Abstract
The conventional anti-Bartonella henselae IgM enzyme-linked immunosorbent assay (IgM-ELISA) methods for diagnosing cat scratch disease (CSD) remain poor in both sensitivity and specificity. We sought to develop an IgM-ELISA with improved accuracy in the serodiagnosis of CSD by exploring the antigens that are most suitable for an ELISA. We prepared 5 different protein antigens: antigen I (sonicated B. henselae whole-cell antigen), antigen II (N-lauroyl-sarcosine-insoluble antigen), antigen III (processed sarcosine-soluble antigen), and antigen IV and antigen V (sarcosine-insoluble and sarcosine-soluble antigens refined by DEAE-Sepharose Fast Flow ion-exchange chromatography). The IgM antibodies in the sera of 47 patients with clinically suspected CSD (24 definite, 23 suspected) and of 85 healthy individuals were examined by ELISAs using the 5 antigens, and the results were compared with those of an IgM indirect fluorescent antibody assay (IgM-IFA). In a reference panel, which consisted of 5 positive and 5 negative sera, antigen I and antigen III failed to distinguish between the two statuses, whereas the other three antigens succeeded in distinguishing between them. When the cutoff value was set at the 98th percentile of the ELISA index for healthy individuals, the sensitivity of IgM-IFA for the 24 cases of definite CSD was 54%, whereas the sensitivities of the IgM-ELISAs with antigen II, IV, and V were 75%, 83%, and 75%, respectively. The sensitivities of these three IgM-ELISAs for all 47 of the clinically suspected cases were 49%, 64%, and 51%, respectively. In contrast, the sensitivity of IgM-IFA was 28%. These results indicate that the refined sarcosine-insoluble proteins (antigen IV), which possessed the highest specificity among the 5 antigens, are the most appropriate for developing an IgM-ELISA for the highly specific serodiagnosis of CSD.
INTRODUCTION
Cat scratch disease (CSD), an infection which is caused by the Gram-negative bacillus Bartonella henselae, is a zoonotic infection which occurs worldwide and which is associated with diverse clinical manifestations (1–3). Although the predominant clinical feature at the onset of CSD is a regional lymphadenopathy, atypical manifestations may occur, along with systemic symptoms, such as hepatic-splenic granuloma, endocarditis, encephalopathy, and neuroretinitis (1–3). The laboratory diagnosis of CSD mainly depends on a serological analysis because it is difficult to isolate B. henselae from patients.
An indirect fluorescent antibody assay (IFA) for anti-B. henselae IgG is regarded as the gold standard for detecting antibodies to B. henselae. However, although this method has high specificity, the sensitivity varies widely in different reports (4–6). Although the detection of IgM antibodies is crucial for the diagnosis of active B. henselae infection, the sensitivity of IFA for anti-B. henselae IgM (IgM-IFA) is reported to be especially low (5–8).
The detection of anti-B. henselae IgM by various enzyme-linked immunosorbent assays (IgM-ELISA) has been proposed as an alternative to IgM-IFA. Several IgM-ELISAs use sonically disrupted B. henselae antigens (whole-cell proteins) (6, 7, 9, 10) or the putative outer membrane proteins which correspond to antigens fractionated from surfactant (i.e., N-lauroyl-sarcosine)-disrupted B. henselae (sarcosine-insoluble proteins) (11–13). However, the constituents of the crude preparations vary widely depending on the methods used for the isolation of the antigens. Thus, it is necessary to increase the sensitivity and specificity of the assay in order to improve its clinical utility.
With this objective, we developed a new anti-B. henselae IgM-ELISA, which uses a sarcosine-insoluble antigen which was refined by passage through exchange chromatography on a DEAE-Sepharose Fast Flow column to improve the serodiagnosis of CSD. Using the IgM-IFA test results as a guide, we evaluated the performance of the new IgM-ELISA in comparison to ELISAs using other antigen preparations, which were developed by incorporating other fractions of sarcosine-soluble or -insoluble proteins.
MATERIALS AND METHODS
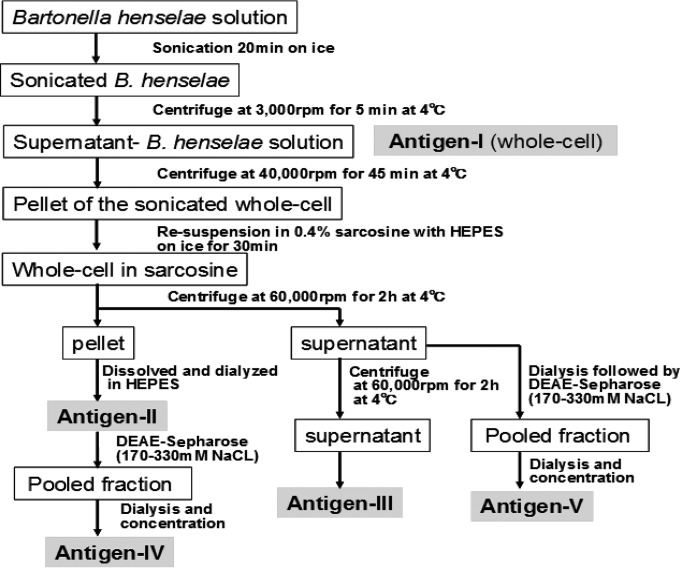
Five different antigen preparations of B. henselae for IgM-ELISA (Fig. 1) were used.
