Abstract
Zika virus (ZIKV) is an Aedes mosquito-borne flavivirus that emerged in Brazil in 2015 and then rapidly spread throughout the tropical and subtropical Americas. Based on clinical criteria alone, ZIKV cannot be reliably distinguished from infections with other pathogens that cause an undifferentiated systemic febrile illness, including infections with two common arboviruses, dengue virus and chikungunya virus. This minireview details the methods that are available to diagnose ZIKV infection.
INTRODUCTION
Zika virus (ZIKV) is an enveloped, positive-sense, single-stranded RNA virus that is a constituent of the Flaviviridae family, Flavivirus genus, and is one of two members of the clade containing Spondweni virus (1, 2). Like several other mosquito-borne flaviviruses, including yellow fever virus (YFV) and dengue virus (DENV), as well as the alphavirus chikungunya virus (CHIKV), ZIKV is transmitted by Aedes species mosquitos. ZIKV is the causative agent of Zika fever, an undifferentiated systemic febrile illness that may present with rash, conjunctivitis, and arthralgia but that may also go undetected or be confused with other causes of febrile illness, like DENV or CHIKV. During the 60 years following its discovery in 1947, ZIKV remained an obscure pathogen, confined to areas of Africa and Asia, and it was thought to be responsible for very little human disease. In 2007, however, ZIKV emerged from obscurity, causing an outbreak of febrile illness on the Yap Islands in the Federated States of Micronesia. By 2014, ZIKV had spread throughout the Pacific Islands, and in early 2015, ZIKV was identified for the first time in Brazil. By year's end, ZIKV had spread throughout continental South America and into Central America, the Caribbean, and Mexico. Though ZIKV was initially thought to cause only a mild febrile illness, with limited morbidity and without mortality, reports from Brazil indicate that infection during pregnancy may be associated with severe birth defects, most notably fetal microcephaly. This minireview describes the discovery of ZIKV, its spread throughout the Pacific Islands and the Americas, and, in particular, the methods that are available to diagnose ZIKV infection.
THE DISCOVERY AND EARLY CHARACTERIZATION OF ZIKA VIRUS
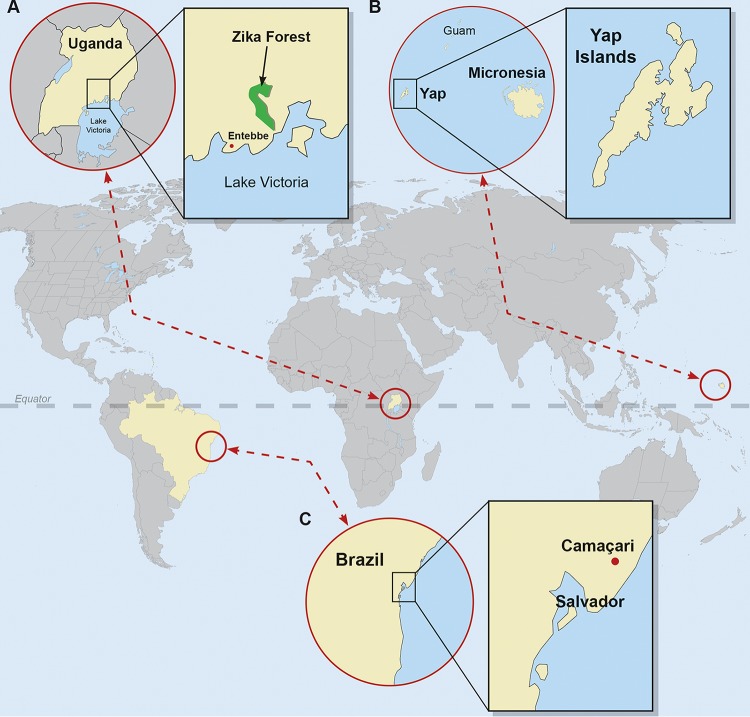
Zika virus was first isolated in 1947 by Dick, Kitchen, and Haddow from the serum of a febrile rhesus monkey, Rhesus 766, that had been caged on a forest canopy platform as part of a Rockefeller Foundation-sponsored sylvatic yellow fever study in the Zika Forest of Uganda (3) (Fig. 1). Intracerebral mouse inoculation of serum from Rhesus 766 resulted in illness at day 10, and a filterable agent isolated from the mouse brain was then neutralized by convalescent serum but not by preinfection serum obtained from Rhesus 766 and another rhesus monkey (771) that had been experimentally infected (3). The same group at the Virus Research Institute in Entebbe, Uganda, isolated ZIKV from the yellow fever mosquito vector Aedes africanus, also collected from sentinel platforms in the Zika forest (3), and provided the first evidence of human ZIKV infection via neutralization experiments using serum from Ugandans taking part in ongoing yellow fever seroprevalence studies (4). The first human isolate of ZIKV was obtained from the serum of a 10-year-old Nigerian girl who presented on day 5 of illness with fever and headache during a suspected outbreak of yellow fever (5).
FIG 1.
A geographic history of Zika virus. The virus was discovered in 1947 in the Zika forest of Uganda (A). The 2007 outbreak on the Yap Islands in the Federated States of Micronesia heralded the introduction of Zika virus to Oceania (B). The identification of Zika virus in Camaçari in the State of Bahia, Brazil, in 2015 marked the emergence of Zika virus in continental South America (C). World map obtained from FreeVectorMaps.com (http://freevectormaps.com).
Throughout the second half of the 20th century and into the early years of the 21st century, ZIKV serologic surveys indicated that human infection was confined to central and western Africa and to areas of South and Southeast Asia (6–15). In addition, phylogenetic analysis was consistent with two distinct introductions from Uganda into West Africa and with one introduction from Uganda into Malaysia (16). Though experimental inoculation of ZIKV in a human volunteer resulted in an undifferentiated systemic febrile illness (17), during this time ZIKV was rarely considered in the differential diagnosis of febrile patients, even in those areas where serology suggested that Zika fever was endemic. In one study investigating arboviral infections in hospitalized Indonesian patients with a recent onset of acute fever, serologic evaluation of paired acute-phase and convalescent-phase sera identified seven patients (2 adults and 5 children) with a >4-fold increase in ZIKV neutralizing antibody titers consistent with Zika fever (15). In addition, a small number of human cases were confirmed by virus isolation, including the 10-year-old febrile Nigerian girl described above (5), five additional febrile Nigerian children (12, 18, 19), and two adults, one in Nigeria and the other in Uganda, who had laboratory-acquired infections (20, 21).
