Abstract
Aims
Non-fluoroscopic imaging (NFI) devices are increasingly used in ablations. The objective was to determine the utility of intracardiac echocardiography (ICE) in ablating paediatric supraventricular tachycardias (SVTs) and assess whether its integrated use with electroanatomic mapping (EAM) resulted in lower radiation exposure than use of EAM alone.
Methods and results
Prospective, controlled, single-centre study of patients (pts) age ≥10 years, weight ≥35 kg, with SVT and normal cardiac anatomy. Patients were randomized to ICE + EAM (ICE) or EAM only (no ICE). Both had access to fluoroscopy as needed. Eighty-four pts were enroled (42 ICE, 42 no ICE). Median age was 15 years (range 10.4–23.7 years); 57% had accessory pathways, 42% atrioventricular nodal reentry tachycardia. There was no difference in radiation dose (9 mGy ICE vs. 23 mGy no ICE, P = 0.37) or fluoroscopy time (1.1 min ICE vs. 1.5 min no ICE, P = 0.38). Transseptal punctures were performed in 25 pts (16 ICE, 9 no ICE), with ICE reducing radiation (8 mGy ICE vs. 62 mGy no ICE, P = 0.002) and fluoroscopy time (1.1 min ICE vs. 4.5 min no ICE, P = 0.01). Zero fluoroscopy was achieved in 13 pts (15% of total, 5 ICE, 8 no ICE), and low-dose cases (<50 mGy) in 57 pts (68% of total, 33 ICE, 24 no ICE). Acute success was 95% for ICE, 88% for no ICE.
Conclusion
Use of an integrated EAM/ICE system was no better than EAM alone in limiting radiation, but can be helpful for transseptal punctures. Given the low dose savings, use of ICE may be weighed against its financial cost. Low-fluoroscopy cases are performed in most NFI procedures.
Keywords: Ablation, Intracardiac echocardiography, Paediatrics, Radiation, Supraventricular tachycardia
What's new?
There was no difference in radiation dose between patients who had supraventricular tachycardia ablations performed using electroanatomic mapping (EAM) alone vs. those using an integrated system of EAM + intracardiac echocardiography (ICE).
A significant reduction in radiation was noted in ICE patients requiring transseptal punctures.
Low-fluoroscopy cases are being performed in the majority of ablations utilizing non-fluoroscopic imaging tools.
Introduction
Fluoroscopy has been used for decades to guide cardiovascular interventions. This has required the use of ionizing radiation, which has led to increasing awareness of its stochastic effects. As such, the guiding principle for the clinical use of ionizing radiation has been to keep radiation ‘as low as reasonably achievable’ or ALARA.1 Non-fluoroscopic imaging (NFI) tools have been employed in electrophysiology (EP) labs to reduce radiation exposure. A recent study by Miyake et al.,2 noted that the use of electroanatomic mapping (EAM) and intracardiac echocardiography (ICE) can reduce fluoroscopy time by over 50% compared with conventional fluoroscopy-only studies, with no difference in acute success or adverse events. As EP practices begin to shift towards the regular use of NFI, the usefulness of specific tools needs to be defined. The primary aim of this study was to determine whether the integrated use of EAM and ICE was no better than the use of EAM alone in reducing radiation exposure for paediatric patients undergoing ablation for supraventricular tachycardia (SVT).
Methods
Study design
This was a prospective, randomized, controlled, single-centre study performed at Boston Children's Hospital. All patients presenting for electrophysiological study (EPS) and ablation for SVT were evaluated. Those who were ≥10 years of age, weighed ≥35 kg, and had normal cardiac anatomy were included, as were those with trivial structural heart defects such as bicuspid aortic valve or a left superior vena cava. Patients were excluded if they had more than trivial congenital heart disease, prior ablation, or prior cardiac surgery. The study was approved by the Institutional Review Board at Boston Children's Hospital. Consent was obtained from one parent and/or the patient (if age appropriate).