FIG 1.
The flow diagram for preparation of antigen I to V. B. henselae harvested from the agar medium was sonicated and treated with sarcosine. The preparation was variously processed to obtain antigen I through antigen V.
(i) Whole-cell antigen (antigen I).
B. henselae 49882 was grown on chocolate agar plates for 4 to 5 days at 35°C in CO2. The bacteria were harvested in 10 mM HEPES (Sigma) (pH 7.4) and sonicated for 20 min on ice. The lysate was centrifuged at 3,000 rpm for 5 min at 4°C, and the supernatant was obtained as a whole-cell antigen.
(ii) Sarcosine-insoluble antigen (antigen II).
The supernatant was transferred to new tubes and centrifuged for another 45 min at 40,000 rpm at 4°C. The pellet was resuspended in 0.4% N-lauroyl-sarcosine (sarcosine) with HEPES buffer and then placed on ice for 30 min. After another centrifugation at 60,000 rpm for 2 h at 4°C, the pellet (the insoluble fraction) was dissolved in HEPES buffer, and the sarcosine was removed by filtration through a dialysis membrane (Viskase Companies, Inc.) at 4°C overnight. We named the fraction attached to the membrane “antigen II.”
(iii) Processed sarcosine-soluble antigen (antigen III).
The sarcosine-soluble proteins obtained as the supernatant of the treatment described above were dialyzed by the same method. Another centrifugation was done at 60,000 rpm for 2 h at 4°C, and the supernatant was collected as a highly processed antigen (antigen III) (14).
(iv) Sarcosine-insoluble and sarcosine-soluble antigens refined by DEAE-Sepharose chromatography (antigen IV and antigen V).
In a preliminary experiment, the whole-cell proteins of B. henselae 49882 were fractionated by ion-exchange chromatography on a DEAE-Sepharose Fast Flow column (DEAE-Sepharose; GE Healthcare Bio-Sciences AB) as follows. A 10-by-200-mm column packed with DEAE-Sepharose was filled with 10 mM Tris-HCl buffer (pH 8.3) without NaCl, and 2 ml of the whole-cell proteins from B. henselae 49882 was placed on top of the column. Next, the same Tris-HCl buffer (pH 8.3) was run at 1 ml/min mixed with an NaCl gradient of 0 to 1,000 mM. The eluate was continuously collected into 1-ml tubes by a fraction collector at room temperature. The protein concentration of each tube was measured at an optical density (OD) of 280 nm. Each fraction was tested for antigens that reacted with pooled B. henselae IgM-positive (IgM+) sera (IgM-IFA titer, ≥1:40) from 3 patients with CSD. The concentration of NaCl that provided the peak antigen concentrations was then determined. Following the evaluation of the optimal NaCl concentration, the DEAE-Sepharose mixture was refreshed in each of the subsequent experiments by washing with 1,000 mM NaCl, after which it was filled with 10 mM Tris-HCl buffer (pH 8.3) without NaCl.
The sarcosine-insoluble proteins (antigen II) were applied to the refreshed column and run with 10 mM Tris-HCl buffer (pH 8.3) containing the previously determined optimal NaCl concentrations. The fractions from the optimal range of NaCl concentrations were collected. The pooled fractions were then dialyzed and concentrated to obtain antigen IV. The same method was applied to the sarcosine-soluble proteins to obtain antigen V.
The reference serum panel for the preliminary evaluation of the 5 antigens.
To estimate the titers of anti-B. henselae IgM by the ELISAs using the 5 different antigens (antigen I to antigen V), a reference panel of 10 serum samples was prepared that was composed of 5 positive serum samples (positive control [PC]), which were selected from clinical specimens with B. henselae IgM titers of ≥1:20 by an in-house IgM-IFA (5, 15), and 5 negative serum samples (negative control [NC]), which were obtained from healthy individuals.
The wells of Polysorb microtiter plates (Sumilon; Sumitomo Bakelite, Japan) were coated overnight at 4°C with 100 μl of an optimally diluted preparation of B. henselae antigen in 50 mM carbonate-bicarbonate coating buffer (pH 9.2). Each antigen was serially diluted using carbonate-bicarbonate coating buffer (pH 9.2) to make protein concentrations of 0.1, 0.05, 0.025, and 0.013 measured at 280 nm. The distinction of the reactivities for the positive and negative portions of the panel was examined at each dilution to find out the optimal dilution. The result showed that the optimal concentration was 0.05 for antigen I to antigen V. The plates were washed, blocked with 5% skim milk (Wako Pure Chemical Industries, Japan)–phosphate-buffered saline (PBS)–0.1%Tween 20 (PBS-T), and incubated for 4 h at room temperature. Control panel sera (PC and NC sera) were diluted 1:100 in PBS-T, incubated for 1 h at 37°C, and washed with PBS-T. After 100 μl of horseradish peroxidase-labeled goat anti-human IgM (Sigma) was added, the sera were incubated for 1 h. Citrate buffer (pH 5.0) containing ortho-phenylenediamine was added, and the mixture was incubated at room temperature. Color development was stopped after 30 min by the addition of 4 N H2SO4. The OD of the plates was read at 490 nm. All of the controls were tested in triplicate. The PC and NC in the panel for the IgM-ELISAs using the 5 different antigens were tested in triplicate, and the average ODs for the PC and NC were calculated for each of the 5 ELISAs.
