Abstract
Molecular detection methods, such as quantitative PCR (qPCR), have found their way into clinical microbiology laboratories for the detection of an array of pathogens. Most routinely used methods, however, are directed at specific species. Thus, anything that is not explicitly searched for will be missed. This greatly limits the flexibility and universal application of these techniques. We investigated the application of a rapid universal bacterial molecular identification method, IS-pro, to routine patient samples received in a clinical microbiology laboratory. IS-pro is a eubacterial technique based on the detection and categorization of 16S-23S rRNA gene interspace regions with lengths that are specific for each microbial species. As this is an open technique, clinicians do not need to decide in advance what to look for. We compared routine culture to IS-pro using 66 samples sent in for routine bacterial diagnostic testing. The samples were obtained from patients with infections in normally sterile sites (without a resident microbiota). The results were identical in 20 (30%) samples, IS-pro detected more bacterial species than culture in 31 (47%) samples, and five of the 10 culture-negative samples were positive with IS-pro. The case histories of the five patients from whom these culture-negative/IS-pro-positive samples were obtained suggest that the IS-pro findings are highly clinically relevant. Our findings indicate that an open molecular approach, such as IS-pro, may have a high added value for clinical practice.
INTRODUCTION
For a long time, clinical bacterial diagnostic testing has been performed almost exclusively using cultivation-based techniques. Over time, these techniques have been highly optimized for the efficient cultivation of known clinically relevant species. Currently, almost all information we have on clinical microbiology has been generated by cultivation-based techniques. Like all techniques, cultivation has its advantages and drawbacks. Its advantages include the ability to simultaneously detect multiple species and the universal nature of the system: while selective medium may be employed to single out specific species, nonselective medium incubated under different conditions can sustain a very broad range of bacteria, which obviates having to decide in advance which specific microbe(s) should be sought. The most notable drawbacks of culture for clinical application are the long time needed for bacteria to grow (1), the inability to culture bacteria after patients have received antibiotics, and the inability to detect bacterial species that are refractory to cultivation.
With the advent of PCR, molecular techniques, and particularly quantitative PCR (qPCR), have been introduced into the field of bacterial diagnostics as an alternative to culture. While qPCR overcomes some of the limitations of culture, it introduces its own limitations. qPCR needs no cultivation, so it is faster than culture, it can detect bacteria even in samples obtained from patients receiving antibiotic treatment, and it can be used to detect cultivation-refractory species. However, qPCR is typically a selective technique, in that only the species at which it is specifically directed can be detected. Eubacterial PCRs and qPCRs have been described, but these methods typically require an additional sequencing step to classify PCR products (2). Current diagnostic schemes based on qPCR therefore often require either the application of multiple qPCRs performed simultaneously or sequentially, or an additional sequencing step, which makes these schemes expensive, slow, or both. Finally, because most qPCRs are built upon knowledge gained by cultivation techniques, they are generally aimed at known cultivable species.
This issue of being aimed at cultivable species, which thus applies to culture and qPCR, has become highly relevant in recent years, as it has become clear that our indigenous microbiota consists mostly of bacteria that are refractory to routine cultivation techniques (3). As many infections are caused by our indigenous microbiota (4–6), it is very well possible that many pathogens have been missed using standard bacterial diagnostics (7).
The combination of amplification and Sanger sequencing of 16S RNA with universal bacterial primers is a common solution to this issue, as this approach allows for the detection of uncultivable bacterial species. However, this approach is applicable only to samples containing one or two bacterial species. When multiple species are present, the different Sanger reads will be superimposed and cannot be interpreted (8).
Next-generation sequencing approaches are able to simultaneously identify multiple sequences. However, large batch sizes are necessary to reduce costs, and workflows are typically complex: while it is has been described to be theoretically possible to process a sample within 24 h (9), a working system has yet to be described.
Moreover, the 16S sequence composition is often not divergent enough to distinguish between individual bacterial species (10), which is essential for clinical diagnostics, as only specific species within a genus may be pathogenic, and the presence and expression of chromosomal antibiotic resistance genes may vary largely between species within the same genus (e.g., AmpC, which is expressed in Citrobacter freundii but not in other Citrobacter species) (11).
We developed a PCR-based method, IS-pro, which combines rapid and cultivation-independent detection of bacterial DNA, irrespective of bacterial species, and, by a careful choice of target and primers, is capable of identifying all bacteria in a sample. IS-pro includes the advantages of qPCR over cultivation techniques, like limited sample processing steps, fast results, detection of bacteria after antibiotic administration, and detection of uncultivable species. IS-pro relies on length and sequence polymorphisms of the 16S-23S ribosomal interspace regions. As these regions are present in all bacteria and their polymorphisms are highly species specific, IS-pro is a universal system that can detect all bacterial species within a sample. Furthermore, as the technique has been set up to minimize interspecies PCR competition, IS-pro can simultaneously detect and identify many species, rendering the technique applicable to single-species detection and complex microbiota analysis alike (12). In this study, we applied the IS-pro technique to samples from infections of normally sterile body sites and fluids commonly caused by indigenous microbiota, as routinely received in a clinical microbiology laboratory. We found that IS-pro generally detected not only the species found by culture but also many additional bacterial species. We confirmed these findings with a next-generation sequencing approach and clinical data.
MATERIALS AND METHODS
Design.
The study was set up as a comparative study of culture techniques as used in daily routine in our laboratory compared to IS-pro. Discrepancies between the results of culture and IS-pro were further investigated by analysis of the samples by next-generation sequencing and the use of clinical information.
Samples.
Samples were collected between October 2012 and May 2013 at the department of medical microbiology of the VU University Medical Center, Amsterdam, the Netherlands. Sequential samples from normally sterile body sites and fluids sent for routine bacterial culture were included. For this study, we selected samples from abscesses and empyemas. Approval from an ethics board was not needed for this study.
Sample processing and cultivation.