THE EMERGENCE OF ZIKA VIRUS—OCEANIA
In 2007, physicians on the Yap Islands in the Federated States of Micronesia (Fig. 1) reported an outbreak of febrile illness characterized by rash, conjunctivitis, and arthralgia (22). Of 185 suspected cases identified through retrospective medical record review and prospective surveillance at the hospital and health centers, 49 confirmed and 59 probable cases of ZIKV disease were identified using IgM enzyme-linked immunosorbent assays (ELISA), plaque-reduction neutralization tests (PRNT), and real-time, reverse transcriptase PCR (rRT-PCR) (22, 23). A community survey using simple, random, one-stage cluster sampling estimated that 73% (95% confidence interval of 68% to 77%) of Yap residents 3 years of age or older had been recently infected with ZIKV (22). No hospitalizations, hemorrhagic complications, or deaths due to ZIKV infection occurred during the Yap outbreak.
The spread of ZIKV throughout Oceania continued in late 2013, at which time surveillance for acute febrile illness in French Polynesia identified three sentinel cases of patients who presented with fever, arthralgia, headache, and rash (24). Over the next 10 weeks, there were an estimated 19,000 suspected ZIKV cases, and through March 2014, there were more than 30,000 suspected cases (24, 25). During the outbreak, there was an increased incidence of Guillain-Barre syndrome (GBS) and there were other severe neurologic complications, suggesting an association with ZIKV infection (26, 27). A number of cases of fetal microcephaly were also noted and are being further investigated to establish linkage with ZIKV infection (28).
ZIKV outbreaks subsequently occurred throughout 2014 on other Pacific islands, including New Caledonia, Cook Islands, Easter Island, Vanuatu, and Solomon Islands; many of the outbreaks occurred concurrently with outbreaks of DENV and CHIKV (25, 29–34). Phylogenetic analysis of sequences of ZIKV strains, including the Yap outbreak strain, identified the presence of two major lineages, African and Asian, and revealed that the Yap strain was of the Asian lineage (35). The introduction of ZIKV on the Yap Islands was thought to be due to a viremic traveler or the importation of infected mosquitos, as has been speculated with regard to the spread of ZIKV throughout the Pacific Islands and to the Americas (22, 29).
ZIKA VIRUS IN THE AMERICAS—BRAZIL AND BEYOND
In March 2015, 24 patients presented with a febrile illness characterized by rash, arthralgia, and conjunctivitis to Santa Helena Hospital in the city of Camaçari, approximately 50 kilometers from Salvador, the capital of the State of Bahia, Brazil (Fig. 1) (36). Seven patients had detectable ZIKV RNA in serum whereas three patients had detectable CHIKV RNA (36), confirming the spread of ZIKV to continental South America and highlighting how difficult Zika fever is to diagnose based on clinical characteristics alone. The Salvador Epidemiologic Surveillance Office further investigated 14,835 cases of indeterminate acute exanthematous illness reported in the 12 districts of Salvador, revealing the apparent cocirculation of ZIKV, CHIKV, DENV-1, and DENV-3 (37). ZIKV was also retrospectively identified by RT-PCR in serum specimens collected from eight patients in Natal, State of Rio Grande do Norte, Brazil, who presented with a “dengue-like illness” in early 2015 (38). Finally, an HIV-positive man in Rio de Janeiro, Brazil, presented in May 2015 with 1 day of rash, myalgia, malaise, and conjunctival hyperemia; acute Zika fever was confirmed by Flavivirus genus RT-PCR and sequencing (39). As of this writing, autochthonous ZIKV transmission has been reported throughout continental South America, Central America, and Mexico, as well as the Caribbean, including Puerto Rico (40). For the most up-to-date information about the spread of ZIKV in the Americas, please consult the reports of the Pan American Health Organization (PAHO) at www.paho.org.
Phylogenetic analysis of ZIKV sequences obtained from Brazil and Suriname revealed that the virus was of the Asian lineage (38, 41), and it is speculated that it was introduced from the Pacific Islands during the summer of 2014 during the World Cup or the Va'a World Sprint Championship canoe race (29, 38).
ZIKA VIRUS TRANSMISSION
The primary mode of ZIKV transmission to humans is via the bite of Aedes species mosquitos, including Ae. aegypti and Ae. albopictus, the most important vectors globally for the transmission of DENV and CHIKV. In early mosquito studies in Uganda, including the manuscript describing its discovery, ZIKV was isolated from Ae. africanus mosquitos (3, 42–44). Consistent with the hypothesis of a primate reservoir and sylvatic cycle, it was demonstrated that ZIKV could be transmitted to a rhesus monkey via the bite of laboratory-infected Ae. aegypti mosquitos (45). The virus was subsequently isolated from Ae. aegypti mosquitos that were collected in west central Malaysia (46), and Ae. aegypti as well as Ae. albopictus mosquitos in Singapore were shown to be orally susceptible to ZIKV infection (47, 48). In addition, ZIKV RNA was most frequently detected in A. albopictus mosquitos during outbreaks of febrile illness in Gabon in 2007 and 2010 (49). In the Yap outbreak, Ae. hensilli was identified as the predominant mosquito species and was considered the probable vector (22, 50). Though ZIKV was not detected in any mosquito samples collected during the outbreak, Ae. hensilli mosquitos were subsequently shown to be orally susceptible to ZIKV infection (22, 50). In the French Polynesia outbreak, Ae. polynesiensis was the suspected vector, though, similarly to Yap studies, ZIKV-infected mosquitos were not reported (24). The vectors responsible for the Brazil outbreak have not yet been confirmed but are suspected to be Ae. aegypti and Ae. albopictus. Importantly, these vectors are endemic throughout the southeastern United States.