Determination of the antigen most appropriate for use in the IgM-ELISA.
The candidate antigens were selected based on the reference panel results. The performance of the ELISAs using the selected antigens was subsequently evaluated with the sera from 47 patients in whom CSD was clinically suspected due to lymphadenopathy and/or fever of unknown origin with a history of contact with a cat or dog. These specimens were obtained when a serological diagnosis for CSD using IFA or a real-time PCR for B. henselae DNA was ordered in our clinical laboratory at Yamaguchi University. Of the 47 serum samples, 24 were judged to be definite cases of CSD when an IgG-IFA to B. henselae or an IgM-IFA yielded a titer of ≥1:256 (n = 21) or ≥1:20 (n = 13), respectively, or when a real-time PCR yielded a positive result (n = 5); i.e., expressed in another way, they were broken down into 10 cases that were IgM+ and IgG+; 3 cases that were IgM+ and IgG−; 8 cases that were IgM− and IgG+; and 3 cases that were IgM−, IgG−, and PCR+. The remaining 23 sera, which were obtained from patients in whom CSD was clinically suspected, were found to be serologically negative when the IgG-IFA and IgM-IFA yielded titers of <1:256 and <1:20, respectively. Thus, these 23 cases were treated as “suspected CSD.” The serum samples of 85 healthy individuals who had no history of overt lymphoadenopathy or cat scratches or bites and who had negative IgG-IFA (titer, <1:64) and IgM-IFA (titer, <1:20) results were used as controls.
All specimens were tested in duplicate. The PC and NC were included in each assay run. The ELISA index for each specimen was calculated as previously reported (14). The cutoff value was set at the 98th percentile of the distribution of the ELISA indexes for the 85 control specimens.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Structural analyses of the 5 different proteins of B. henselae were conducted by SDS-PAGE and Western blotting. These procedures, which were essentially the same as those reported previously (14), were used with minor modifications. Briefly, the protein staining for SDS-PAGE was performed with the use of a Silver Stain Plus kit (Bio-Rad), while polyclonal rabbit anti-human IgM/horseradish peroxidase (HRP) antibody (Dako) was used as the antibody and Super Signal West Dura substrate (Thermo Fisher Scientific) was used as the chemiluminescent substrate for the Western blotting.
Immunofluorescence assay.
The in-house antigen slides were prepared for the IgG-IFA using B. henselae ATCC 49882 (at approximately 108 cells/ml) which was cocultivated with Vero cells. The slides for the IgM-IFA were prepared using B. henselae ATCC 49882 without cocultivation with Vero cells. The details of the procedures have been described elsewhere (5, 14). The antibody titer was expressed as the highest dilution of serum that yielded a positive staining result. Sera with IgG titers of ≥1:256 or IgM titers of ≥1:20 were regarded as positive, whereas sera with IgG titers of <1:256 or IgM titers of <1:20 were regarded as negative (5, 15). The IFAs were performed by one technician. The ELISA was performed by another technician. Both technicians were blind to the clinical and laboratory findings for the specimens.
Real-time PCR.
Specific B. henselae virB4 DNA from whole-blood specimens or lymph node specimens from suspected patients was detected by a real-time PCR assay as reported previously (16) using specific primers (forward, 5′-AGCGAAGAAAACACAATCTGAA-3; reverse, 5′-TCCATAGCTTTCCAATCCTTCT-3′) and Universal ProbeLibrary Probe 135 (Roche Applied Science).
Data analysis.
The sensitivity of the IgM-ELISA was calculated as the proportion of positive sera among the 47 sera from the CSD patients (24 definite, 23 suspected). The specificity, which corresponded to the proportion of negative results among the control sera from the 85 healthy individuals, was always 98% because the cutoff value was deliberately set at the 98th percentile of ELISA indexes for those control sera.
RESULTS
Preparation of refined sarcosine-insoluble and sarcosine-soluble proteins.
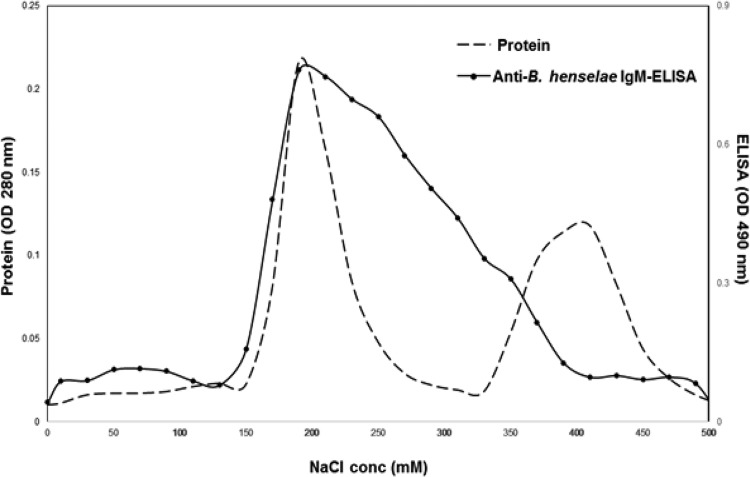
Whole-cell proteins (antigen I) from B. henselae were fractionated by DEAE-Sepharose Fast Flow ion-exchange chromatography using an NaCl gradient from 0 to 1,000 mM. As shown in Fig. 2, two peaks were identified from the protein concentration curve (indicated by the dashed line), at the NaCl concentration ranges of 150 to 250 mM and 350 to 450 mM NaCl. By analyzing the reactivity of each elution fraction to the anti-B. henselae IgM by an ELISA, a profile of the serial changes in IgM reactivity was obtained (the curve is indicated by a solid line). It had a broad peak at the concentration range of 150 to 400 mM NaCl. From this observation, we chose 170 to 330 mM NaCl as the optimal concentration range for the refinement of the sarcosine-insoluble and sarcosine-soluble proteins (antigen IV and antigen V) by DEAE-Sephadex chromatography.