Samples were sent by routine transport to the microbiological laboratory of the VU University Medical Center (VUMC) and processed immediately. For routine bacteriological culture, all samples were processed according to the standard operating procedures of the VUMC laboratory. This encompassed a Gram stain and inoculation and incubation at 37°C of the following media: chocolate agar (bioMérieux, Marcy l'Étoile, France), 2 days of incubation in CO2; sheep blood agar (bioMérieux), 2 days of aerobic incubation; CAP agar (sheep blood agar with colistin and aztreonam; Oxoid, Landsmeer, the Netherlands), 2 days of aerobic incubation; MacConkey 3 agar (bioMérieux), 2 days of aerobic incubation; brain heart infusion (BHI) broth (Mediaproducts BV, Groningen, the Netherlands), 2 days of aerobic incubation; Schaedler agar (Oxoid), 3 days of anaerobic incubation; and Schaedler agar supplemented with phenyl ethyl alcohol (Oxoid), 3 days of anaerobic incubation. If the BHI broth did not show growth after 2 days, it was subcultured on sheep blood agar and Schaedler agar; these were incubated as described above. Identification of bacterial colonies was done by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Vitek MS system; bioMérieux).
DNA isolation.
DNA was isolated directly from all specimens, according to routine molecular diagnostic protocols. Briefly, 200 μl of sample (pus or other) was added to 400 μl of buffer AL (Qiagen, Hilden, Germany) and 40 μl of proteinase K in an Eppendorf container. This mixture was centrifuged for 10 s at 9, 000 × g and then vortexed and incubated at 56°C while shaking at 1,400 rpm for 1 h. An easyMAG automated DNA isolation machine (bioMérieux) was used for further DNA extraction.
Sample mixtures (each 640 μl) were transported to an 8-well easyMAG container and suspended in 2 ml of NucliSENS lysis buffer, as provided by the manufacturer. After incubation at room temperature for 1 h, 70 μl of magnetic silica beads was added, as provided with the easyMAG machine. Afterward, the mixture was inserted in the easyMAG machine, and the “specific A” protocol was chosen, selecting the off-board workflow and eluting DNA in 110 μl of NucliSens easyMAG extraction buffer 3, as provided by the manufacturer (bioMérieux). All DNA was stored at −20°C. For all separate DNA isolation runs, negative controls were included that were analyzed with IS-pro. The same DNA isolate was used for IS-pro and PacBio analyses.
IS-pro.
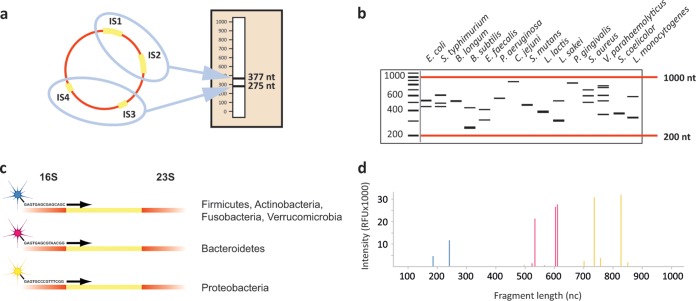
Amplification of intergenic spacer (IS) regions was performed with the IS-pro assay (IS-Diagnostics, Amsterdam, the Netherlands), according to the protocol provided by the manufacturer. IS-pro differentiates bacterial species by the length of the 16S-23S rRNA gene IS region, with taxonomic classification made using phylum-specific fluorescently labeled PCR primers (12) (Fig. 1). The procedure consists of two separate standard PCRs: the first PCR mixture contains two different fluorescently labeled forward primers targeting different bacterial groups and three reverse primers providing universal coverage for those groups. The first forward primer is specific for the phyla Firmicutes, Actinobacteria, Fusobacteria, and Verrucomicrobia (FAFV), and the second labeled forward primer is specific for the phylum Bacteroidetes. A separate PCR with a labeled forward primer combined with seven reverse primers is specific for the phylum Proteobacteria. Amplifications were carried out on a GeneAmp 9700 PCR system (Applied Biosystems, Foster City, CA). After PCR, 5 μl of PCR product was mixed with 20 μl of formamide and 0.2 μl of custom size marker (IS-Diagnostics). DNA fragment analysis was performed on an ABI Prism 3500 genetic analyzer (Applied Biosystems). Data were analyzed with the IS-pro proprietary software suite (IS-Diagnostics), and the results are presented as microbial profiles. Automated species calling of IS-pro peaks was done with the dedicated IS-pro software suite (IS-Diagnostics), in which peaks are linked to a database containing IS-profile information of >500 microbial species.
FIG 1.
Schematic representation of the concept behind the IS-pro procedure. (A) All bacterial species contain at least one IS region in their chromosome. However, many species contain multiple alleles of the IS region. These regions may vary between different alleles. Depicted here is the schematic situation in E. faecalis that contains four alleles of the IS region. Two alleles have a length of 275 nt, and the other two have a length of 377 nt. When amplified, a profile specific for E. faecalis is obtained. (B) IS profiles are highly diverse between different species. The fact that species commonly have multiple alleles with different lengths dramatically increases the differential potential between species. S. typhimurium, Salmonella enterica serovar Typhimurium; B. longum, Bifidobacterium longum; B. subtilis, Bacillus subtilis; C. jejuni, Campylobacter jejuni; S. mutans, Streptococcus mutans; L. lactis, Lactococcus lactis; L. sakei, Lactobacillus sakei; P. gingivalis; Porphyromonas gingivalis; V. parahaemolyticus; Vibrio parahaemolyticus; S. coelicolor; Streptomyces coelicolor; L. monocytogenes, Listeria monocytogenes. (C) By amplifying IS fragments with phylum-specific fluorescently labeled primers, a next layer of information is added. (D) When an IS profile is made of a sample containing multiple species from different phyla, peaks with different lengths, height, and color are found. These correspond to species, abundance, and phylum. A peak profile may be translated to a list of bacterial species by a software algorithm linked to a database of IS profiles of known bacterial species. The whole IS-pro procedure from unprocessed sample to analyzed data can be performed in 5 h. nc, nucleotides.
