Patient retention within the first publicly funded methadone program on the sub-Saharan Africa mainland is comparable to that in North America, Europe, and Asia. Optimized retention strategies include higher doses and strengthened support for clients at risk of attrition.
Keywords: HIV, methadone, sub-Saharan Africa, people who inject drugs, implementation science
Abstract
Background. People who inject drugs (PWID) in Dar es Salaam, Tanzania, have an estimated human immunodeficiency virus (HIV) prevalence of 42%–50% compared with 6.9% among the general population. Extensive evidence supports methadone maintenance to lower morbidity, mortality, and transmission of HIV and other infectious diseases among PWID. In 2011, the Tanzanian government launched the first publicly funded methadone clinic on the mainland of sub-Saharan Africa at Muhimbili National Hospital.
Methods. We conducted a retrospective cohort study of methadone-naive patients enrolling into methadone maintenance treatment. Kaplan-Meier survival curves were constructed to assess retention probability. Proportional hazards regression models were used to evaluate the association of characteristics with attrition from the methadone program.
Results. Overall, 629 PWID enrolled into methadone treatment during the study. At 12 months, the proportion of clients retained in care was 57% (95% confidence interval [CI], 53%–62%). Compared with those receiving a low dose (<40 mg), clients receiving a medium (40–85 mg) (adjusted hazard ratio [aHR], 0.50 [95% CI, .37–.68]) and high (>85 mg) (aHR, 0.41 [95% CI, .29–.59]) dose of methadone had a lower likelihood of attrition, adjusting for other characteristics. Older clients (aHR, 0.53 per 10 years [95% CI, .42–.69]) and female clients (aHR, 0.50 [95% CI, .28–.90]) had a significantly lower likelihood of attrition, whereas clients who reported a history of sexual abuse (aHR, 2.84 [95% CI, 1.24–6.51]) had a significantly higher likelihood of attrition.
Conclusions. Patient retention in methadone maintenance is comparable to estimates from programs in North America, Europe, and Asia. Future implementation strategies should focus on higher doses and flexible dosing strategies to optimize program retention and strengthened efforts for clients at higher risk of attrition.
(See the Editorial Commentary by Guise et al on pages 743–4.)
In the mid-1980s, East Africa became an important stop along international drug trafficking routes [1]. In 2009, 40–45 tons of heroin was trafficked into Africa, mostly through the East African countries of the United Republic of Tanzania, Kenya, Mozambique, Ethiopia, and Somalia. Of the 40–45 tons of heroin trafficked into Africa, an estimated 34 of those tons were consumed in the region. Currently, an estimated 533 000 opiate users live in East Africa [2]. Since the late 1990s, injection drug use, primarily of heroin, has become widespread in Dar es Salaam and is spreading throughout the country, [3, 4] with an estimated 50 000 people who inject drugs (PWID) in Tanzania [5].
Approximately 60% of PWID in Dar es Salaam reported injecting 3 times per day, with 41% reporting needle sharing in the preceding 30 days [6]. Sexual risk behaviors are known to accompany drug use risks. Male PWID reported an average of 2 sexual partners, and female PWID reported an average of 25 sexual partners in the last 30 days, with only 42% reporting condom use [7]. As a result of these risky behaviors in the context of a generalized human immunodeficiency virus (HIV) epidemic in Tanzania, PWID in Dar es Salaam experience a high HIV burden with an estimated HIV prevalence of 42%–50% compared with 6.9% among the general population in the city [6, 8, 9]. A recent survey highlighted the astonishing prevalence of HIV among female injectors at 71% [9].
Extensive evidence supports the use of methadone maintenance treatment (MMT) as a life-saving, long-term, chronic healthcare intervention [10]. MMT has been shown to lower mortality, morbidity, and illegal income-generating activities associated with opioid dependence [11]. Among PWID, MMT can prevent transmission of HIV, hepatitis C virus, and other infectious diseases by reducing injection-related and sexual risk behaviors such as needle sharing, unprotected sex, and multiple sex partners [12, 13].