FIG 2.
The reactivity of fractions from DEAE-Sepharose to anti-B. henselae IgM antibody. The sonicated B. henselae whole-cell proteins were processed by DEAE-Sepharose Fast Flow ion-exchange chromatography. The profiles of the protein concentrations (conc) (optical density [OD] at 280 nm) and reactivity to anti-B. henselae IgM antibody are depicted by dashed and solid lines, respectively.
The SDS-PAGE and Western blot analyses of the 5 B. henselae protein preparations.
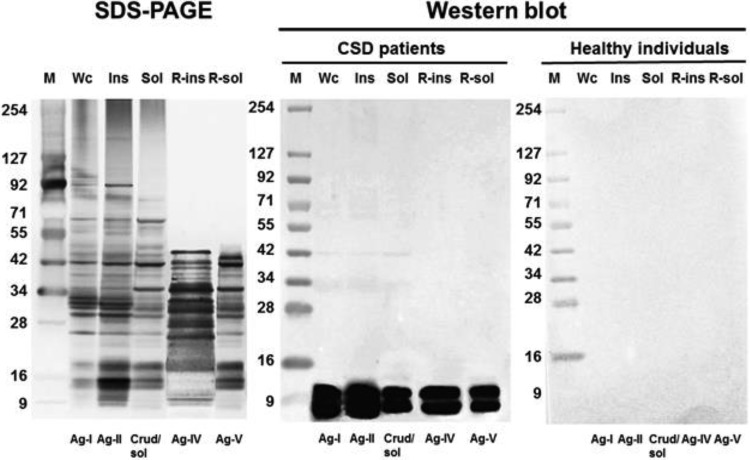
The SDS-PAGE analyses of the whole-cell and sarcosine-insoluble proteins showed similar patterns, with bands corresponding to a molecular mass ranging from 8 to 175 kDa; whereas that of the sarcosine-soluble proteins showed a different pattern, with fewer bands in the higher molecular mass range (Fig. 3). In contrast, the SDS-PAGE analysis of the refined sarcosine-insoluble and sarcosine-soluble proteins showed a markedly different pattern, with a complete absence of bands in the region above 45 kDa.
FIG 3.
SDS-PAGE and Western blot analyses of 5 proteins associated with Bartonella henselae. An SDS-PAGE analysis was performed with the use of silver staining for 5 different protein preparations. Wc, whole-cell B. henselae; Ins, 0.4% N-lauroyl sarcosine-insoluble proteins; Sol, 0.4% N-lauroyl sarcosine-soluble proteins; R-ins, sarcosine-insoluble proteins refined by DEAE-Sepharose chromatography; R-sol, sarcosine-soluble proteins refined by DEAE-Sepharose chromatography. A Western blot analysis using a chemiluminescent reaction was performed to examine the reactivity to anti-B. henselae IgM of two pools of sera, one composed of sera from 3 CSD patients and the other composed of sera from 3 healthy individuals (shown in the right two panels). Molecular size markers (kDa) are indicated at the left at each panel. Ag, antigen.
A Western blot analysis was performed using IgM-IFA-positive and IgM-IFA-negative pooled sera to examine the IgM reactivity of the 5 different protein preparations (whole-cell, crude sarcosine-insoluble, crude sarcosine-soluble, refined sarcosine-insoluble, and refined sarcosine-soluble protein preparations) of B. henselae (Fig. 3). For all of the 5 proteins, the positive pooled sera revealed two conspicuous bands of IgM reactivity in the region of 8 to 10 kDa. In contrast, the negative pooled serum showed no visible bands for any of the 5 protein preparations, demonstrating the complete lack of a nonspecific reaction.
The reactivity to the reference serum panel of the anti-B. henselae IgM-ELISAs with the 5 different antigens.
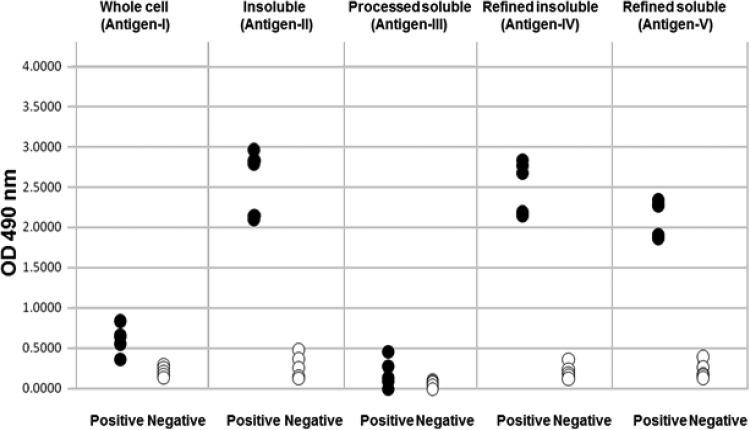
We set up 5 separate anti-B. henselae IgM-ELISAs using antigen I to antigen V to compare their abilities to detect B. henselae IgM using a reference panel composed of sera with a known IgM status. The OD values of the IgM-positive sera that were determined by the ELISAs using antigen I and antigen III were much lower than those determined using antigen II, antigen IV, and antigen V as shown in Fig. 4. It is also noteworthy that the separation of the OD values between the positive and negative sera was incomplete. In contrast, the ELISAs using antigen II, IV, and V resulted in the complete separation of the positive and negative sera.