Randomization and study intervention
Randomization was stratified by the operator to account for variation in practice and to ensure that the total number of ICE and no ICE patients randomized to each operator were similar. Patients randomized to the ICE group underwent an ablation procedure with the operator having access to fluoroscopy, EAM, and ICE (Carto3, Biosense Webster, Inc.). Patients randomized to the ‘no ICE’ group had access to fluoroscopy and EAM (Carto3, Biosense Webster, Inc.), but no ICE. Operators were instructed to use as much fluoroscopy as necessary to perform a safe and effective ablation, but they were aware that the goal in both groups was to minimize radiation exposure. The use of NFI devices was encouraged but not required.
Primary and secondary outcomes
The primary outcome was radiation dose [measured in milligrey (mGy)], as calculated by the fluoroscopy system based on tube output, distance from the image intensifier, and patient chest depth. Secondary outcomes included fluoroscopy time (in minutes), procedural times, acute success, and adverse events. Follow-up was obtained at 1–6 months after the procedure.
Clinical management
All patients underwent a preablation electrocardiogram (ECG) and echocardiogram. Procedures were performed with patients under general anaesthesia with endotracheal intubation performed by a cardiac anaesthesiologist. Fluoroscopic settings were not standardized but the maximal frame rates used in each study were 7.5 frames/s. Patients underwent placement of four catheters via femoral venous access (7F sheath in the right femoral vein, two 6F and one 5F sheath in the left femoral vein); patients in the ICE group had an additional 10F sheath placed, most often in the right femoral vein, for the ICE catheter. Right internal jugular venous access was performed at the discretion of the operator for either coronary sinus (CS) and/or tricuspid valve annulus mapping. Electroanatomic shells of the inferior vena cava, right atrium, CS, and left atrium (if needed) were created. The His position was marked on the right atrial shell. Catheters were then placed in the CS, His bundle position, right ventricular (RV) apex, and high right atrium. The SVT mechanism was then determined and mapping was performed in pre-excited sinus rhythm, SVT, or during steady-rate ventricular pacing for accessory pathway-mediated tachycardia, or by anatomical landmarks and electrograms for atrioventricular (AV) nodal reentrant tachycardia (AVNRT). Radiofrequency energy was used for ablations unless the operator felt cryoablation was necessary, either for paranodal locations or within the middle cardiac vein. Procedural times (clock time and fluoroscopy time) and radiation exposure were documented for each segment of the procedure (Figure 1). Senior EP fellows began all cases, obtained venous access, and placed all catheters. The attending electrophysiologist would scrub-in at their discretion, but was standardized such that if the fellow had reached 3 min of fluoroscopy time, the attending would enter the case. Furthermore, use of ICE required two operators at the table; therefore, both the attending and the fellow worked together more during these cases.
Figure 1.
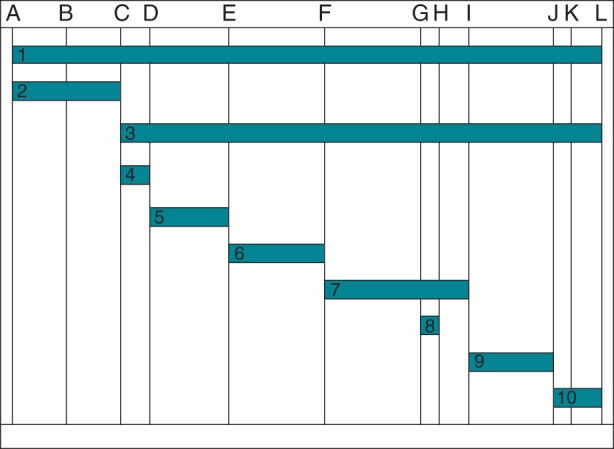
Breakdown of procedure components. A, patient enters EP lab; B, patient intubated; C, start of vascular access; D, vascular access complete; E, all catheters in position; F, start of mapping; G, start of transseptal; H, end of transseptal; I, first ablation lesion; J, end of 30 min waiting period; K, all vascular sheaths removed; L, patient exits room; 1, total case time; 2, set-up; 3, total EP procedure time; 4, vascular access; 5, creation of electroanatomic shells/catheter placement; 6, diagnostic EPS; 7, mapping; 8, transseptal; 9, ablation and 30 min wait period; 10, post-procedure care and transfer to recovery room.