Research demonstrates that MMT is most effective as a long-term, chronic healthcare intervention [13, 14]. Patient retention in the program is essential to optimize the HIV prevention benefits offered by methadone treatment. In addition, for PWID living with HIV, enrollment and continued engagement in MMT has been shown to further enhance linkage and adherence to antiretroviral therapy [15, 16].
To combat the growing HIV prevalence among PWID in Tanzania, the first publicly funded methadone clinic on the mainland of sub-Saharan Africa was opened at Muhimbili National Hospital in Dar es Salaam in February 2011. We sought to assess the retention of clients in MMT, as well as program and patient characteristics associated with attrition from the program.
METHODS
Study Setting
In February 2011, the Ministry of Health and Social Welfare, Muhimbili University of Health and Allied Sciences, and the Drug Control Commission, with support from Pangaea Global AIDS Foundation (Pangaea) and funding from the US President's Emergency Plan for AIDS Relief via the US Centers for Disease Control and Prevention (CDC), launched the first public MMT clinic on the mainland of sub-Saharan Africa at Muhimbili National Hospital in Dar es Salaam, Tanzania. Enrollment into the MMT program required referral from a community-based organization (CBO). In preparation for MMT initiation, individuals were also required to attend a series of educational sessions on HIV, sexually transmitted infections, medication adherence, and supportive services provided by CBOs. To be eligible for MMT, individuals had to (1) present with opioid dependence, (2) have evidence of recent drug injection, and (3) test positive for opiates through urine screening. Once enrolled in MMT, methadone was provided to clients 7 days a week at the clinic. Clients visited the clinic on a daily basis to receive in-person, directly observed methadone dosing. The pharmacy did not allow clients to take methadone doses away from the clinic for dosing at a later point in time. Integrated services such as HIV counseling and testing, tuberculosis testing, and psychosocial support were also offered to MMT clients. Upon enrollment in MMT, clients were encouraged to obtain rapid HIV screening via provider-initiated testing and counseling. However, clients could opt out of HIV screening. If an initial rapid test was reactive, a second rapid test was used to confirm results. In the event of the first 2 rapid tests yielding discordant results, a third rapid test was used as a tiebreaker. Clients who were identified as HIV positive were referred to the hospital to receive appropriate clinical care. Once a patient was initiated on HIV treatment, he/she would return to the MMT clinic to continue picking up his/her medications. In addition, MMT clients continued to receive support services (eg, skills building, nutrition, support groups) from the CBOs.
Study Population
Study subjects included clients who initiated MMT from February 2011 to January 2013 at Muhimbili National Hospital.
Data Sources
The study utilized the electronic database, typically used for routine clinical and program monitoring, from the MMT clinic at Muhimbili National Hospital. Clients who enrolled into MMT were asked to complete a comprehensive baseline survey to collect demographic, drug history, legal history, mental health, and HIV risk behavior data. In addition, daily methadone dosing data were collected by the pharmacy on each MMT client. In January 2013, the databases were closed and collected for the purposes of this research.
Measures
Exposures
Individual-level characteristics were obtained from the MMT electronic database. Demographic risk factors included age (in years), sex (male/female), education level (primary-level schooling or less/more than primary-level schooling) and marital status (currently married/not married). Sexual risk factors included multiple sex partners in the last 6 months (defined as >1 sex partner) and risky sexual behavior in the last 6 months (defined as no or inconsistent condom use). Injection-related risk factors included flashblood (injecting blood from another drug user who has recently injected heroin), [17] shared needles at last injection, shared other equipment at last injection, cleaned needles with bleach if sharing at the last injection, and polysubstance use (heroin and alcohol, cocaine, benzodiazepine or amphetamine). Mental health domains of interest included high substance dependence [18, 19] (defined as the maximum score of the substance dependence scale) and depression and anxiety from the Hopkins Symptom Checklist-25 [20], which has been previously validated in Tanzania [21, 22]. Violence history included any history of physical abuse and any history of sexual abuse.