Peaks of <128 relative fluorescence units (RFU) were regarded as background noise and were discarded from further analysis. The whole procedure, from DNA isolation to analyzed data, can be done within 5 h.
Next-generation sequencing.
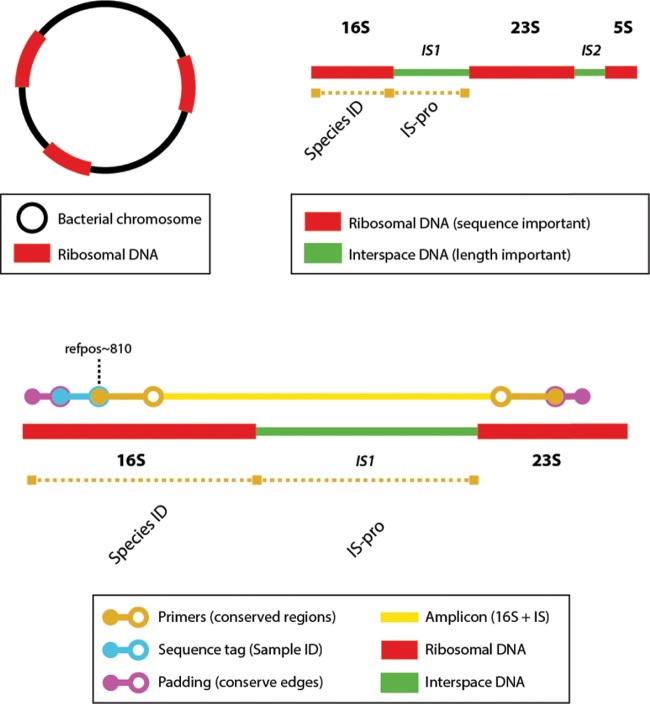
All samples were further analyzed by circular consensus sequencing with a SMRTbell template on the PacBio RS II machine (Pacific Biosciences, CA, USA). For circular consensus PacBio sequencing, a forward primer was selected in a conserved region of the 16S rRNA gene (5′-GGATTAGATACCCBGGTAGTCC-3′, Escherichia coli reference position ∼810). Four reverse primers were selected in the 23S rRNA gene (5′-CCATCCTTTTCACCTTTCCTTCACAGTAC-3′, 5′-CCATCCTTTTCGCCTTTCCTTCACAGTAC-3′, 5′-CCATCCTTTTCGCCTTTCCCTCACGGTAC-3′, and 5′-CCATCCTTTTCACCTTTCCCTCACGGTAC-3′), together providing very broad coverage of bacterial species. Amplicons consisted of the V6 to V9 regions of the 16S rRNA gene, the 16S-23S interspace region (IS1), and a part of the 23S rRNA gene region (Fig. 2). Forward primers were barcoded at the 5′ side for multiplexing on the PacBio single-molecular real-time (SMRT) cell. To ensure optimal SMRT cell ligation, padding sequences of 5 nucleotides were added to the primer sequences at the 5′ side. The PCR mixture for amplification consisted of 10.325 μl of high-performance liquid chromatography (HPLC) water, 2.5 μl of SuperTaq buffer (Sphaero Q, Gorinchem, the Netherlands), 0.8 μl of 0.04% bovine serum albumin (BSA) (Sigma), 0.975 μl of 16S-23S reverse primers (10 μm), 0.2 μl of 0.2 mM deoxynucleoside triphosphate (dNTP) (Invitrogen, Carlsbad, CA, USA), 0.2 μl of SuperTaq enzyme (5 U/μl; Sphaero Q), 10 μl of DNA, and 1 μl of forward primer (10 μM). The GeneAmp 9700 PCR system (Applied Biosystems, Foster City, CA) was used for amplification, with 35 cycles of 30 s of melting at 94°C, 45 s of annealing at 60°C, 140 s of elongation at 72°C, and a final elongation for 11 min at 72°C. Sequencing was performed on the PacBio RS II instrument. The data collected from the PacBio RS II instrument were processed and filtered using the SMRT Analysis software suite. The continuous long-read (CLR) data were filtered by read length (>50 bp), subread length (>50 bp), and read quality (Q >0.75). Quality analysis of the filtered FASTQ reads was performed with CLC Genomics Workbench version 6.0.4. The resulting fragments were again filtered by length (>1,000 bp and <3,000 bp). Sequences in which no tag was found were discarded, and the remaining sequences were oriented in the same direction by using the tag orientation as a reference. Virtual IS-pro was performed on the resulting sequences with an in-house-built algorithm. This algorithm identified best-matching IS-pro primers and their binding sites. Thus, the fluorescent color label and length of corresponding IS fragments could be predicted. These virtual IS-pro fragments were used to match to actual IS-pro data. Subsequently, the first 50 nucleotides were trimmed from all sequences, and the following 700 nucleotides were aligned and taxonomically classified with the Silva Incremental Aligner (SINA) version 1.2.11 (13). The settings for taxonomical classification were as follows: minimal identity with query sequence, 0.9; number of neighbors per query sequence, 10; identity for rejection of sequences, <70%. Comparisons were made against five different databases (Greengenes, RDP, EMBL, Living Tree Project [LTP], and Silva SSU [SLV]) to identify ambiguous classifications.
FIG 2.
Schematic representation of the PacBio sequencing approach. A bacterial chromosome always contains one or more ribosomal regions (top left, red bars). These regions each consist of a 16S, 23S, and 5S region and two interspace regions (IS1 and IS2). The identity of the bacterial species can be assessed either by the sequence of the 16S fragment or by the length of the IS1 region (top right). Amplification primers were chosen to cover a large part (∼700 nt) of the 16S region, containing four variable regions (V6 to V9) for sequence-based species identification. Each amplicon thus consists of both the 16S region and the IS1 region, enabling a comparison of identifications. Forward primers had sequence tags for multiplexing in the PacBio run. Padding sequences were added to prevent damage to the sequence tags and for optimal SMRTbell ligation (bottom). By performing a virtual IS-pro on full-length sequences, best-matching (labeled) IS-pro primers and predicted length of resulting IS fragments was determined. The resulting virtual IS fragments were matched to actual IS fragments. In this fashion, bacterial identifications by 16S sequence analysis could be directly matched to corresponding IS fragments. ID, identification; refpos, E. coli reference position.