Patients with right-sided ablations were discharged the same day, whereas patients with left-sided ablations remained overnight for heparin infusion and were discharged the following morning. All patients were examined within 4 h after the procedure and again prior to discharge if they had been admitted overnight. An ECG was obtained for all patients prior to discharge. Nursing staff were instructed to inform study investigators of any patient complaints or adverse events.
Non-fluoroscopic imaging techniques
The procedures were identical in both groups except for the additional ability to use ICE in one group. The Carto3 system was used to create electroanatomic shells of the inferior vena cava, right atrium, and CS using the mapping catheter. With these EAM shells, reference catheters were placed within the heart. For patients randomized to ICE, echocardiography was used as needed to augment the EAM shells, most commonly for identification of the CS os and creation of a CS shell to assist with its cannulation. Intracardiac echocardiography was also used for visualization of the atrial septum for Brockenbrough procedures, the AV groove, and confirming catheter contact with the tissue. Fluoroscopy was used as needed for catheter manipulation, with frame rates decreased to a maximum of 7.5 frames/s. In general, the Carto system was used for catheter manipulation and movement around the AV groove.
Statistical analysis
Patient characteristics and outcomes are summarized as number (percent) for categorical variables and median (range) for continuous variables. Fisher's exact test was used for comparisons between categorical variables and Wilcoxon rank sum test for continuous variables. The primary analyses were based on intention-to-treat, with a secondary analysis performed based on whether the ICE catheter was actually used during the procedure (no-intention-to-treat). If imbalances were noted between treatment groups in any patient or operator characteristics, linear regression was used to examine associations between treatment group and outcome variables adjusting for these factors. The occurrences of adverse events were compared between groups using Fisher's exact test. Natural logarithms were used as the outcome variables as radiation exposure and fluoroscopy time were not normally distributed. Statistical analysis was performed using STATA 100 (StataCorp).
Results
Patient characteristics
Over a study period of 14 months, a total of 221 patients presented for an EPS and ablation for SVT. Patients excluded consisted of those <10 years of age or <35 kg (n = 51), had a prior ablation (n = 30), had congenital heart disease (n = 9), were enroled in a concurrent anaesthesiology study (n = 4), or other (n = 12). Of the 115 patients eligible for enrolment, 84 consented and 31 declined.
Baseline characteristics are summarized in Table 1. Although the patients who did not have ICE during their procedure were older (14.7 vs. 16.1 years), there was no significant difference in patient weight, height, body surface area, body mass index, and chest depth. The distribution of SVT mechanisms and the occurrence of right- or left-sided accessory pathway location were not different between the two groups. Case difficulty, as subjectively assessed by the attending and fellow after performing the case (on a scale from 1 to 10, with 10 being most difficult), was no different between the ICE and no ICE groups.
Table 1.