FIG 4.
Determinations of contents of the sera in the reference panel by the 5 IgM-ELISAs. The reference serum panel (5 positive and 5 negative sera) was tested by the 5 IgM-ELISAs. The test results of each ELISA are expressed as optical density (OD). The 5 different antigen preparations were sonically disrupted B. henselae (Whole cell [antigen I]); 0.4% N-lauroyl sarcosine-insoluble and -soluble proteins of B. henselae (Insoluble [antigen II] and Processed soluble [antigen III], respectively); and sarcosine-insoluble and -soluble proteins refined by DEAE-Sepharose chromatography (Refined insoluble [antigen IV] and Refined soluble [antigen V], respectively).
The performance of three selected anti-B. henselae IgM-ELISAs in comparison with IgM-IFA.
Sera from the 47 patients with clinically suspected CSD (24 definite, 23 suspected) and the 85 healthy adults were tested by three optimal anti-B. henselae IgM-ELISAs specific for antigen II, IV, and V. The test results were compared with those determined by the IgM-IFA. For the three ELISAs with better performance, the cutoff indexes which provided 98% negative results for the 85 healthy individuals were 0.71, 0.58, and 0.60, respectively.
As shown in Table 1, the IgM-IFA yielded positive results in only 54% (13/24) of the 24 definite cases of CDS and in none of the 23 suspected cases. In contrast, the three IgM-ELISAs using antigen II, IV, and V yielded positive results in 75%, 83%, and 75% of the definite cases, respectively. These rates were much higher than those obtained by the IgM-IFA. Regarding the 23 suspected cases, the three IgM-ELISAs yielded positive results in 22%, 43%, and 26% of the cases, respectively; this was in sharp contrast to the completely negative results of the IgM-IFA. As a whole, among the 47 clinically suspected cases, the sensitivities of the three IgM-ELISAs were 49% (23/47), 64% (30/47), and 51% (24/47), respectively. In contrast, the sensitivity of the IgM-IFA was 28% (13/47). However, both the IgM-IFA and the three IgM-ELISAs did not yield any false-positive results in the healthy control sera.
TABLE 1.
The sensitivity of anti-B. henselae IgM-ELISAs performed with three antigens in comparison to IgM-IFA in the diagnosis of patients with clinically suspected cat scratch disease
| Subject category | Total no. of subjects | No. (%) of IgM-IFA-positive subjectsa | No. (%) of IgM-ELISA positive subjects |
||
|---|---|---|---|---|---|
| Ag-IIb | Ag-IVc | Ag-Vd | |||
| Patients | |||||
| Definite CSDe | 24 | 13 (54) | 18 (75) | 20 (83) | 18 (75) |
| Suspected CSD | 23 | 0 (0) | 5 (22) | 10 (43) | 6 (26) |
| Total | 47 | 13 (28) | 23 (49) | 30 (64) | 24 (51) |
| Healthy individuals | 85 | 0 | 0 | 0 | 0 |
IgM-IFA-positive titer, ≥1:20.
Ag-II, sarcosine-insoluble antigen.
Ag-IV, refined sarcosine-insoluble antigen.
Ag-V, refined sarcosine-soluble antigen.
CSD, cat scratch disease.
The results of the 24 definite cases of CSD were subjected to a detailed evaluation (Table 2). All of the cases were determined to be positive by an IgG-IFA, by an IgM-IFA, or by a real-time PCR for B. henselae. Thirteen cases (54%) were found to be positive by the IgM-IFA. Of the remaining 11 IgM-IFA-negative sera (cases 14 to 24), 5 were found to be positive by all three IgM-ELISAs, demonstrating the superior sensitivity of the three ELISAs over the IgM-IFA. For the remaining six IgM-IFA-negative sera that are listed at the bottom of the table (cases 19 to 24), the IgM-ELISA using antigen IV detected two additional positive results, both of which were PCR positive.
TABLE 2.