All downstream data analyses, visualizations, and comparisons of resulting data from culture, IS-pro, and PacBio analyses were done with the Spotfire software package (Tibco, Palo Alto, CA, USA).
RESULTS
Culture versus IS-pro.
In total, 66 samples were included from different bodily locations, falling into nine distinct categories (Table 1). Of these samples, 56 were culture positive and 10 were culture negative. All samples that yielded a positive culture result were also positive by IS-pro. Of the 10 culture-negative samples, five were also negative with IS-pro. However, the other five culture-negative samples were positive with IS-pro (Table 1). For 20 samples, outcomes at the species level were identical between culture and IS-pro (Table 2). For 31 samples, more species were found with IS-pro than with culture. For two samples, fewer species were found with IS-pro than with culture. Finally, for 13 samples, comparisons could not be made for all species, because some species were not identified by culture, either because species did not have final identifications or, more commonly, because species identification was deemed to be clinically irrelevant in the context of the presence of known pathogens that alone might account for the clinical picture. In 12 of these 13 samples, the dominant species that were identified by culture were all confirmed by IS-pro. Because the results were identical as far as was analyzable, we displayed these samples as being identical in Table 2. For one sample in this subset, none of the cultured species were identified, making a comparison impossible.
TABLE 1.
Sample types and outcomes of culture and IS-proa
| Sample type | No. of samples by result |
||||
|---|---|---|---|---|---|
| Total | Only culture positive | Culture negative/IS-pro positive | Both negative | Both positive | |
| Intraperitoneal abscess | 18 | 0 | 1 | 1 | 16 |
| Head/neck abscess | 11 | 0 | 3 | 1 | 7 |
| Mamma abscess | 4 | 0 | 0 | 0 | 4 |
| Septic arthritis | 2 | 0 | 0 | 0 | 2 |
| Lymph node | 1 | 0 | 0 | 1 | 0 |
| Osteomyelitis/spondylodiscitis | 9 | 0 | 1 | 0 | 8 |
| Perianal abscess | 1 | 0 | 0 | 0 | 1 |
| Pulmonary abscess | 1 | 0 | 0 | 0 | 1 |
| Urogenital abscess | 5 | 0 | 0 | 0 | 5 |
| Wound/subcutaneous infection | 14 | 0 | 0 | 2 | 12 |
| Total | 66 | 0 | 5 | 5 | 56 |
No culture-positive samples were negative by IS-pro. Six culture-negative samples were positive by IS-pro. In four of these samples, the same species that was identified by IS-pro was detected with PacBio sequencing. The two remaining culture-negative and IS-pro-positive samples showed a very low load (<500 RFU).
TABLE 2.
Comparison of identified species between culture and IS-pro
| Sample type | No. of samples identified by test |
|||
|---|---|---|---|---|
| Total | Identical | More in culture | More in IS-proa | |
| Intraperitoneal abscess | 18 | 10 | 0 | 8 |
| Head/neck abscess | 11 | 5 | 0 | 6 |
| Mamma abscess | 4 | 1 | 0 | 3 |
| Septic arthritis | 2 | 2 | 0 | 0 |
| Lymph node | 1 | 1 | 0 | 0 |
| Osteomyelitis/spondylodiscitis | 9 | 3 | 1 | 5 |
| Perianal abscess | 1 | 0 | 0 | 1 |
| Pulmonary abscess | 1 | 0 | 1 | 0 |
| Urogenital abscess | 5 | 4 | 0 | 1 |
| Wound/subcutaneous infection | 14 | 7 | 0 | 7 |
| Total | 66 | 33 | 2 | 31 |
More species were generally found with IS-pro for samples in which more complex microbiota was expected.
In total, 76 species were identified by culture. Seventy-two of these species (95%) were also found with IS-pro. The four cultured strains that were not found by IS-pro came from two samples that harbored multiple species. The first sample came from a thoracic abscess post-esophagus surgery and contained Enterococcus faecium and, in lesser abundance, Staphylococcus aureus, Serratia marcescens, and Achromobacter denitrificans (all in a single sample). IS-pro detected E. faecium, and, in lesser abundance, Enterococcus faecalis and Streptococcus anginosus, but not the additional three species found by culture. The second sample derived from a subcutaneous infection and contained Aggregatibacter actinomycetemcomitans and, in lesser abundance, Actinomyces israelii and multiple anaerobic species. With IS-pro, A. actinomycetemcomitans and multiple anaerobic species were found, but Actinomyces israelii was not found. The presence of these strains additionally found by culture in these two samples was not confirmed by sequencing.