Baseline characteristics
| ICE (n = 42) | No ICE (n = 42) | P value | |
|---|---|---|---|
| Age (years) | 14.7 (10.4–18.4) | 16.1 (10.8–23.7) | 0.02 |
| Male | 24 (57%) | 22 (52%) | 0.83 |
| Weight (kg) | 55.6 (35.0–107.0) | 62.8 (43.4–103.6) | 0.06 |
| Height (cm) | 163 (135–186) | 168 (147–183) | 0.16 |
| BSA | 1.64 (1.17–2.28) | 1.69 (1.18–2.22) | 0.09 |
| BMI | 21.8 (16.6–35.3) | 23.1 (17.2–35.4) | 0.1 |
| Chest circumference (cm) (n = 42, 38) | 18 (12–23) | 18 (13–22) | 0.38 |
| Diagnosis | 0.71 | ||
| AVNRT | 15 (36%) | 20 (48%) | |
| WPW | 18 (43%) | 13 (31%) | |
| URAP | 8 (19%) | 8 (19%) | |
| FAT or Mahaim | 1 (2%) | 1 (2%) | |
| Accessory pathway | 0.25 | ||
| Left-sided | 15 (55%) | 8 (36%) | |
| Right-sided | 12 (44%) | 14 (64%) | |
| Case difficulty | |||
| Attending (n = 40, 38) | 5.0 (1.0–9.0) | 4.5 (2.0–8.0) | 0.49 |
| Fellow (n = 40, 42) | 5.5 (2.0–9.0) | 6.0 (2.0–10.0) | 0.97 |
Primary and secondary outcomes
The radiation dose and fluoroscopy time used during the entire case and each of its components is shown in Table 2. Overall, these levels are lower than previously reported in Miyake et al. Specifically, catheter placement required little or no need for X-ray with the creation of an anatomical shell of the inferior vena cava allowing the operator to guide the catheters into the heart. The greatest contrast between the two groups was during transseptal access. With the use of ICE, radiation dose decreased from 62 to 8 mGy (P = 0.002), allowing the operator to visualize the atrial septum on echo while the needle passed into the left atrium rather than using fluoroscopy. The amount of radiation exposure during mapping was also higher in the no ICE group, as transseptal punctures are incorporated within the ‘mapping’ period of the study.
Table 2.
Radiation dose and fluoroscopy times
| Total | ICE | No ICE | P value | |
|---|---|---|---|---|
| Radiation dose (mGy) | ||||
| Total case (42 ICE, 42 no ICE) | 14.5 (0–1014) | 9 (0–611) | 23 (0–1014) | 0.37 |
| Cath placement (42 ICE, 41 no ICE) | 1 (0–580) | 1 (0–580) | 1 (0–112) | 0.57 |
| Mapping (42 ICE, 37 no ICE) | 0 (0–325) | 0 (0–52) | 2 (0–325) | 0.02 |
| Ablation (42 ICE, 38 no ICE) | 2 (0–738) | 2 (0–476) | 5 (0–738) | 0.39 |
| Transseptal (16 ICE, 9 no ICE) | 7 (0–757) | 8 (0–257) | 62 (13–757) | 0.002 |
| Fluoroscopy time (min) | ||||
| Total case (42 ICE, 42 no ICE) | 1.3 (0–33.8) | 1.1 (0–19.7) | 1.5 (0–33.8) | 0.38 |
| Cath placement (42 ICE, 41 no ICE) | 0.0 (0–11.5) | 0.1 (0–11.5) | 0.0 (0–7.6) | 0.41 |
| Mapping (42 ICE, 37 no ICE) | 0.0 (0–14.0) | 0.0 (0–3.7) | 0.1 (0–14.0) | 0.08 |
| Ablation (42 ICE, 38 no ICE) | 0.2 (0–22.2) | 0.1 (0–14.2) | 0.3 (0–22.2) | 0.25 |
| Transseptal (16 ICE, 9 no ICE) | 1.4 (0–33.8) | 1.1 (0–17.5) | 4.5 (1.5–33.8) | 0.01 |
Among the entire cohort of patients, there were 13 (15%) studies that used no radiation during the procedure, 5 in the patients with ICE, 8 in the patients without ICE (P = 0.38). In fact, the majority of patients in both groups were low-fluoroscopy cases, with 33 (79%) ICE patients having radiation doses <50 mGy, compared with 24 (57%) patients in the no ICE group (P = 0.06). High-fluoroscopy cases, arbitrarily assigned as cases using >500 mGy of radiation, were low, with only two patients noted in each group (539 and 611 mGy in the ICE group, 757 and 1014 mGy in the no ICE group). In terms of fluoroscopy time and the potential of performing low-fluoroscopy cases of <60 s, 26 (62%) ICE and 24 (57%) no ICE patients were able to obtain that goal.