Potential non-vector-borne modes of ZIKV transmission include sexual (51, 52), transfusion-associated (53, 54), and perinatal (55) transmission. Most notably, the Zika virus pandemic in the Americas corresponded with a dramatic upsurge in the number of reported cases of fetal and pediatric microcephaly throughout Brazil (56–59), suggesting intrauterine transmission. This hypothesis was supported by the work of Oliveira Melo et al., who described two cases of fetal microcephaly in which ZIKV RNA was detected in amniotic fluid, but not peripheral blood, from two women who reported symptoms consistent with Zika fever during their pregnancy (57). Ocular manifestations in three Brazilian infants with presumed ZIKV-associated microcephaly have also been described (60). The risk of severe fetal neurological manifestations associated with ZIKV infection during pregnancy has prompted the U.S. Centers for Disease Control and Prevention (CDC) to issue a travel alert to women who are pregnant or who are trying to become pregnant to postpone traveling to countries where there is ongoing ZIKV transmission (http://www.cdc.gov/zika/) (61). For pregnant women in countries where the outbreak is ongoing, infection prevention through mosquito control is recommended, though several countries also advise postponing pregnancy until the outbreak subsides and the risk of fetal Zika disease is better understood.
CLINICAL PRESENTATION AND ROUTINE LABORATORY TESTING
The clinical presentation of patients with acute ZIKV infection typically includes a combination of fever, headache, retro-orbital pain, conjunctivitis, a maculopapular rash, myalgias, and/or arthralgias (22, 38). Fever is often low grade (∼38°C), though cases with fever of up to 40°C have been reported (15, 22, 33, 38). Symptom duration is generally 2 to 7 days, but the rash and arthralgias may last 2 weeks or longer (22, 38). The description of routine laboratory findings in patients with ZIKV infections is limited to case reports and small case series. The complete blood count and routine chemistries are reported to be normal for most patients. Abnormal laboratory findings, when present, are typically mild and include leukopenia, thrombocytopenia, and elevation of liver transaminases (33, 34, 38, 39, 62).
Patients with ZIKV disease are often suspected of having DENV or a mild presentation of CHIKV disease. Conjunctivitis and peripheral edema are reported to be more common in ZIKV infections than in DENV and CHIKV disease, while leukopenia and thrombocytopenia are less common (27). However, no studies have directly compared the clinical and laboratory findings for patients with these infections in a defined cohort. Beyond DENV and CHIKV, the differential diagnosis for patients with ZIKV is broad and includes infections with Plasmodium species, herpesviruses (cytomegalovirus, Epstein-Barr virus, human herpesvirus 6 [HHV-6]), and other arboviral infections such as West Nile virus (WNV) and yellow fever virus infections. Notably, it is estimated that 80% of patients with a ZIKV infection remain asymptomatic or develop only mild clinical manifestations (22). These infections may go undetected, which may skew the description of ZIKV cases in the literature toward more-severe presentations.
ZIKA VIRUS DIAGNOSTICS
There is relatively limited literature describing the performance characteristics of diagnostic tests for ZIKV. This section describes the methods that have been used to identify ZIKV infections in human specimens.
Viral culture.
Culture-based methods for ZIKV detection are used in public health and research laboratories but are not generally available for clinical purposes. The reference method for the isolation of ZIKV and other arboviruses is intracerebral mouse inoculation (3, 63). ZIKV is also culturable in several cell lines, including African green monkey (Vero) and rhesus monkey kidney (LLC-MK2), as well as Aedes pseudoscutellaris (MOS61 or AP-61) and Aedes albopictus (C6/36) (35, 63).
Antibody detection.
Evidence of human ZIKV infection was obtained primarily via identification of ZIKV neutralizing antibodies in human serum (4, 6–13). In serologic surveys conducted in Nigeria, Macnamara et al. (10) noted that sera capable of neutralizing ZIKV in mouse protection experiments were also strongly associated with neutralization of DENV and YFV, suggesting serologic overlap or cross-reactivity. In fact, DENV was considered in the differential for identification of the causative agent of the 2007 Yap Islands ZIKV outbreak due in part to false-positive rapid DENV IgM test results, despite a clinical syndrome that was not entirely consistent with dengue (22, 23, 64). The manufacturer of this DENV IgM assay was not disclosed. Further serologic evaluation of Yap outbreak specimens at the Arbovirus Diagnostic Laboratory at the CDC revealed that acute-phase specimens collected within the first 9 days of illness from patients with previous flavivirus infection demonstrated extensive cross-reactivity with other flaviviruses in both IgM capture ELISA and plaque reduction neutralization tests (PRNT) (23). In several cases, a higher level of serologic reactivity was observed for the non-Zika virus flaviviruses, in particular, DENV and Japanese encephalitis virus (JEV) (23). These results prompted the CDC to caution that serologic data alone are insufficient to confirm acute ZIKV infection in patients with secondary flavivirus infection. False-positive DENV IgM results in cases of acute ZIKV infections have also been observed in specimens collected from travelers returning from Indonesia (65), Thailand (62, 66, 67), French Polynesia (68, 69) and Maldives (70). DENV IgM assays demonstrating false-positive results in ZIKV infection include Focus Diagnostics DENV IgM Capture (62, 70) and SD Bioline Dengue Duo NS1 Ag + Ab Combo (66).
As ZIKV spreads throughout the areas of the world where DENV infection is endemic, detection of DENV IgM antibodies in patients with a dengue-like illness, particularly in the absence of DENV nonstructural protein 1 (NS1) or DENV RNA, should prompt consideration of the possibility of ZIKV infection. While DENV serologic tests are widely used, assays for ZIKV antibodies are currently available only through public health laboratories such as the CDC laboratories. Given the association of ZIKV with fetal microcephaly, improved accessibility to ZIKV-specific serology may be important to assess the risk of fetal transmission and to identify women requiring amniocentesis for confirmation of infection, in analogy to the use of serologic testing for congenital infections with cytomegalovirus (CMV) and Toxoplasma gondii. Furthermore, the development of commercial ZIKV serologic tests for use in clinical laboratories and at the point of care will require careful evaluation of test specificity, particularly in populations with a high prevalence of DENV and of infections with other flaviviruses.
Antigen detection.
Unlike the DENV diagnostics field, where NS1 antigen assays are widely available, antigen assays for the diagnosis of acute ZIKV infection have not yet been developed.