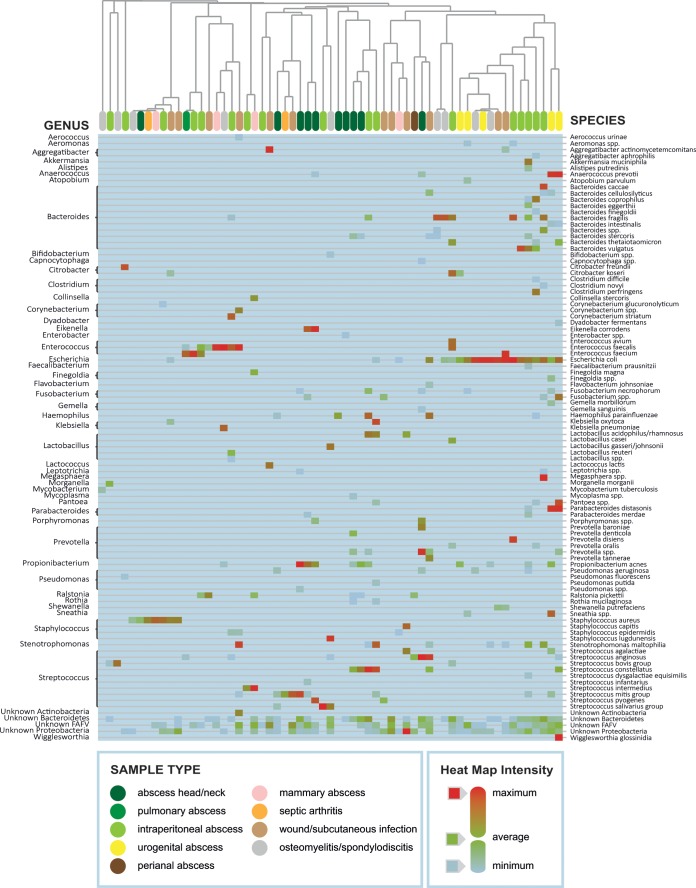
Overall, common pathogens were found in samples in which they were expected, such as Escherichia coli in abdominal and urogenital abscesses and S. aureus in subcutaneous infections. For all abscess-derived samples, IS-pro found significantly more species than culture, including bacterial species that are less familiar as pathogens, such as Akkermansia muciniphila and Alistipes putredinis, which are well-known gut commensals that are refractory to standard culture techniques. Species considered to be normal commensals were generally found in locations close to their normal niche, such as abdominal abscesses for species commonly found in the intestines. However, these species were sometimes also found in less-expected locations, such as an Aggregatibacter sp. in a subcutaneous infection or Bacteroides fragilis in an osteomyelitis sample. A clustered heat map of all IS-pro results is shown in Fig. 3.
FIG 3.
IS-pro results of all positive samples depicted as a clustered heat map. Common pathogens known from culture clearly play an important role when considering all species found by IS-pro. In intraperitoneal abscesses and abscesses in the urogenital region, gut microbiota clearly play an important role, as can be seen by the high presence of Bacteroides and other anaerobic species in the rightmost cluster.
PacBio sequencing.
To aid in the interpretation of discrepancies between culture and IS-pro, PacBio sequencing data were used as a third unrelated comparator technique. Because error rates are known to be high in PacBio sequence data (14), even when performing circular consensus sequencing, these data were interpreted only in the context of culture and IS-pro. In practice, this meant that PacBio data were used only as a confirmatory technique: when species were found with IS-pro that were not found with culture, the presence of those species in the PacBio data was determined. The basis of this approach lies in the negligible chance that a series of sequencing errors would lead to exactly the same species as was found with an unrelated technique (IS-pro). We chose to use the PacBio platform, as we wanted to be certain that species identification by sequence analysis truly matched the IS-pro data. Therefore, we needed to sequence the 16S and the adjacent IS region (Fig. 3). From these long sequences (1,500 to 2,000 nucleotides [nt]), we could match sequence identification by 16S V6 to V9 regions to predicted IS fragments by virtual IS-pro. These predicted IS-fragments were subsequently used to match against actual IS-pro data.
For all but one of the samples that were identical in culture and IS-pro, the presence of all species was also confirmed by PacBio data, at least to the family level. A Mycobacterium tuberculosis isolate present in a sample from a spondylodiscitis was found by culture and IS-pro but was not identified by PacBio. For the samples that were positive in culture and IS-pro, of the total of 129 species found with IS-pro, 94 species (73%) were also found by PacBio. The two samples in which more species were found by culture than by IS-pro were discussed in “Culture Versus IS-pro,” above.
Three of the five samples that were negative by culture and positive by IS-pro were also positive with PacBio sequencing. The two IS-pro-positive samples that were not positive with PacBio showed a very low bacterial load in IS-pro (<500 relative fluorescence units [RFU]).
For samples with the most relevant discrepancies (i.e., those that were discrepant in positivity between culture and IS-pro or for which fewer species were found in IS-pro than those in culture), outcomes compared to PacBio results and clinical records (see below) are given in Table 3.
TABLE 3.
Samples with most relevant discrepancies between culture and IS-pro and the associated PacBio and clinical data
| Case no. | Sample type | Discrepancy | Culture resulta | IS-pro result | PacBio result | Clinical record |
|---|---|---|---|---|---|---|
| 1 | Infected thoracolumbar osteosynthesis device | Culture negative, IS-pro positive | Negative | B. fragilis | B. fragilis | Patient first ameliorated after surgical debridement of osteosynthesis device combined with antibiotics but subsequently declined. Following surgery, presence of B. fragilis in culture was revealed |
| 2 | Punctate from cervical fluid collection after radical neck dissection | Culture negative, IS-pro positive | Negative | S. mitis group, S. salivarius group, H. parainfluenzae, P. aeruginosa, Bacteroidetes species | Streptococcus species, Bacteroides species | Patient recovered after aspiration of fluid and antibiotic treatment |
| 4 | Punctate from resolving paracolic abscess after left-sided hemicolectomy | Culture negative, IS-pro positive | Negative | Unidentified Firmicutes (low signal, <500 RFU) | Negative | Sample was taken from a resolving paracolic abscess, which was encountered during surgery for a different issue. Patient recovered further under antibiotic treatment |
| 5 | Persisting delirium and intracerebral soft-tissue mass | Culture negative, IS-pro positive | Negative | Bacteroidetes and Enterobacter species (low signal, <500 RFU) | Negative | Persistent delirium and elevated infection parameters following recurrent episodes of otitis. Patient did not respond to penicillin, ceftazidime, and voriconazole and died 7 wk after admission |
| x | Pulmonary abscess | Fewer species found in IS-pro than culture | E. faecium, S. aureus, Achromobacter denitrificans, S. marcescens | E. faecium, E. faecalis, S. anginosus | Enterococcus species, Streptococcus species | S. aureus found in sputum samples but not in pleural punctate taken 3 wk later. Patient recovered under vancomycin treatment |
| x | Pus from spondylodiscitis Th5 | Fewer species found in IS-pro than culture | Multiple unidentified species, regarded as contamination | Streptococcus bovis | negative | Not available |
In four of the five cases with negative culture, patients had been treated with antibiotics. x, the two samples with case number x were the samples in which more species were found by culture than by IS-pro.