Case duration
Use of ICE did not affect the length of the EPS (128 min ICE vs. 133 min no ICE, P = 0.85), nor did it affect the total time the patient was in the catheterization lab (225 min ICE vs. 219 min no ICE, P = 0.25). The only significant difference was the time required to obtain vascular access (20 min ICE vs. 12 min no ICE, P < 0.001), as ICE patients required the insertion of an extra 10F sheath for the ICE catheter. Although there was no difference between the groups, both were longer than the fluoroscopy-only group reported in Miyake et al. (total laboratory time 180 min). This is probably due to the 23 (5–57) min needed to create the anatomical shells of the inferior vena cava, right atrium, and CS at the beginning of each case.
Clinical outcome
There was no significant difference in the acute success rate between the two groups, achieved in 40 (95%) of ICE patients and 37 (88%) of no ICE patients (P = 0.43). Table 3 summarizes the patients with procedural failure. Four no ICE patients did not undergo any ablation (one low-risk pathway in the right anteroseptal region, one Mahaim fibre with no inducible SVT, one concealed mid-septal pathway with no inducible SVT, and one AVNRT with transient catheter-induced heart block). Radiofrequency ablation alone was used in 38 ICE patients, with 2 using only cryoablation and 2 using a combined approach. In the patients who did not have access to ICE, 34 patients had radiofrequency ablation only, 2 with cryoablation only, and 2 using a combined approach.
Table 3.
Acute procedural failure
| Patients | Study group | Age (years) | Diagnosis | Ablation | Reason for failure |
|---|---|---|---|---|---|
| 4 | ICE | 17.7 | WPW—right anteroseptal | Cryo | Low-risk antegrade only right anteroseptal pathway, no effect with cryo, no AV block |
| 48 | ICE | 16.7 | WPW—right posteroseptal | RF | Immediate recurrence of pathway in the recovery room |
| 24 | No ICE | 16 | AVNRT | None | Transient AV block with catheter manipulation |
| 51 | No ICE | 17 | Concealed pathway—right mid-septal | None | No inducible SVT |
| 57 | No ICE | 15.1 | Mahaim fibre | None | No inducible SVT |
| 58 | No ICE | 17.2 | WPW—right anteroseptal | Cryo | Low-risk pathway, transient AV nodal injury with cryoablation |
| 59 | No ICE | 12.4 | WPW—right anteroseptal | None | Low-risk pathway, no ablation performed |
Peri-procedural adverse events were noted in four patients. This included transient AV block in a no ICE patient with catheter manipulation resulting in an inability to perform a slow pathway modification, looping of a RV catheter in the femoral vein of an ICE patient requiring insertion of a snare to assist removal, bleeding from the ICE venous access site just prior to discharge from the recovery room, and transient AV nodal Wenckebach in an ICE patient at sinus rates of 100 b.p.m. after a slow pathway modification requiring inpatient observation overnight. Although not recorded as adverse events, procedural difficulties due to the use of NFI devices included one patient in whom the ICE catheter could not be passed through the left iliac vein. In another patient, use of the EAM system was abandoned due to patient motion which resulted in deregistration of the anatomical shells from the patient's true anatomy.
Seventy-nine (94%) patients returned for follow-up at a median of 2.1 months after their procedure. Late complications were noted in three patients: One ICE patient had visual changes 4 days after her AVNRT ablation (later diagnosed as migraines), another ICE patient had intermittent headaches after a left-sided ablation and subsequently had a normal brain magnetic resonance imaging, and one no ICE patient had a persistent haematoma 1 week post-EPS. Excluding the patients who had no ablation performed or an unsuccessful procedure, 4 of 77 patients (5.2%) had a recurrence of their arrhythmia substrate. Three ICE patients had return of either their SVT (two AVNRT patients) or Wolff–Parkinson–White (WPW) on ECG (right posteroseptal pathway). One no ICE patient had a recurrence with intermittent pre-excitation seen on ECG after ablation of a right anteroseptal pathway.
Operator characteristics and preferences
Figure 2 shows the overall radiation dose for each operator. Some operators showed more benefit in using ICE to reduce radiation, although the overall radiation dose was quite low for each operator, with none having a median exposure over 150 mGy. Post-procedural surveys were performed to determine how each attending physician viewed using NFI devices and whether it affected their practice. In general, the operators found EAM to be helpful in reducing radiation whereas ICE was not. The EAM system was helpful not only for catheter visualization, but for lesion localization as well. One notable downside to EAM was if the patient were to move significantly during the case, the resulting unreliability of the anatomical shells in depicting true anatomical landmarks led to the operators abandoning EAM and using fluoroscopy only.