RNA detection.
Laboratory diagnosis of acute ZIKV infection currently relies upon the detection of ZIKV RNA in patient specimens, including serum, plasma, urine, saliva, and amniotic fluid specimens. Perhaps the best-studied ZIKV reverse transcriptase PCR assay was designed by the CDC using sequence derived from the 2007 Yap Islands epidemic (23). The CDC ZIKV assay is comprised of two one-step real-time RT-PCR (rRT-PCR) reactions targeting the ZIKV premembrane (prM) and envelope (E) genes, respectively (Table 1). The compositions of the two reaction mixtures, including primer/probe concentrations, reaction volumes, and eluate volumes, are not clearly stated here but are available through collaboration with the CDC. Interpretation requires evaluation of both reactions; a sample is considered positive only if both reactions demonstrate amplification with crossing threshold (CT) values of <38.5 cycles, and an equivocal result indicates that a sample amplified in only one of the two reactions or that the CT value in either reaction was >38.5 cycles. Assay exclusivity was confirmed by testing RNA from a variety of flaviviruses and alphaviruses, and no cross-reactions were identified. Analytical sensitivity was determined using a quantitated ZIKV RNA transcript, and the lower limits of detection were estimated as 100 copies and 25 copies for the prM and E gene targets, respectively. A protocol distributed by PAHO for use in the Americas replaces the less sensitive prM assay with another reaction targeting a region of the NS2B gene designed for detection of the ZIKV Asian lineage (Angel Balmaseda, personal communication).
TABLE 1.
RT-PCR assays for the detection of Zika virusa
| Reference or source | Yr | RT-PCR type | Target | Primer/probe name | Sequence 5′–3′ | Position |
|---|---|---|---|---|---|---|
| Lanciotti et al. (23) | 2008 | One step, real time | prM | ZIKV 835 | TTGGTCATGATACTGCTGATTGC | 835–857c |
| ZIKV 911c | CCTTCCACAAAGTCCCTATTGC | 911–890 | ||||
| ZIKV 860-FAMb | CGGCATACAGCATCAGGTGCATAGGAG | 860–886 | ||||
| Lanciotti et al. (23) | 2008 | One step, real time | E | ZIKV 1086 | CCGCTGCCCAACACAAG | 1086–1102c |
| ZIKV 1162c | CCACTAACGTTCTTTTGCAGACAT | 1162–1139 | ||||
| ZIKV 1107-FAMb | AGCCTACCTTGACAAGCAGTCAGACACTCAA | 1107–1137 | ||||
| PAHO | 2015 | One step, real time | Zika 4481 | CTGTGGCATGAACCCAATAG | 4434–4453e | |
| Zika 4552c | ATCCCATAGAGCACCACTCC | 4524–4505 | ||||
| Zika 4507c-FAMd | CCACGCTCCAGCTGCAAAGG | 4479–4460 | ||||
| Faye et al. (71) | 2008 | One step, conventional | E | ZIKVENVF | GCTGGDGCRGACACHGGRACT | 1538–1558c |
| ZIKVENVR | RTCYACYGCCATYTGGRCTG | 1883–1902 | ||||
| Balm et al. (72) | 2012 | One step, conventional | NS5 | ZIKVF9027 | CCTTGGATTCTTGAACGAGGA | 9121–9141c |
| ZIKVR9197c | AGAGCTTCATTCTCCAGATCAA | 9312–9290 | ||||
| Faye et al. (73) | 2013 | One step, real time | NS5 | Forward primer | AARTACACATACCARAACAAAGTGGT | 9365–9390c |
| Reverse primer | TCCRCTCCCYCTYTGGTCTTG | 9466–9446 | ||||
| Probef | CTYAGACCAGCTGAAR | 9398–9413 | ||||
| Tappe et al. (66) | 2014 | One step, real time | NS3 | ZIKAf | TGGAGATGAGTACATGTATG | 6001–6020c |
| ZIKAr | GGTAGATGTTGTCAAGAAG | 6095–6077 | ||||
| ZIKApg | CTGATGAAGGCCATGCACACTG | 6039–6060 | ||||
| Pyke et al. (74) | 2014 | One step, real time | E | Zika E For | AAGTTTGCATGCTCCAAGAAAAT | 1222–1244e |
| Zika E Rev | CAGCATTATCCGGTACTCCAGAT | 1293–1271 | ||||
| Zika E probeh | ACCGGGAAGAGCATCCAGCCAGA | 1246–1268 | ||||
| Pyke et al. (74) | 2014 | One step, real time | NS1 | Zika NS1 For | GCACAATGCCCCCACTGT | 3329–3346e |
| Zika NS1 Rev | TGGGCCTTATCTCCATTCCA | 3394–3375 | ||||
| Zika NS1 probeh | TTCCGGGCTAAAGATGGCTGTTGGT | 3349–3373 |
RT-PCR, reverse transcriptase PCR; PAHO, Pan American Health Organization; prM, precursor membrane; E, envelope; NS5, nonstructural protein 5; NS3, nonstructural protein 3; NS1, nonstructural protein 1; For, forward; Rev, reverse.
The probe was labeled with 5-carboxyfluorescein (5-FAM) at the 5′ end. The 3′ quencher was not specified.
Numbering according to Zika virus strain MR-766 (GenBank accession number AY632535).
The probe was labeled with fluorescein; the isomer was not specified. The quencher was not specified.
Numbering according to Zika virus strain Yap 2007 (GenBank accession number EU545988).
The probe was labeled with 6-carboxyfluorescein (6-FAM) at the 5′ end. The 3′ quencher is listed as 5-carboxytetramethylrhodamine (TAMRA) in the article text and black berry quencher (BBQ) in the article table. The probe is listed as containing locked nucleic acids (LNA). The residues utilizing LNA chemistry were not specified.
The probe was labeled with 6-carboxyfluorescein (6-FAM) at the 5′ end. The 3′ quencher was black hole quencher-1 (BHQ-1).
The probe was labeled with fluorescein; the isomer was not specified. TAMRA was used as quencher.