Clinical cases.
As it can be difficult to assess the value of a new technique against that of a gold standard to which it is potentially superior, we focused on the five cases that were culture-negative and IS-pro positive (Table 3). The case reports are discussed briefly below.
The first case was a child with an infected thoracolumbar osteosynthetic device, which had been placed to correct scoliosis. The infection was treated with intravenous broad-spectrum antibiotics, and the osteosynthetic device was surgically cleaned. A sample was taken during surgery for microbiological analysis. This sample was culture negative but showed the presence of B. fragilis by IS-pro (reproduced by PacBio data). The child was discharged with oral antibiotics (not directed specifically toward B. fragilis, as IS-pro analysis was not performed in the acute setting). However, the child did not recover and returned for further surgical cleaning of the osteosynthetic device 2 months later. During this procedure, two samples were taken for microbiological analysis. These samples were analyzed by culture only, and both showed a high load of B. fragilis.
The second case was a 63-year-old female who had undergone a modified radical neck dissection for an adenoid carcinoma. She had received perioperative gentamicin and clindamycin. However, infection parameters rose postoperatively (15.9 × 10e9 leukocytes/liter, C-reactive protein [CRP] level, 119 mg/liter), and a fluid collection was found in the operated region of the neck. This collection was aspirated, after which the aspirate was sent in for microbiological analysis. Culture was negative, but IS-pro showed the presence of multiple bacterial species, all typical residents of the pharyngeal mucosa: Streptococcus mitis group, Streptococcus salivarius group, Haemophilus parainfluenzae, Pseudomonas aeruginosa, and a Bacteroidetes species. The presence of streptococci and Bacteroidetes species was also found by PacBio sequencing. The patient recovered following aspiration of the fluid and a course of amoxicillin-clavulanate.
The third case was a patient who had undergone an operation for a laryngeal carcinoma. Postoperatively, the patient developed aspiration pneumonia. In a sputum sample, S. aureus, H. parainfluenzae, and S. mitis group organisms were found by culture. The patient was treated with a 7-day course of intravenous flucloxacillin. After an initial good response to the antibiotic therapy, infection parameters rose again after 5 days during flucloxacillin treatment (20.1 × 109 leukocytes/liter; CRP, 269 mg/liter). A course of ceftriaxone combined with amoxicillin-clavulanate was subsequently initiated, after which the infection parameters decreased only slowly. A fluid collection was found in the operated area; this was punctured and drained. The punctate was sent in for microbiological analysis. Culture results were negative, while IS-pro showed H. parainfluenzae. This IS-pro finding was confirmed by PacBio analysis. The infection subsided after drainage of the fluid collection and continuation of the antibiotic therapy.
The fourth case was a patient with a colonic carcinoma who underwent a left-sided hemicolectomy. The operation was complicated by leakage of the intestinal anastomosis, followed by septicemia, for which admission to the intensive care unit was necessary. There, the patient was treated with a broad-spectrum antibiotic regimen consisting of metronidazole, ceftazidime, and fluconazole. While the infection subsided under this regimen, the patient underwent another abdominal operation to correct a fascial dehiscence. Perioperatively, a remnant of a paracolic abscess was found. The contents were aspirated and sent in for microbiological analysis; culture was negative. IS-pro showed a low signal of an unidentified Firmicutes species, and the PacBio result was negative. The patient recovered slowly and was discharged 2 months later.
The fifth case was a patient who developed a persisting delirium after recurrent episodes of otitis media. On magnetic resonance imaging (MRI), a soft-tissue mass was seen close to the jugular foramen. A punctate was taken from this lesion and sent in for cytological and microbiological examination. By cytology, an infectious process was suspected; however, microbiological culture was negative. IS-pro showed the low-level presence of a Bacteroidetes species and an Enterobacter species (not found by PacBio). While IS-pro results were not available at the time of the decision, the cytology results combined with elevated infection parameters (13.6 leukocytes; CRP, 113) prompted the attending physician to initiate a course of penicillin based on a suspicion of Actinomyces infection. However, the patient did not respond to this treatment. Subsequently, a course of voriconazole was given for a suspected fungal infection and a course of ceftazidime, because P. aeruginosa was found in culture of a sputum sample. The patient did not respond to any of these treatments and died 7 weeks after initial admission. It should be noted here that both Enterobacter species and Bacteroides species are generally insensitive to penicillin and ceftazidime.
DISCUSSION
In this study, we demonstrate the application of IS-pro, an open molecular approach that can be performed rapidly on clinical samples, and we compared it to culture. IS-pro was able to reproduce the results of culture and additionally found many species that were not detected by culture, possibly reflecting the actual in vivo microbial situation more accurately. Not only did IS-pro detect additional bacteria in culture-positive samples, but bacteria were also found in culture-negative samples. These additional IS-pro findings were largely (73% of strains found by IS-pro) confirmed by an independent sequencing approach, and the case records further underline that they may be highly relevant for clinical diagnosis and appropriate treatment.
We are not the first to find that molecular techniques can detect bacteria that have not been detected by culture (2) and that these additional species are not necessarily uncultivable species (15). However, studies performed to date did not include a third (and unrelated) comparator technique to further evaluate discrepancies. Moreover, while various approaches have been explored to detect microorganisms by an open molecular approach, no suitable means of doing this in a clinical setting has been demonstrated to date: amplification and detection of 16S fragments do not give species identification (16), Sanger sequencing is restricted to one or maximally two species being present in a sample, and next-generation sequencing approaches, even though the first system was described 25 years ago (17), still have not led to applications that may be performed in a clinical routine setting in a microbiological laboratory.