Figure 2.
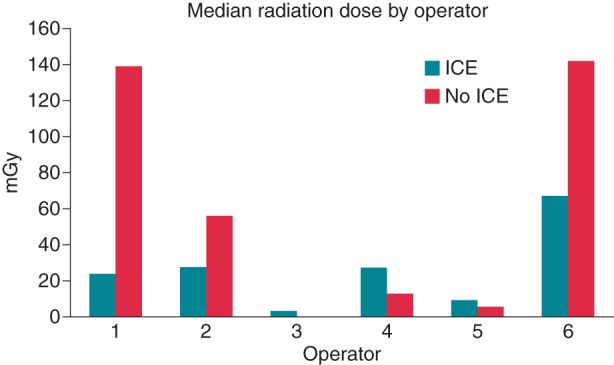
Median radiation dose by operator. Total number of procedures per operator broken down by ICE/no ICE are: Operator 1: 2/1; Operator 2: 10/9; Operator 3: 10/11; Operator 4: 5/6; Operator 5: 9/9; Operator 6: 6/6.
Of importance, overall radiation exposure was decreased not only from the use of NFI, but also with modification of the fluoroscopy system. Although not studied directly, from the surveys the operators reported ensuring that the frame rate was decreased on all cases (to a maximum of 7.5 frames/s), collimating the images, and closing the shutters as tightly as possible.
Use of intracardiac echocardiography
The amount of time ICE used in a procedure was recorded in 40 of 42 cases. The median time for ICE use was 10 (1–40) min during a procedure. Using the post-procedural survey, ICE was identified as useful in 24 (57%) cases, most commonly for transseptal punctures (n = 14) and visualizing the CS (n = 6). There was also some benefit in visualizing the left AV groove during ablation of left-lateral pathways, although it was noted that the left posteroseptal region was difficult to see. Of the 18 cases in which ICE was deemed unhelpful, there were 4 cases in which the 10F sheath was placed but ICE was not used, 3 due to operator preference, and 1 because the ICE catheter could not be manoeuvred through the left iliac vein. Difficulty in navigating the left iliac vein with the ICE catheter was also noted on several other cases, requiring the use of some fluoroscopy to pass the catheter into the IVC. The majority of cases in which ICE was unhelpful were for AVNRT (n = 10) or right posteroseptal pathways (n = 4), with the ICE images unable to visualize in a better manner Koch's triangle and the posterior septum, respectively. Intracardiac echocardiography was found to be unhelpful in one transseptal puncture due to inadequate visualization of the atrial septum due to its close proximity to the catheter.
Based on the post-procedure surveys, patients randomized to the ICE group but in whom the ultrasound probe was never used were grouped with the no ICE patients and the analysis repeated. With the resulting 38 ICE patients, there remained no significant difference in radiation dose (9 mGy ICE vs. 23 mGy no ICE, P = 0.29) or fluoroscopy time (0.9 min ICE vs. 1.6 min no ICE, P = 0.35) between the two groups.
Discussion
With awareness of radiation risk increasing, numerous reports have been published on the benefits of NFI in catheterization procedures.3–10 This is the first prospective study examining the use of an integrated EAM/ICE platform (Carto3). Overall, there was no significant difference in radiation exposure or fluoroscopy time with or without the use of ICE, although intracardiac echo had a measurable effect of reducing radiation and was generally deemed to be useful in cases requiring transseptal puncture.
Prior studies have shown that the use of EAM and ICE can decrease fluoroscopy time; however, ICE has been noted to be difficult to use in children, lacking any discernible benefit in some cases, while in others having a high utility.2 To optimize ICE with the potential of making it useful for all cases, ultrasound images could be integrated directly into an EAM system using the Carto3 platform. Despite this advancement, our study found that the use of EAM alone was not inferior to the combined use of EAM/ICE.