The detailed test results of the 24 cases of cat scratch disease which were proven by IgG-IFA, IgM-IFA, or PCRa
| CSD case no. | IgG-IFA titer | IgM-IFA titer | IgM-ELISA result (titer index value) |
PCR result | ||
|---|---|---|---|---|---|---|
| Ag-II | Ag-IV | Ag-V | ||||
| 1 | 1:256 | 1:80 | + (1.52) | + (1.50) | + (1.68) | +b |
| 2 | 1:256 | 1:40 | + (1.32) | + (1.48) | + (1.07) | −c |
| 3 | 1:128 | 1:20 | + (1.07) | + (1.21) | + (1.35) | NT |
| 4 | 1:1,024 | 1:160 | + (0.81) | + (0.96) | + (1.09) | NT |
| 5 | 1:256 | 1:40 | + (2.15) | + (2.00) | + (2.83) | NT |
| 6 | >1:1,024 | 1:20 | + (1.08) | + (1.06) | + (0.87) | NT |
| 7 | 1:512 | 1:20 | + (1.89) | + (1.86) | + (1.54) | +b |
| 8 | >1:1,024 | 1:40 | + (2.78) | + (2.73) | + (2.26) | NT |
| 9 | 1:256 | 1:20 | + (1.99) | + (1.83) | + (2.06) | NT |
| 10 | 1:256 | 1:20 | + (2.85) | + (2.84) | + (2.73) | +b |
| 11 | 1:128 | 1:20 | + (1.11) | + (1.24) | + (0.98) | NT |
| 12 | 1:256 | 1:20 | + (1.11) | + (1.00) | + (1.38) | NT |
| 13 | 1:128 | 1:20 | + (0.88) | + (0.90) | + (0.69) | NT |
| 14 | 1:1,024 | − | + (1.14) | + (1.21) | + (1.41) | +b |
| 15 | 1:1,024 | − | + (1.91) | + (1.91) | + (2.48) | NT |
| 16 | 1:512 | − | + (0.75) | + (0.91) | + (0.99) | NT |
| 17 | >1:1,024 | − | + (2.63) | + (2.68) | + (2.42) | +c |
| 18 | >1:1,024 | − | + (2.78) | + (2.65) | + (2.40) | NT |
| 19 | 1:1,024 | − | − (0.46) | − (0.39) | − (0.53) | −c |
| 20 | 1:512 | − | − (0.30) | − (0.30) | − (0.19) | NT |
| 21 | 1:1,024 | − | − (0.18) | − (0.29) | − (0.14) | NT |
| 22 | <1:64 | − | − (0.54) | − (0.46) | − (0.42) | +c |
| 23 | <1:64 | − | − (0.57) | + (0.74) | − (0.49) | +c |
| 24 | 1:64 | − | − (0.64) | + (0.82) | − (0.52) | +c |
CSD, cat scratch disease; NT, not tested; PCR, real-time PCR. Within-assay coefficients of variation (CVs) for Ag-II, Ag-IV, and Ag-V were 3.27%, 3.22%, and 4.3%, respectively. Sensitivities of the IgM-IFA, Ag-II IgM-ELISA, Ag-IV IgM-ELISA, and Ag-V IgM-ELISA were 54%, 75%, 83%, and 75%, respectively.
Lymph node.
Whole blood.
DISCUSSION
The serological detection of B. henselae is notoriously difficult and is always hampered by both the low sensitivity and the low specificity of the assays that are conventionally used in routine laboratory setting. The presence of IgM in patients is clear evidence of recent contact with B. henselae; however, the sensitivity of assays in the detection of the IgM is low (5–10, 12). IgM remains in the serum for approximately 3 months (7, 13). The development of a more sensitive as well as more specific IgM assay for B. henselae is crucial for improving the clinical diagnosis of CSD. Obviously, the preparation of very specific antigens from B. henselae is a key to success. In this study, we explored the antigens most suitable for developing an anti-B. henselae IgM-ELISA that is highly sensitive and also specific for the serodiagnosis of CSD.
With this objective, we attempted to refine the sonically dissolved proteins from B. henselae. We first processed the proteins with a surfactant (sarcosine) to separate them into soluble and insoluble fractions. These were further treated separately by DEAE-Sepharose Fast Flow ion-exchange chromatography (17). To the best of our knowledge, there have been no previous attempts to use chromatography to refine sarcosine fractions for use in the development of a specific IgM-ELISA.
In the preliminary experiment using DEAE-Sepharose chromatography and pooled sera that were shown to be positive by anti-B. henselae IgM-IFA, we found fractions (eluted at 150 to 400 mM NaCl) of whole-cell proteins of B. henselae that showed a specific reaction to the IgM. Based on this result, in the subsequent refinement of the sarcosine-insoluble and sarcosine-soluble proteins by DEAE-Sepharose chromatography, we adopted 170 to 330 mM NaCl as the optimal condition. The refined status of the DEAE-Sepharose-treated antigens (antigen IV and V) was confirmed by the disappearance of the bands corresponding to proteins larger than 45 kDa in the SDS-PAGE analysis. As a result, we chose to evaluate 5 antigens as candidates for the development of the specific IgM-ELISA: antigen I (whole-cell B. henselae), antigen II (sarcosine-insoluble proteins), antigen III (processed sarcosine-soluble proteins), antigen IV (sarcosine-insoluble proteins refined with DEAE-Sephadex), and antigen V (sarcosine-soluble proteins refined with DEAE-Sephadex). The diagnostic utility of each ELISA was evaluated with the use of the reference panel of sera (5 positive and 5 negative sera).