As IS-pro is straightforward to implement and fast to execute (<5 h from sample to result), we demonstrate here a fully molecular approach, which performs better than culture, has a turnaround time of less than a day, and is performed directly on clinical specimens without the need for sequencing. We confirmed the accuracy of this approach with an independent sequencing approach.
An issue with this study was that all peaks cannot yet be identified to the species level by IS-pro, nor could all sequences be identified to species or genus level with PacBio sequencing. Comparisons were therefore sometimes necessarily made on higher taxonomic levels, which may have led to incorrect matching of species. When IS-pro peaks could not be identified to the species level, they were classified to the phylum level. Therefore, the results of unknown species could be given as a molecular Gram stain, which could still guide antibiotic choice, as discussed previously by Kobayashi et al. (18). We designed this study to demonstrate the possibilities and results of a broad-range bacterial PCR with direct species identification to clinical practice on a wide range of clinical specimens. We decided not to include samples from locations that harbor resident microbiota, such as sputum or feces. While it is of course technically feasible to perform IS-pro on these sample types, our goal was to perform a head-on comparison between IS-pro and culture. This would not have been feasible for samples that contain many species that are difficult to detect by routine culture. Moreover, a comparison of sequence-based techniques to highly selective culture approaches as are applied routinely in fecal diagnostics is a wholly different issue altogether, and it falls outside the scope of this study.
Furthermore, in this study, we did not examine all human sample types; important omissions were blood and cerebrospinal fluid (CSF), both of which are sample types that typically harbor low bacterial loads. We did not perform analyses on blood, as it has been shown that the sensitivity of molecular assays for detecting pathogens in blood is largely dependent on the volume of blood from which DNA is isolated and requires specialized DNA extraction methods (19). CSF was not included in this study, as CSF sample volumes are often low and are used in total for culture. In the period of sample collection of this study, no samples with enough excess CSF were collected. However, another sample type that typically harbors a low bacterial load is joint aspirate. The two joint aspirates that were included in this study showed full concurrence between culture and IS-pro results (both positive and the same species was identified). The choice to omit certain sample types, however, also precluded an accurate estimation of analytical performance characteristics by sample type.
Furthermore, this study highlights one of the most important issues of an open molecular approach in routine diagnostics: the interpretation of species that have not been found by culture. To assess whether these additional species are truly present in the site of pathology in the patient and have a clinical implication, the possibility of contamination of samples should first be ruled out. The contamination of the DNA isolation reagents or PCR mixture was ruled out by the negative controls, which were taken along with each DNA isolation run throughout the whole process, and were all negative. Contamination of the samples themselves was more difficult to rule out. However, a number of ways to make contamination very unlikely are demonstrated in the case reports: in the first case, an initial finding of B. fragilis was confirmed in a completely independent follow-up sample taken from the same patient 2 months later, making contamination very unlikely. In the second case report, laboratory contamination was unlikely, as all bacteria found were common residents of the pharyngeal mucosa. The abscess from which the punctate was taken was situated adjacent to the pharynx, making pharyngeal mucosal residents the most likely causative agents. In the fifth case report, the clinical relevance of the species found was most likely because the patient did not respond to antibiotics to which the species found by IS-pro were intrinsically resistant. The third case report makes the species found likely to be clinically relevant by a combination of the factors at play in the first and fifth case report: in the first sample, H. parainfluenzae, S. aureus, and S. mitis were found, while an unrelated second sample confirmed the presence of H. parainfluenzae after a course of flucloxacillin, to which it is generally resistant, while the other two species are generally sensitive to flucloxacillin. The culture media used for the two samples were identical.
Of course, the contamination of samples from the patient's own microbiota is more difficult to assess. However, this issue has always been a known factor in culture-based microbiology, most notably a factor that clinical microbiologists have successfully learned to deal with. Here, extensive experience with bacterial cultures in a clinical situation has led to an understanding and correct interpretation of results. It is to be expected that this will also happen for open-molecular techniques as microbiologists gain more experience with this approach in the clinical routine.
Many studies have now confirmed that routine culture techniques cannot detect many species that are common in endogenous complex microbial niches, such as the gut, oral cavity, or urogenital tract (20–22). It is also well established that bacteria deriving from endogenous microbiota are common causative agents of infections in normally sterile body sites (5, 6, 15, 23). In addition, this study and previous studies show that cultivable organisms can also be missed by routine culture (2, 15).
The importance of a fast open molecular approach for clinical practice thus seems evident. Examples of clinical situations in which such an approach may be of added value include situations in which a rapid diagnosis is required (e.g., septic arthritis), in hard-to-diagnose clinical syndromes (e.g., fever of unknown origin), when patients have already received antibiotics prior to sampling, for samples with a high false-negative rate in culture (e.g., joint aspirates [24]), and for diseases caused by fastidious or uncultivable organisms (e.g., strict anaerobes). In addition, an open molecular technique can detect unknown novel pathogens and has great potential for the promising field of complex microbiota-based diagnostics.
We conclude that an open molecular approach that can be performed in a clinical routine setting, such as IS-pro, may have a high added value for clinical practice. IS-pro outperforms culture in terms of speed and detection of bacteria, and it has the advantage over current PCR-based approaches in that it is not limited to a predefined set of bacteria that has to be decided on in advance.
Of course, microbiologists will need to gain experience with such a novel approach to optimally interpret results for clinical decision-making, but it is very likely that this will happen, just as it has been done for culture-based microbiology.
ACKNOWLEDGMENTS
We thank Malieka Degen and Suzanne Jeleniewski for their invaluable support with labwork and data analysis.