Methadone dose, our primary exposure of interest, was obtained from the electronic pharmacy log and was treated as a time-varying exposure over 3-month intervals beginning with when a patient initiated methadone treatment. For each interval, we took the average of the daily methadone doses for each of the clients as the estimates. The 3-month time interval was chosen to allow for sufficient follow-up time for outcomes to occur for a given exposure level. Methadone dose was categorized into low (<40 mg), medium (40–85 mg), and high (>85 mg) categories to allow for testing of a dose-response effect. The methadone dose categories were based on a recent Cochrane review of methadone maintenance dosing [23].
Outcome
The pharmacy log contained information regarding the initiation date of methadone, daily methadone dose, and client outcomes. Outcomes of methadone clients were defined as (1) remaining on treatment: receiving methadone treatment through the end of the study period; (2) involuntary discharge: discharged from the methadone program due to inappropriate behavior; (3) medical withdrawal: client initiated tapering of methadone dose; (4) death: death of the client; and (5) attrition: dropping out of the methadone program. For clients who stopped coming to the clinic for methadone dosing, the clinic would contact the CBO that referred the client to conduct follow-up. Outreach workers ascertained whether the client had died or was choosing to not return to the clinic. Family members verified deaths occurring outside of a medical facility. For deaths occurring within a medical facility, deaths were verified based on information recorded within the client's medical record by the clinician responsible for their care. Attrition from the program was defined as 21 consecutive missed doses, and a client's last pharmacy refill was assigned as the date of attrition. Clients were followed up through 31 January 2013.
Statistical Methods
The χ2 statistic was used to assess for differences between methadone dose groups. Confirmed HIV test results were used to estimate prevalence, expressed as a percentage with corresponding 95% confidence intervals (CIs), among methadone clients. Kaplan-Meier survival curves were constructed to assess the probability of retention over multiple time points during the follow-up period. Cox proportional hazards regression was used to test for associations between exposures and time to attrition. Follow-up time began at initiation of methadone and ended with the date of death, involuntary discharge, medical withdrawal, attrition, or 31 January 2013, whichever came first. Patients who died, were involuntary discharged, or were medically withdrawn were censored at their last pharmacy refill. Patients who remained on methadone were censored on 31 January 2013.
We assessed the univariate relationship of methadone dose, demographics, sexually related and injection-related risk factors, and histories of mental health, abuse, and arrests with attrition. Our primary exposure of interest was methadone dose. Backward stepwise regression with a criterion P value of .2 was used to select the variables for the multivariable Cox proportional hazards regression model to test for the independent relationship between methadone dose and time to attrition. Forward stepwise regression with a criterion P value of .2 was used to confirm variables for inclusion in the final multivariable model. With the stepwise procedures, the final multivariable model included age, sex, history of risky sexual behavior, history of sexual abuse, and methadone dose. In sensitivity analysis, the proportional hazards assumption was tested using the Schoenfeld residuals. Hazard ratios (HRs) and 95% CIs were calculated to compare outcomes between groups. All statistical analyses were conducted using Stata software version 12.1 (StataCorp, College Station, Texas).
The use of data for this study was approved as nonhuman subjects research by the CDC and Institutional Review Board of Ethical and Independent Review Services.