We proved that the ELISAs using antigen II, IV, and V could clearly distinguish between the two sets of sera, whereas the ELISAs with antigen I and III showed overlapping results for the two sets, indicating that these two antigens were not suitable for use in the IgM-ELISA. There have been many previous reports on the development of IgM-ELISAs using whole-cell protein (antigen I) (6–10), which have generally shown poor sensitivity. We confirmed that whole-cell protein was inappropriate for use in the IgM-ELISA. We previously reported the usefulness of antigen III in developing an IgG-ELISA for detection of B. henselae which had sensitivity and specificity as high as 95.7% and 97.7% (14), respectively. However, we had not tested its suitability for developing an IgM-specific ELISA, and we found it to be unsuitable in this study. Thus, separate antigens should be used, one for IgG-ELISA and the other for IgM-ELISA. This logic is analogous with an IFA method (5) which uses an antigen from B. henselae cocultivated with Vero cells for IgG-IFA and an antigen from B. henselae derived from an agar medium for IgM-IFA.
To confirm the specificity of the other three antigens, antigen II, IV, and V, we performed a Western blot analysis and tested reactivity to IgM-IFA-positive and -negative pooled sera. To compare the assays, antigen I (whole-cell) and the sarcosine-soluble proteins were run in parallel. The analysis revealed that each of the three antigens displayed two conspicuous bands corresponding to 8 to 10 kDa in the positive pooled serum, as did antigen I and the sarcosine-soluble proteins, but no specific bands were recognized with the use of the negative pooled serum. To our knowledge, there is only a single report of a Western blot analysis of B. henselae-related proteins which describes the use of its IgM antibody. The report by Litwin et al. (18) showed that the IgM Western blot analysis of IFA-positive serum displayed a clear distinct band at the 8-kDa protein which matched the reactivity of both the IgM-ELISA and IgM-IFA.
To compare the performances of the IgM-ELISAs using the three antigens (antigen II, antigen IV, and antigen V) with that of IgM-IFA, we analyzed the sera from the 47 patients clinically suspected of having CSD to the sera from 85 healthy individuals. In deciding the cutoff value for each assay, we regard it as more important to increase the confidence in the ability of the assay to detect active CSD infections by increasing its specificity. In contrast, confidence in the results would be diminished if the assay were known to sometimes return false-positive results. With this reasoning, we chose the cutoff value at the 98th percentile of the ELISA indices of healthy individuals. The specificity for each of the IgM-ELISAs using antigen II, IV, and V was 98%, with cutoff values of 0.71, 0.58, and 0.60, respectively. Those of antigen IV and V refined by DEAE-Sepharose chromatography were lower than that of crude sarcosine-insoluble antigen II. This difference in the cutoff values indicates the improved refinement that was achieved with the use of DEAE-Sepharose chromatography.
The three IgM-ELISAs using antigen II, IV, and V yielded positive results in the 24 definite CSD cases at rates of 75%, 83%, and 75%, respectively, which were much higher than that seen with IgM-IFA (54%). Besides, the three IgM-ELISAs yielded positive results for 49%, 64%, and 51% of the 47 patients with clinically suspected CSD, respectively, which is in sharp contrast to the rate obtained with IgM-IFA (28%). In particular, the sensitivity was better with the new IgM-ELISA using antigen IV, which was refined from the crude sarcosine-insoluble proteins of B. henselae (antigen II) by DEAE-Sepharose chromatography. The use of antigen II for IgM-ELISA was previously reported by Giladi et al. (12), but its sensitivity in well-defined CSD cases was only 48% (positive PCR, skin test, or B. henselae culture), although its specificity was 98% to 100%. We have now proven that antigen IV is far superior to antigen II for use in the IgM-ELISA. Further proof of the superiority of the new ELISA using antigen IV is that it yielded positive results for two of the three sera that gave PCR-positive but IgG-IFA/IgM-IFA-negative results.
The sensitivity of the new ELISA using antigen IV was 83% when cases were limited to patients with definite CSD, despite the specificity being deliberately set to 98%. However, the sensitivity for all of the clinically suspected patients was reduced to 64%. This figure appears to be similar to those of previous reports. However, we indiscriminately included all of the cases of patients who were alleged to have had unknown fever and lymphadenopathy as “clinically suspected CSD cases”—symptoms that may have been caused by infections other than CSD. In any case, we believe that the superiority of the ELISA using antigen IV is best demonstrated by the fact that it showed the highest performance among the 5 candidate antigens, which were compared in parallel under the same conditions using the same sets of specimens.
In summary, to improve the sensitivity and specificity of the conventional anti-B. henselae IgM-IFA or IgM-ELISA, we prepared 5 different antigens from sonicated B. henselae whole cells for use in the IgM-ELISA. Based on the analyses of a reference serum panel and sera from 47 patients who were clinically suspected of having CSD and from 85 healthy individuals, we identified antigen IV as the antigen fraction that provided the highest sensitivity and specificity for the sarcosine-insoluble proteins that were refined by DEAE-Sepharose chromatography. The use of the new IgM-ELISA is expected to greatly improve the serodiagnosis of CSD.
ACKNOWLEDGMENTS
This work was supported in part by JSPS KAKENHI Grant no. 26460678 to Hidehiro Tsuneoka and by JSPS KAKENHI Grant no. 15K15226 and the YU Grant Program for Epoch Research in Commemoration of the 200th Anniversary of Foundation to Ken-ichiro Otsuyama.
We thank the patients and doctors for allowing us to analyze the blood and/or lymph node specimens of the suspected CSD patients.