A.E.B. and P.H.M.S. have proprietary rights to the IS-pro technique and are co-owners of the spin-off company IS-Diagnostics.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Livermore DM, Wain J. 2013. Revolutionising bacteriology to improve treatment outcomes and antibiotic stewardship. Infect Chemother 45:1–10. doi: 10.3947/ic.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sontakke S, Cadenas MB, Maggi RG, Diniz PP, Breitschwerdt EB. 2009. Use of broad range 16S rDNA PCR in clinical microbiology. J Microbiol Methods 76:217–225. doi: 10.1016/j.mimet.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claridge JA, Banerjee A, Kelly KB, Leukhardt WH, Carter JW, Haridas M, Malangoni MA. 2014. Bacterial species-specific hospital mortality rate for intra-abdominal infections. Sur Infect (Larchmt) 15:194–199. doi: 10.1089/sur.2011.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moazzam AA, Rajagopal SM, Sedghizadeh PP, Zada G, Habibian M. 2015. Intracranial bacterial infections of oral origin. J Clin Neurosci 22:800–806. doi: 10.1016/j.jocn.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Schindlbeck C, Dziura D, Mylonas I. 2014. Diagnosis of pelvic inflammatory disease (PID): intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch Gynecol Obstet 289:1263–1269. doi: 10.1007/s00404-014-3150-7. [DOI] [PubMed] [Google Scholar]
- 7.Schlaberg R, Simmon KE, Fisher MA. 2012. A systematic approach for discovering novel, clinically relevant bacteria. Emerg Infect Dis 18:422–430. doi: 10.3201/eid1803.111481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dmitriev DA, Rakitov RA. 2008. Decoding of superimposed traces produced by direct sequencing of heterozygous indels. PLoS Comput Biol 4:e1000113. doi: 10.1371/journal.pcbi.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salipante SJ, Sengupta DJ, Rosenthal C, Costa G, Spangler J, Sims EH, Jacobs MA, Miller SI, Hoogestraat DR, Cookson BT, McCoy C, Matsen FA, Shendure J, Lee CC, Harkins TT, Hoffman NG. 2013. Rapid 16S rRNA next-generation sequencing of polymicrobial clinical samples for diagnosis of complex bacterial infections. PLoS One 8:e65226. doi: 10.1371/journal.pone.0065226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox GE, Wisotzkey JD, Jurtshuk P Jr. 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol 42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 11.Normark S, Lindquist S, Lindberg F. 1986. Chromosomal beta-lactam resistance in enterobacteria. Scand J Infect Dis Suppl 49:38–45. [PubMed] [Google Scholar]
- 12.Budding AE, Grasman ME, Lin F, Bogaards JA, Soeltan-Kaersenhout DJ, Vandenbroucke-Grauls CM, van Bodegraven AA, Savelkoul PH. 2010. IS-pro: high-throughput molecular fingerprinting of the intestinal microbiota. FASEB J 24:4556–4564. doi: 10.1096/fj.10-156190. [DOI] [PubMed] [Google Scholar]
- 13.Pruesse E, Peplies J, Glöckner FO. 2012. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, Peluso P, Rank D, Baybayan P, Bettman B, Bibillo A, Bjornson K, Chaudhuri B, Christians F, Cicero R, Clark S, Dalal R, Dewinter A, Dixon J, Foquet M, Gaertner A, Hardenbol P, Heiner C, Hester K, Holden D, Kearns G, Kong X, Kuse R, Lacroix Y, Lin S, Lundquist P, Ma C, Marks P, Maxham M, Murphy D, Park I, Pham T, Phillips M, Roy J, Sebra R, Shen G, Sorenson J, Tomaney A, Travers K, Trulson M, Vieceli J, Wegener J, Wu D, Yang A, Zaccarin D, et al. 2009. Real-time DNA sequencing from single polymerase molecules. Science 323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 15.Song YG, Shim SG, Kim KM, Lee DH, Kim DS, Choi SH, Song JY, Kang HL, Baik SC, Lee WK, Cho MJ, Rhee KH. 2014. Profiling of the bacteria responsible for pyogenic liver abscess by 16S rRNA gene pyrosequencing. J Microbiol 52:504–509. doi: 10.1007/s12275-014-4241-7. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi T, Reesink HW, Vandenbroucke-Grauls. CMJE, Savelkoul PHM. 2003. Optimization of real-time PCR assay for rapid and sensitive detection of eubacterial 16S ribosomal DNA in platelet concentrates. J Clin Microbiol 41:4796–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner S, Johnson M, Bridgham J, Golda G, Lloyd DH, Johnson D, Luo S, McCurdy S, Foy M, Ewan M, Roth R, George D, Eletr S, Albrecht G, Vermaas E, Williams SR, Moon K, Burcham T, Pallas M, DuBridge RB, Kirchner J, Fearon K, Mao J, Corcoran K. 2000. Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat Biotechnol 18:630–634. doi: 10.1038/76469. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi N, Bauer TW, Togawa D, Lieberman IH, Sakai H, Fujishiro T, Tuohy MJ, Procop GW. 2005. A molecular Gram stain using broad range PCR and pyrosequencing technology: a potentially useful tool for diagnosing orthopaedic infections. Diagn Mol Pathol 14:83–89. doi: 10.1097/01.pas.0000162753.38284.1a. [DOI] [PubMed] [Google Scholar]
- 19.Loonen AJ, Bos MP, MBvan Neerken S, Catsburg A, Dobbelaer I, Penterman R, Maertens G, van de Wiel P, Savelkoul P, van den Brule AJ. 2013. Comparison of pathogen DNA isolation methods from large volumes of whole blood to improve molecular diagnosis of bloodstream infections. PLoS One 8:e72349. doi: 10.1371/journal.pone.0072349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):S4680–S4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sears CL. 2005. A dynamic partnership: celebrating our gut flora. Anaerobe 11:247–251. doi: 10.1016/j.anaerobe.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Sibley CD, Church DL, Surette MG, Dowd SE, Parkins MD. 2012. Pyrosequencing reveals the complex polymicrobial nature of invasive pyogenic infections: microbial constituents of empyema, liver abscess, and intracerebral abscess. Eur J Clin Microbiol Infect Dis 31:2679–2691. doi: 10.1007/s10096-012-1614-x. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Ghanem E, Sharkey P, Aggarwal A, Burnett RS, Barrack RL. 2008. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res 466:2628–2633. doi: 10.1007/s11999-008-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]