 2015. Performance comparison of three rapid tests for the diagnosis of drug-resistant tuberculosis. PLoS One 10(8):e0136861. doi: 10.1371/journal.pone.0136861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hain Lifescience GmbH. 2012. GenoType MTBDRplus version 2.0: instruction manual. Hain Lifescience GmbH, Nehren, Germany. [Google Scholar]
- 23.Hain Lifescience GmbH. 2009. GenoType MTBDRsl version 1.0: instruction manual. Hain Lifescience GmbH, Nehren, Germany. [Google Scholar]
- 24.Hillemann D, Rusch-Gerdes S, Richter E. 2007. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol 45:2635–2640. doi: 10.1128/JCM.00521-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SY, Desmond EP. 2014. Molecular diagnosis of tuberculosis and drug resistance. Clin Lab Med 34:297–314. doi: 10.1016/j.cll.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Lin SY, Rodwell TC, Victor TC, Rider EC, Pham L, Catanzaro A, Desmond EP. 2014. Pyrosequencing for rapid detection of extensively drug-resistant Mycobacterium tuberculosis in clinical isolates and clinical specimens. J Clin Microbiol 52:475–482. doi: 10.1128/JCM.01821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newcombe RG. 1998. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 17:2635–2650. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Tracevska T, Jansone I, Broka L, Marga O, Baumanis V. 2002. Mutations in the rpoB and katG genes leading to drug resistance in Mycobacterium tuberculosis in Latvia. J Clin Microbiol 40:3789–3792. doi: 10.1128/JCM.40.10.3789-3792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singhal R, Myneedu VP, Arora J, Singh N, Sah GC, Sarin R. 2014. Detection of multi-drug resistance & characterization of mutations in Mycobacterium tuberculosis isolates from North-Eastern States of India using GenoType MTBDRplus assay. Indian J Med Res 140:501–506. [PMC free article] [PubMed] [Google Scholar]
- 30.Brossier F, Veziris N, Truffot-Pernot C, Jarlier V, Sougakoff W. 2006. Performance of the genotype MTBDR line probe assay for detection of resistance to rifampin and isoniazid in strains of Mycobacterium tuberculosis with low- and high-level resistance. J Clin Microbiol 44:3659–3664. doi: 10.1128/JCM.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huyen MN, Tiemersma EW, Lan NT, Cobelens FG, Dung NH, Sy DN, Buu TN, Kremer K, Hang PT, Caws M, O'Brien R, van Soolingen D. 2010. Validation of the GenoType MTBDRplus assay for diagnosis of multidrug resistant tuberculosis in South Vietnam. BMC Infect Dis 10:149. doi: 10.1186/1471-2334-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buu TN, Huyen MN, van Soolingen D, Lan NT, Quy HT, Tiemersma EW, Borgdorff MW, Cobelens FG. 2010. The Mycobacterium tuberculosis Beijing genotype does not affect tuberculosis treatment failure in Vietnam. Clin Infect Dis 51:879–886. doi: 10.1086/656410. [DOI] [PubMed] [Google Scholar]
- 33.Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. 2010. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol 48:1683–1689. doi: 10.1128/JCM.01947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang WL, Chi TL, Wu MH, Jou R. 2011. Performance assessment of the GenoType MTBDRsl test and DNA sequencing for detection of second-line and ethambutol drug resistance among patients infected with multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 49:2502–2508. doi: 10.1128/JCM.00197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]