Among the results from 157 serum samples from the Yap epidemic tested using the CDC ZIKV rRT-PCR, 10.8% (17/157) were positive, 6.4% (10/157) were equivocal, and 82.8 (130/157) were negative (23). Among the positive samples, 88.2% (15/17) were collected within the first 3 days of illness, suggesting that the duration of detectable ZIKV viremia is relatively short following the development of clinical symptoms. These findings were also observed during the 2013–2014 French Polynesia outbreak, where the mean time point of illness for patients that were serum positive by the CDC ZIKV rRT-PCR was 3.3 days (standard deviation, 1.8 days) (75). Though neither of these studies provided day-of-illness statistics for the sample set as a whole or for the negative and equivocal specimens, the 5-day cutoff recommended for performing CDC DENV PCR assays also seems appropriate for the CDC ZIKV rRT-PCR assay. Similarly to DENV diagnostics, as more-sensitive ZIKV tests are developed, recommendations regarding the timing of sample collection will need to be reassessed (76–78).
ZIKV RNA was also quantitated in the Yap outbreak specimens using the CDC ZIKV rRT-PCR, revealing relatively low levels of viremia (23). Future studies with larger numbers of positive specimens will be required to further characterize the dynamics of ZIKV levels in blood and determine if there are correlations of virus load with disease severity and immune responses.
In addition to tests using serum or plasma, the CDC ZIKV rRT-PCR has been evaluated using several alternative specimen types. For example, ZIKV RNA was more frequently detected in saliva than in serum when this assay was used to test paired specimens (n = 182) from the 2013–2014 French Polynesia outbreak (75). Of 182 cases with paired samples, 28.6% (52/182) were positive in saliva and serum, 19.2% (35/182) in saliva only, and 8.8% (16/182) in serum only. A total of 43.4% (79/182) were negative in both specimen types. However, the mean day of illness seen with samples with ZIKV RNA detectable only in saliva was not significantly different from the mean day of illness seen with those samples detectable only in serum (day 3.5 versus day 3.3). These data suggest that optimal diagnosis of acute ZIKV infection may require testing of multiple specimen types but that the addition of saliva does not extend the window for viral RNA detection.
To investigate the potential detection of ZIKV RNA later in the course of illness, the CDC ZIKV rRT-PCR was used to test serial urine and serum samples collected from a case series of six patients infected during the 2013–2014 New Caledonia outbreak (79). DENV RNA (80) and WNV RNA (81) have previously been shown to be detected later in urine than in serum in the course of illness; similarly, the New Caledonia study demonstrated that ZIKV RNA was detected in urine 7 or more days after becoming undetectable in serum (79). Specimens described in a case report of a traveler returning from French Polynesia were also evaluated as being ZIKV RNA positive in urine but negative in serum when paired specimens were collected on day of illness 10 (82). Given the specificity challenges of ZIKV IgM assays, testing for viral RNA in urine may allow diagnosis of acute infection after viremia has resolved and after RNA is also no longer detectable in saliva. Future studies will be required to further establish the clinical utility of urine testing for ZIKV infection.
The CDC rRT-PCR was also used to detect ZIKV RNA in the amniotic fluid from the two cases of fetal microcephaly described above (57). ZIKV rRT-PCR analysis of amniotic fluid will be an important diagnostic tool to confirm ZIKV congenital infection and evaluate its association with fetal neurological abnormalities.
Other ZIKV molecular diagnostics include conventional, one-step RT-PCR assays targeting the E gene (71) and the nonstructural protein 5 (NS5) gene (72), as well as one-step, rRT-PCR assays targeting the NS5 (73), NS3 (66), E (74), and NS1 (74) genes (Table 1). Both the conventional E gene and real-time NS5 gene assays were developed by the same group at the Institute Pasteur in Dakar, Senegal, and were evaluated using cultured isolates primarily of mosquito origin and/or field-caught mosquito pools (71, 73). During the Brazil outbreak, the Dakar conventional E gene RT-PCR was used to detect ZIKV RNA in specimens from Camaçari (36) and Natal (38).
A ZIKV rRT-PCR targeting the NS3 gene was also developed at the Bernhard Nocht Institute for Tropical Medicine in Hamburg, Germany (66), and was utilized to evaluate returning travelers (66, 83, 84), though only one of the three patients described had detectable viremia. Similarly, ZIKV rRT-PCR assays targeting the E and NS1 genes were developed in Queensland, Australia, and both assays were positive in tests of a serum specimen collected from a returning traveler from the Cook Islands (74). Complete analytical and clinical validations of the NS3, E, and NS1 assays have not yet been reported. Unlike the singleplex ZIKV assays described thus far, the conventional NS5 RT-PCR is a duplex assay that also includes internal-control primers targeting the human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene (72). In addition to the analytical evaluation, this assay was used to screen archived RNA from adult patients presenting to National University Hospital, Singapore, who tested negative for DENV or CHIKV RNA by RT-PCR. Because no ZIKV RNA-positive specimens were identified, the clinical sensitivity of this assay is not currently known.
Commercial tests are also becoming available, including RealStar Zika Virus RT-PCR kit 1.0, developed by Altona Diagnostics (Hamburg, Germany), an internally controlled one-step, real-time RT-PCR assay now with CE marking. While the Altona test demonstrates excellent analytical performance, it has not yet been evaluated with clinical specimens in the literature.
PERSPECTIVES ON AN ONGOING PANDEMIC
The spread of ZIKV to the Americas and its association with a marked increase in the incidence of fetal neurologic abnormalities has led to unprecedented interest in this once-esoteric pathogen. Large epidemiologic studies and intensification of basic and translational research will result in increased understanding of ZIKV pathogenesis and immunology as well as in important breakthroughs in sorely needed ZIKV therapeutics and vaccines. The sensitive and specific diagnosis of patients with Zika fever is critical to this work, to ongoing epidemiologic surveillance, and to the care of patients with an undifferentiated systemic febrile illness. Significantly, improved availability of validated diagnostic tests, including tests for the detection of ZIKV antibodies, antigens, and RNA, will be critical to understand and respond to this pandemic. Moreover, tests that combine the diagnosis of Zika with diagnosis of dengue and chikungunya are likely to be of great utility, as are tests that can be performed at or near the point of care. The CDC has issued detailed recommendations for the testing of pregnant women returning to the United States from areas of ZIKV transmission as well as for the testing of infants with suspected congenital infection (61, 85). However, future work will be required to clearly detail test performance characteristics in these patient groups and to determine whether these recommendations meet the needs of pregnant women and their neonates and infants in countries where ZIKV transmission is ongoing. As this pandemic evolves, the further development, evaluation, and widespread implementation of ZIKV diagnostics will be critical to monitoring, preventing, and eventually treating Zika disease.