REFERENCES
- 1.Maurin M, Birtles R, Raoult D. 1997. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis 16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 2.Murakami K, Tsukahara M, Tsuneoka H, Iino H, Ishida C, Tsujino K, Umeda A, Furuya T, Kawauchi S, Sasaki K. 2002. Cat scratch disease: analysis of 130 seropositive cases. J Infect Chemother 8:349–352. doi: 10.1007/s10156-002-0194-6. [DOI] [PubMed] [Google Scholar]
- 3.Anderson BE, Neuman MA. 1997. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev 10:203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regnery RL, Olson JG, Perkins BA, Bibb W. 1992. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet 339:1443–1445. doi: 10.1016/0140-6736(92)92032-B. [DOI] [PubMed] [Google Scholar]
- 5.Tsuneoka H, Fujii R, Yamamoto K, Fujisawa K, Iino H, Matsuda M, Tsukahara M. 1998. Determination of anti-Bartonella henselae antibody by indirect fluorescence antibody test—comparison of two types of antigen: non-cocultivated B. henselae and cocultivated B. henselae with Vero cells. Kansenshogaku Zasshi 72:801–807. (In Japanese.) doi: 10.11150/kansenshogakuzasshi1970.72.801. [DOI] [PubMed] [Google Scholar]
- 6.Bergmans AM, Peeters MF, Schellekens JF, Vos MC, Sabbe LJ, Ossewaarde JM, Verbakel H, Hooft HJ, Schouls LM. 1997. Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J Clin Microbiol 35:1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermeulen MJ, Herremans M, Verbakel H, Bergmans AM, Roord JJ, van Dijken PJ, Peeters MF. 2007. Serological testing for Bartonella henselae infections in The Netherlands: clinical evaluation of immunofluorescence assay and ELISA. Clin Microbiol Infect 13:627–634. doi: 10.1111/j.1469-0691.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen MJ, Verbakel H, Notermans DW, Reimerink JH, Peeters MF. 2010. Evaluation of sensitivity, specificity and cross-reactivity in Bartonella henselae serology. J Med Microbiol 59:743–745. doi: 10.1099/jmm.0.015248-0. [DOI] [PubMed] [Google Scholar]
- 9.Herremans M, Bakker J, Vermeulen MJ, Schellekens JF, Koopmans MP. 2009. Evaluation of an in-house cat scratch disease IgM ELISA to detect Bartonella henselae in a routine laboratory setting. Eur J Clin Microbiol Infect Dis 28:147–152. doi: 10.1007/s10096-008-0601-8. [DOI] [PubMed] [Google Scholar]
- 10.Herremans M, Vermeulen MJ, Van de Kassteele J, Bakker J, Schellekens JF, Koopmans MP. 2007. The use of Bartonella henselae-specific age dependent IgG and IgM in diagnostic models to discriminate diseased from non-diseased in Cat Scratch Disease serology. J Microbiol Methods 71:107–113. doi: 10.1016/j.mimet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Welch DF, Hensel DM, Pickett DA, San Joaquin VH, Robinson A, Slater LN. 1993. Bacteremia due to Rochalimaea henselae in a child: practical identification of isolates in the clinical laboratory. J Clin Microbiol 31:2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giladi M, Kletter Y, Avidor B, Metzkor-Cotter E, Varon M, Golan Y, Weinberg M, Riklis I, Ephros M, Slater L. 2001. Enzyme immunoassay for the diagnosis of cat-scratch disease defined by polymerase chain reaction. Clin Infect Dis 33:1852–1858. doi: 10.1086/324162. [DOI] [PubMed] [Google Scholar]
- 13.Metzkor-Cotter E, Kletter Y, Avidor B, Varon M, Golan Y, Ephros M, Giladi M. 2003. Long-term serological analysis and clinical follow-up of patients with cat scratch disease. Clin Infect Dis 37:1149–1154. doi: 10.1086/378738. [DOI] [PubMed] [Google Scholar]
- 14.Tsuruoka K, Tsuneoka H, Kawano M, Yanagihara M, Nojima J, Tanaka T, Yamamoto M, Ichihara K. 2012. Evaluation of IgG ELISA using N-lauroyl-sarcosine-soluble proteins of Bartonella henselae for highly specific serodiagnosis of cat scratch disease. Diagn Microbiol Infect Dis 74:230–235. doi: 10.1016/j.diagmicrobio.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Tsuneoka H, Tsukahara M. 2006. Analysis of data in 30 patients with cat scratch disease without lymphadenopathy. J Infect Chemother 12:224–226. doi: 10.1007/s10156-006-0454-Y. [DOI] [PubMed] [Google Scholar]
- 16.Kimura S, Hasegawa S, Yanagihara M, Inoue H, Matsushige T, Tsuneoka H, Ichiyama T, Ohga S. 2015. Cat-scratch disease with severe pleuritis in a 6-year-old girl. Pediatr Int 57:501–503. doi: 10.1111/ped.12680. [DOI] [PubMed] [Google Scholar]
- 17.Senol M, Nadaroglu H, Dikbas N, Kotan R. 2014. Purification of Chitinase enzymes from Bacillus subtilis bacteria TV-125, investigation of kinetic properties and antifungal activity against Fusarium culmorum. Ann Clin Microbiol Antimicrob 13:35. doi: 10.1186/s12941-014-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litwin CM, Martins TB, Hill HR. 1997. Immunologic response to Bartonella henselae as determined by enzyme immunoassay and Western blot analysis. Am J Clin Pathol 108:202–209. [DOI] [PubMed] [Google Scholar]