Intracardiac echocardiography cannot be totally dismissed, however. Its benefit has been shown for transseptal access in adults,11,12 and in our patient population, direct visualization of the atrial septum could be achieved in the majority cases, with the transseptal needle and sheath seen passing through the septum and contrast seen in the left atrium. Using ICE, over 2min of fluoroscopy time can be saved, with a reduction of 50 mGy in radiation exposure. This led to one zero-fluoroscopy case for left-sided pathways, an accomplishment which would be difficult to achieve with EAM alone. Although this study was not powered to assess for adverse events, transseptal puncture with ICE visualization had no increased risk in complications and has been shown in prior studies to be a safer method to obtain left atrial access.13 Zero-fluoroscopy procedures for left-sided pathways were not seen in the study by Miyake et al. Although the integrated EAM/ICE platform may have helped with assisting left-sided access,14 it is also possible that zero fluoroscopy was accomplished due to an operator learning curve and comfort in using NFI tools. There remained a significant amount of variability between operators, however, in the effect EAM and ICE had on radiation reduction. This may improve over time as more EP labs adopt the use of NFI and confidence in these tools continues to grow. In contrast to Miyake et al., none of the operators in this study had median radiation doses >150 mGy, with the overall radiation exposure decreased by 95 mGy and fluoroscopy time by 6 min.
The usefulness of ICE in reducing radiation should be weighed against the cost of this tool, the need for an additional venous entry point, and added time to obtain access for the case. Since a 10F sheath is needed for insertion of the ICE catheter, its utility will be limited in small patients. Some studies have evaluated the use of transoesophageal echocardiography (TEE) in performing EPSs.4 Its lower financial cost and familiarity with cardiologists and anaesthesiologists may make it a valid and more attractive alternative to ICE. The ICE catheter, however, could also be placed via the oesophagus in place of a standard (bulkier) TEE probe to provide anatomical shells of the heart, identify important landmarks and catheter location, and potentially guide transseptal punctures. In our study, there were four cases where the sheath was placed but the ICE was not used for any significant part of the procedure. This put the patient at risk for venous complications for little or no gain. Although not performed in this study, there may be value in adding an ICE catheter on an ‘as needed’ basis, whether for a difficult CS cannulation, transseptal puncture, or other visualization challenge.
Finally, over 50% of our cases were performed using <50 mGy of radiation. Although there has been a dramatic decrease in radiation exposure in EPS with the use of NFI devices, the risk of cancer at even these low doses is still present. Similar radiation doses from CT scans may triple the risk of leukaemia and brain cancer in children.15 Taken in this context, the radiation reduction offered by ICE in specific scenarios may have an important effect in future outcomes. Consistent performance of procedures using <30–60 s of fluoroscopy addresses the goals of ALARA. Although zero-fluoroscopy procedures may not be achievable at this point in every patient, its feasibility is likely to increase as technology advances. Future progress towards these goals may allow cases to be performed in novel, cost-efficient settings, such as a procedure room equipped with a mobile C-arm.
Limitations
Although there were no adverse effects attributed to ICE, our focus was on children over 10 years of age and 35 kg. A 10F sheath required for placement of the ICE catheter may have increased venous complications in younger/smaller patients. Transoesophageal placement of an ICE catheter may be useful in these patients, although further investigation is needed. Finally, our study was not powered to investigate the use of ICE in transseptal needle punctures, although there was a distinct difference in radiation dose noted within our cohort. Ultrasound-guided transseptals have been shown to be safe in the adult population. It should be left to the operator's discretion as to whether the cost of ICE outweighs the benefit of a 50 mGy dose reduction.
Conclusion
In a prospective study, the use of an integrated system combining ICE and ECM for ablation of SVT in paediatric patients resulted in lower fluoroscopy use for transseptal puncture, but did not decrease overall fluoroscopy use, when compared with patients in whom ECM was used alone. Efficacy of ablation therapy and prevalence of adverse events were not different between the two groups. The overall effect of efforts to reduce fluoroscopy use was marked in both groups, with more than half of all patients having fluoroscopy times/radiation doses under 60 s and 50 mGy, and 15% of patients having zero-fluoroscopy procedures.