Biographies

Jesse J. Waggoner is an Instructor in the Division of Infectious Diseases and Geographic Medicine at the Stanford University School of Medicine. He attended medical school and completed his residency in internal medicine at Duke, followed by a fellowship in infectious diseases at Stanford. His interest in global health began in medical school, and his current research focuses on the implementation and evaluation of improved diagnostics for pathogens important to the global health community. His work on a new Zika virus diagnostic, which is currently in the process of clinical evaluation, began in 2013. Overall, Jesse's research has resulted in the publication of 23 manuscripts, including 17 first-author papers. He has received financial awards from Stanford University, the National Institute of Allergy and Infectious Diseases, the Thrasher Research Fund, and the American Society of Tropical Medicine and Hygiene.

Benjamin A. Pinsky is the Medical Director of the Clinical Virology Laboratory for Stanford Health Care and Stanford Children's Health and is an Assistant Professor of Pathology and Medicine in the Division of Infectious Diseases and Geographic Medicine at the Stanford University School of Medicine. Dr. Pinsky earned his M.D. and Ph.D. degrees in the Medical Scientist Training Program at the University of Washington School of Medicine in Seattle and completed residency training in clinical pathology at the Stanford University School of Medicine. He has published over 50 manuscripts and 10 book chapters and is coeditor of the 5th edition of the Clinical Virology Manual. Dr. Pinsky received Young Investigator Awards from the Pan American Society for Clinical Virology and the American Society for Microbiology. His research interests include the design of novel diagnostics and investigation of the clinical impact of molecular infectious diseases testing, particularly for arboviral infections.
REFERENCES
- 1.Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. 1998. Phylogeny of the genus Flavivirus. J Virol 72:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuno G, Chang GJ. 2007. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol 152:687–696. doi: 10.1007/s00705-006-0903-z. [DOI] [PubMed] [Google Scholar]
- 3.Dick GW, Kitchen SF, Haddow AJ. 1952. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 4.Dick GW. 1952. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 5.Macnamara FN. 1954. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 6.Smithburn KC. 1952. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J Immunol 69:223–234. [PubMed] [Google Scholar]
- 7.Smithburn KC. 1954. Neutralizing antibodies against arthropod-borne viruses in the sera of long-time residents of Malaya and Borneo. Am J Hyg 59:157–163. [DOI] [PubMed] [Google Scholar]
- 8.Smithburn KC, Kerr JA, Gatne PB. 1954. Neutralizing antibodies against certain viruses in the sera of residents of India. J Immunol 72:248–257. [PubMed] [Google Scholar]
- 9.Hammon WM, Schrack WD Jr, Sather GE. 1958. Serological survey for a arthropod-borne virus infections in the Philippines. Am J Trop Med Hyg 7:323–328. [DOI] [PubMed] [Google Scholar]
- 10.Macnamara FN, Horn DW, Porterfield JS. 1959. Yellow fever and other arthropod-borne viruses; a consideration of two serological surveys made in South Western Nigeria. Trans R Soc Trop Med Hyg 53:202–212. doi: 10.1016/0035-9203(59)90072-0. [DOI] [PubMed] [Google Scholar]
- 11.Pond WL. 1963. Arthropod-borne virus antibodies in sera from residents of South-East Asia. Trans R Soc Trop Med Hyg 57:364–371. doi: 10.1016/0035-9203(63)90100-7. [DOI] [PubMed] [Google Scholar]
- 12.Fagbami AH. 1979. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 83:213–219. doi: 10.1017/S0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson JG, Ksiazek TG, Gubler DJ, Lubis SI, Simanjuntak G, Lee VH, Nalim S, Juslis K, See R. 1983. A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Ann Trop Med Parasitol 77:131–137. [DOI] [PubMed] [Google Scholar]
- 14.Adekolu-John EO, Fagbami AH. 1983. Arthropod-borne virus antibodies in sera of residents of Kainji Lake Basin, Nigeria 1980. Trans R Soc Trop Med Hyg 77:149–151. doi: 10.1016/0035-9203(83)90053-6. [DOI] [PubMed] [Google Scholar]
- 15.Olson JG, Ksiazek TG, Suhandiman, Triwibowo. 1981. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 75:389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 16.Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, Zanotto PM, Sall AA. 2014. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 8:e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bearcroft WG. 1956. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 50:442–448. doi: 10.1016/0035-9203(56)90091-8. [DOI] [PubMed] [Google Scholar]
- 18.Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, David-West TS, Kemp GE. 1975. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol 69:49–64. [DOI] [PubMed] [Google Scholar]
- 19.Fagbami A. 1977. Epidemiological investigations on arbovirus infections at Igbo-Ora, Nigeria. Trop Geogr Med 29:187–191. [PubMed] [Google Scholar]
- 20.Simpson DI. 1964. Zika virus infection in man. Trans R Soc Trop Med Hyg 58:335–338. doi: 10.1016/0035-9203(64)90200-7. [DOI] [PubMed] [Google Scholar]
- 21.Filipe AR, Martins CM, Rocha H. 1973. Laboratory infection with Zika virus after vaccination against yellow fever. Arch Gesamte Virusforsch 43:315–319. doi: 10.1007/BF01556147. [DOI] [PubMed] [Google Scholar]
- 22.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 23.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D. 2014. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 20:1085–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derraik JG, Slaney D. 2015. Notes on Zika virus—an emerging pathogen now present in the South Pacific. Aust N Z J Public Health 39:5–7. doi: 10.1111/1753-6405.12302. [DOI] [PubMed] [Google Scholar]
- 26.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. 2014. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill 19::pii=20720 http://dx.doi.org/10.2807/1560-7917.ES2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 27.Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. 2014. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 44:302–307. doi: 10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Kucharski AJ, Funk S, Eggo RM, Mallet H-P, Edmunds J, Nilles EJ. 11 February 2016. Transmission dynamics of Zika virus in island populations: a modelling analysis of the 2013–14 French Polynesia outbreak. bioRxiv doi: 10.1101/038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musso D. 2015. Zika virus transmission from French Polynesia to Brazil. Emerg Infect Dis 21:1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musso D, Cao-Lormeau VM, Gubler DJ. 2015. Zika virus: following the path of dengue and chikungunya? Lancet 386:243–244. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 31.Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, Parra B, Mora J, Becerra N, Lagos N, Vera L, Olivares B, Vilches M, Fernandez J. 26 November 2015. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol doi: 10.1007/s00705-015-2695-5. [DOI] [PubMed] [Google Scholar]
- 32.Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, Guillaumot L, Souares Y. 2014. Concurrent outbreaks of dengue, chikungunya and Zika virus infections—an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro Surveill 19:pii=20929 http://dx.doi.org/10.2807/1560-7917.ES2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 33.Dupont-Rouzeyrol M, O'Connor O, Calvez E, Daures M, John M, Grangeon JP, Gourinat AC. 2015. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis 21:381–382. doi: 10.3201/eid2102.141553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villamil-Gómez WE, González-Camargo O, Rodriguez-Ayubi J, Zapata-Serpa D, Rodriguez-Morales AJ. 2 January 2016. Dengue, chikungunya and Zika co-infection in a patient from Colombia. J Infect Public Health doi: 10.1016/j.jiph.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. 2012. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campos GS, Bandeira AC, Sardi SI. 2015. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardoso CW, Paploski IA, Kikuti M, Rodrigues MS, Silva MM, Campos GS, Sardi SI, Kitron U, Reis MG, Ribeiro GS. 2015. Outbreak of exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil. Emerg Infect Dis 21:2274–2276. doi: 10.3201/eid2112.151167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. 2015. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calvet GA, Filippis AM, Mendonca MC, Sequeira PC, Siqueira AM, Veloso VG, Nogueira RM, Brasil P. 2016. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. J Clin Virol 74:1–3. [DOI] [PubMed] [Google Scholar]
- 40.Releveì eìpideìmiologique hebdomadaire/Section d'hygieÌne du Secreìtariat de la Socieìteì des Nations (Weekly epidemiological record/Health Section of the Secretariat of the League of Nations). 2015. Zika virus outbreaks in the Americas. Wkly Epidemiol Rec 90:609–610. (In French and English.)26552108 [Google Scholar]
- 41.Enfissi A, Codrington J, Roosblad J, Kazanji M, Rousset D. 8 January 2016. Zika virus genome from the Americas. Lancet doi: 10.1016/S0140-6736(16)00003-9. [DOI] [PubMed] [Google Scholar]
- 42.Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. 1964. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull World Health Organ 31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 43.Weinbren MP, Williams MC. 1958. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 52:263–268. doi: 10.1016/0035-9203(58)90085-3. [DOI] [PubMed] [Google Scholar]
- 44.McCrae AW, Kirya BG. 1982. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg 76:552–562. doi: 10.1016/0035-9203(82)90161-4. [DOI] [PubMed] [Google Scholar]
- 45.Boorman JP, Porterfield JS. 1956. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg 50:238–242. doi: 10.1016/0035-9203(56)90029-3. [DOI] [PubMed] [Google Scholar]
- 46.Marchette NJ, Garcia R, Rudnick A. 1969. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg 18:411–415. [DOI] [PubMed] [Google Scholar]
- 47.Li MI, Wong PS, Ng LC, Tan CH. 2012. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis 6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. 2013. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis 7:e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, Leroy EM. 2014. Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, Pretrick M, Marfel M, Griggs A, Bel M, Duffy MR, Hancock WT, Ho-Chen T, Powers AM. 2014. Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl Trop Dis 8:e3188. doi: 10.1371/journal.pntd.0003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. 2015. Potential sexual transmission of Zika virus. Emerg Infect Dis 21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau VM, Broult J. 2014. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 19:pii=20761 http://dx.doi.org/10.2807/1560-7917.ES2014.19.14.20761 [DOI] [PubMed] [Google Scholar]
- 54.Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. 2015. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2015:1–6. doi: 10.2450/2015.0066-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. 2014. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 19:pii=20751 http://dx.doi.org/10.2807/1560-7917.ES2014.19.13.20751 [PubMed] [Google Scholar]
- 56.Tetro JA. 14 January 2016. Zika and microcephaly: causation, correlation, or coincidence? Microbes Infect doi: 10.1016/j.micinf.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. 2016. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 58.Dyer O. 2015. Zika virus spreads across Americas as concerns mount over birth defects. BMJ 351:h6983. [DOI] [PubMed] [Google Scholar]
- 59.Fauci AS, Morens DM. 13 January 2016. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 60.Ventura CV, Maia M, Bravo-Filho V, Gois AL, Belfort R Jr. 8 January 2016. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 61.Petersen EE, Staples JE, Meaney-Delman D, Fischer M, Ellington SR, Callaghan WM, Jamieson DJ. 2016. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 65:30–33. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- 62.Fonseca K, Meatherall B, Zarra D, Drebot M, MacDonald J, Pabbaraju K, Wong S, Webster P, Lindsay R, Tellier R. 2014. First case of Zika virus infection in a returning Canadian traveler. Am J Trop Med Hyg 91:1035–1038. doi: 10.4269/ajtmh.14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Digoutte JP, Calvo-Wilson MA, Mondo M, Traore-Lamizana M, Adam F. 1992. Continuous cell lines and immune ascitic fluid pools in arbovirus detection. Res Virol 143:417–422. doi: 10.1016/S0923-2516(06)80135-4. [DOI] [PubMed] [Google Scholar]