Conflict of interest: F.C. has received grants from Honoraria, Medtronic, and St Jude Medical. J.K.T is Consultant for Biosense Webster Inc.
References
- 1.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation; Nuclear and Radiation Studies Board DoEaLS, National Research Council of the National Academies. Health risks from exposure to low levels of ionizing radiation: BEIR VII 2. Washington DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 2.Miyake CY, Mah DY, Atallah J, Oikle HP, Melgar ML, Alexander ME, et al. Nonfluoroscopic imaging systems reduce radiation exposure in children undergoing ablation of supraventricular tachycardia. Heart Rhythm. 2011;8:519–25. doi: 10.1016/j.hrthm.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez M, Tercedor L, Almansa I, Ros N, Galdeano RS, Burillo F, et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 2009;6:1714–20. doi: 10.1016/j.hrthm.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Clark J, Bockoven JR, Lane J, Patel CR, Smith G. Use of three-dimensional catheter guidance and trans-esophageal echocardiography to eliminate fluoroscopy in catheter ablation of left-sided accessory pathways. Pacing Clin Electrophysiol. 2008;31:283–9. doi: 10.1111/j.1540-8159.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson JD, Helms A, Mangrum JM, Mahapatra S, Mason P, Bilchick K, et al. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol. 2009;2:611–9. doi: 10.1161/CIRCEP.109.872093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papagiannis J, Avramidis D, Alexopoulos C, Kirvassilis G. Radiofrequency ablation of accessory pathways in children and congenital heart disease patients: impact of a nonfluoroscopic navigation system. Pacing Clin Electrophysiol. 2011;34:1288–396. doi: 10.1111/j.1540-8159.2011.03170.x. [DOI] [PubMed] [Google Scholar]
- 7.Papagiannis J, Tsoutsinos A, Kirvassilis G, Sofianidou I, Koussi T, Laskari C, et al. Nonfluoroscopic catheter navigation for radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing Clin Electrophysiol. 2006;29:971–8. doi: 10.1111/j.1540-8159.2006.00472.x. [DOI] [PubMed] [Google Scholar]
- 8.Papez AL, Al-Ahdab M, Dick M, II, Fischbach PS. Impact of a computer assisted navigation system on radiation exposure during pediatric ablation procedures. J Interv Card Electrophysiol. 2007;19:121–7. doi: 10.1007/s10840-007-9148-3. [DOI] [PubMed] [Google Scholar]
- 9.Smith G, Clark JM. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing Clin Electrophysiol. 2007;30:510–8. doi: 10.1111/j.1540-8159.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- 10.Tuzcu V. A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing Clin Electrophysiol. 2007;30:519–25. doi: 10.1111/j.1540-8159.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- 11.Daoud EG, Kalbfleisch SJ, Hummel JD. Intracardiac echocardiography to guide transseptal left heart catheterization for radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1999;10:358–63. doi: 10.1111/j.1540-8167.1999.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Adler S, Berman A, Duran A, Loar D. Localization of fossa ovalis and Brockenbrough needle prior to left atrial ablation using three-dimensional mapping with EnSite fusion. J Interv Card Electrophysiol. 2011;30:37–44. doi: 10.1007/s10840-010-9525-1. [DOI] [PubMed] [Google Scholar]
- 13.Epstein LM, Smith T, TenHoff H. Nonfluoroscopic transseptal catheterization: safety and efficacy of intracardiac echocardiographic guidance. J Cardiovasc Electrophysiol. 1998;9:625–30. doi: 10.1111/j.1540-8167.1998.tb00945.x. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd EJ, Gall SA, Furniss SS. Interatrial septal puncture without the use of fluoroscopy-reducing ionizing radiation in left atrial ablation procedures. J Interv Card Electrophysiol. 2008;22:183–7. doi: 10.1007/s10840-008-9263-9. [DOI] [PubMed] [Google Scholar]
- 15.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