- 64.Hancock WT, Marfel M, Bel M. 2014. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 20:1960. doi: 10.3201/eid2011.141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwong JC, Druce JD, Leder K. 2013. Zika virus infection acquired during brief travel to Indonesia. Am J Trop Med Hyg 89:516–517. doi: 10.4269/ajtmh.13-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tappe D, Rissland J, Gabriel M, Emmerich P, Gunther S, Held G, Smola S, Schmidt-Chanasit J. 2014. First case of laboratory-confirmed Zika virus infection imported into Europe, November 2013. Euro Surveill 19:pii=20685 http://dx.doi.org/10.2807/1560-7917.ES2014.19.4.20685. [DOI] [PubMed] [Google Scholar]
- 67.Shinohara K, Kutsuna S, Takasaki T, Moi ML, Ikeda M, Kotaki A, Yamamoto K, Fujiya Y, Mawatari M, Takeshita N, Hayakawa K, Kanagawa S, Kato Y, Ohmagari N. 18 January 2016. Zika fever imported from Thailand to Japan, and diagnosed by PCR in the urines. J Travel Med doi: 10.1093/jtm/tav011. [DOI] [PubMed] [Google Scholar]
- 68.Summers DJ, Acosta RW, Acosta AM. 2015. Zika virus in an American recreational traveler. J Travel Med 22:338–340. doi: 10.1111/jtm.12208. [DOI] [PubMed] [Google Scholar]
- 69.Zammarchi L, Stella G, Mantella A, Bartolozzi D, Tappe D, Gunther S, Oestereich L, Cadar D, Munoz-Fontela C, Bartoloni A, Schmidt-Chanasit J. 2015. Zika virus infections imported to Italy: clinical, immunological and virological findings, and public health implications. J Clin Virol 63:32–35. doi: 10.1016/j.jcv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Korhonen EM, Huhtamo E, Smura T, Kallio-Kokko H, Raassina M, Vapalahti O. 2016. Zika virus infection in a traveller returning from the Maldives, June 2015. Euro Surveill 21:pii=30107. doi: 10.2807/1560-7917.ES.2016.21.2.30107. [DOI] [PubMed] [Google Scholar]
- 71.Faye O, Faye O, Dupressoir A, Weidmann M, Ndiaye M, Alpha Sall A. 2008. One-step RT-PCR for detection of Zika virus. J Clin Virol 43:96–101. doi: 10.1016/j.jcv.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Balm MN, Lee CK, Lee HK, Chiu L, Koay ES, Tang JW. 2012. A diagnostic polymerase chain reaction assay for Zika virus. J Med Virol 84:1501–1505. doi: 10.1002/jmv.23241. [DOI] [PubMed] [Google Scholar]
- 73.Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. 2013. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 10:311. doi: 10.1186/1743-422X-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pyke AT, Daly MT, Cameron JN, Moore PR, Taylor CT, Hewitson GR, Humphreys JL, Gair R. 2014. Imported Zika virus infection from the Cook islands into Australia, 2014. PLoS Curr 6 doi: 10.1371/currents.outbreaks.4635a54dbffba2156fb2fd76dc49f65e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. 2015. Detection of Zika virus in saliva. J Clin Virol 68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 76.Waggoner JJ, Abeynayake J, Sahoo MK, Gresh L, Tellez Y, Gonzalez K, Ballesteros G, Pierro AM, Gaibani P, Guo FP, Sambri V, Balmaseda A, Karunaratne K, Harris E, Pinsky BA. 2013. Single-reaction, multiplex, real-time rt-PCR for the detection, quantitation, and serotyping of dengue viruses. PLoS Negl Trop Dis 7:e2116. doi: 10.1371/journal.pntd.0002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waggoner JJ, Abeynayake J, Sahoo MK, Gresh L, Tellez Y, Gonzalez K, Ballesteros G, Guo FP, Balmaseda A, Karunaratne K, Harris E, Pinsky BA. 2013. Comparison of the FDA-approved CDC DENV-1-4 real-time reverse transcription-PCR with a laboratory-developed assay for dengue virus detection and serotyping. J Clin Microbiol 51:3418–3420. doi: 10.1128/JCM.01359-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waggoner JJ, Pinsky BA. 2014. Reply to “Inconclusive reverse transcription-PCR assay comparison for dengue virus detection and serotyping”. J Clin Microbiol 52:1801–1802. doi: 10.1128/JCM.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. 2015. Detection of Zika virus in urine. Emerg Infect Dis 21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirayama T, Mizuno Y, Takeshita N, Kotaki A, Tajima S, Omatsu T, Sano K, Kurane I, Takasaki T. 2012. Detection of dengue virus genome in urine by real-time reverse transcriptase PCR: a laboratory diagnostic method useful after disappearance of the genome in serum. J Clin Microbiol 50:2047–2052. doi: 10.1128/JCM.06557-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barzon L, Pacenti M, Franchin E, Pagni S, Martello T, Cattai M, Cusinato R, Palu G. 2013. Excretion of West Nile virus in urine during acute infection. J Infect Dis 208:1086–1092. doi: 10.1093/infdis/jit290. [DOI] [PubMed] [Google Scholar]
- 82.Kutsuna S, Kato Y, Takasaki T, Moi M, Kotaki A, Uemura H, Matono T, Fujiya Y, Mawatari M, Takeshita N, Hayakawa K, Kanagawa S, Ohmagari N. 2014. Two cases of Zika fever imported from French Polynesia to Japan, December 2013 to January 2014 [corrected]. Euro Surveill 19:pii=20683 http://dx.doi.org/10.2807/1560-7917.ES2014.19.4.20683. [DOI] [PubMed] [Google Scholar]
- 83.Tappe D, Nachtigall S, Kapaun A, Schnitzler P, Gunther S, Schmidt-Chanasit J. 2015. Acute Zika virus infection after travel to Malaysian Borneo, September 2014. Emerg Infect Dis 21:911–913. doi: 10.3201/eid2105.141960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wæhre T, Maagard A, Tappe D, Cadar D, Schmidt-Chanasit J. 2014. Zika virus infection after travel to Tahiti, December 2013. Emerg Infect Dis 20:1412–1414. doi: 10.3201/eid2008.140302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Staples JE, Dziuban EJ, Fischer M, Cragan JD, Rasmussen SA, Cannon MJ, Frey MT, Renquist CM, Lanciotti RS, Munoz JL, Powers AM, Honein MA, Moore CA. 2016. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 65:63–67. doi: 10.15585/mmwr.mm6503e3. [DOI] [PubMed] [Google Scholar]