RESULTS
MMT Clients
A total of 629 individuals initiated MMT between February 2011 and January 2013, providing 432 person-years of follow-up. The attrition rate was 58.37 per 100 person-years. At the end of the study period, 365 (58%) of participants were alive and receiving methadone treatment. Of the remaining 264 patients not receiving methadone treatment, 95% was due to attrition, 2% was due to involuntary discharge, and 3% was due to death. At 6, 12, and 24 months, the proportion of patients retained in methadone was 67% (95% CI, 63%–71%), 57% (95% CI, 53%–62%), and 48% (95% CI, 42%–53%), respectively. Among clients who attrited from the program, 51% dropped out within 3 months from methadone initiation, 24% between 3 and 6 months after methadone initiation, and 25% after 6 months of methadone treatment. Among 469 (75%) individuals who received screening for HIV as of January 2013, 185 tested positive for HIV, resulting in an overall estimated prevalence of 39% (95% CI, 35%–44%). Among patients living with HIV, 156 (84%) were male and 29 (16%) were female. Estimated HIV prevalence among male and female clients who received screening was 36% (95% CI, 32%–41%) and 74% (95% CI, 60%–88%), respectively (P < .001).
Characteristics of enrolled patients are outlined in Table 1. The average age at enrollment was 32 years (SD, 6 years) and the majority (93%) of clients were male. Overall, 64% had primary level schooling or less; 13% were married; and 31% had at least 1 child at the time of MMT enrollment. Clients in the higher methadone dose groups tended to be less educated (P < .001), more likely to have risky sex (P = .005) and higher levels of substance dependence (P = .003), and less likely to have been arrested (P = .006).
Table 1.
Characteristics of Patients Initiating Methadone Treatment
| Characteristic | Initial Methadone Dosea |
Total (N = 629) | ||
|---|---|---|---|---|
| Low (< 40 mg) | Medium (40–85 mg) | High (>85 mg) | ||
| Demographics | ||||
| Age, y | ||||
| ≤25 | 7 (3) | 19 (8) | 11 (7) | 37 (6) |
| 26–35 | 115 (51) | 140 (58) | 94 (59) | 349 (56) |
| 36–45 | 94 (41) | 75 (31) | 47 (22) | 216 (34) |
| >45 | 11 (5) | 6 (3) | 8 (5) | 25 (4) |
| Male sex | 213 (94) | 225 (94) | 147 (92) | 585 (93) |
| More than primary-level education | 113 (50) | 57 (26) | 38 (29) | 208 (36) |
| Married | 38 (17) | 24 (10) | 20 (13) | 82 (13) |
| At least 1 child | 74 (33) | 62 (29) | 37 (29) | 173 (31) |
| Sexual risk factors | ||||
| Multiple sex partners in last 6 mo | 41 (18) | 44 (19) | 32 (21) | 117 (19) |
| Risky sexual behavior in last 6 mo | 87 (39) | 122 (52) | 84 (55) | 293 (48) |
| Injection risk factors | ||||
| Ever used flashblood | 17 (8) | 19 (8) | 8 (5) | 44 (7) |
| Shared needles at last injection | 34 (15) | 27 (11) | 20 (13) | 81 (13) |
| Cleaned shared needles with bleach | 15 (44) | 8 (30) | 6 (30) | 29 (36) |
| Shared other equipment at last injection | 29 (13) | 20 (8) | 16 (10) | 65 (11) |
| Polysubstance use | 73 (32) | 79 (37) | 39 (31) | 191 (34) |
| Mental health history | ||||
| High substance dependence | 150 (74) | 167 (87) | 132 (83) | 449 (81) |
| Depression in last 30 d | 57 (25) | 47 (22) | 26 (20) | 130 (23) |
| Anxiety in last 30 d | 55 (24) | 46 (21) | 26 (20) | 127 (22) |
| History of abuse | ||||
| Any history of physical abuse | 24 (11) | 28 (13) | 20 (16) | 72 (13) |
| Any history of sexual abuse | 1 (<1) | 5 (2) | 5 (4) | 11 (2) |
| Criminal history | ||||
| Ever arrested | 138 (61) | 114 (53) | 64 (49) | 316 (55) |
Data are presented as No. (%).
a Average dose of the patient's first 3 months of treatment.
Factors Associated With Attrition
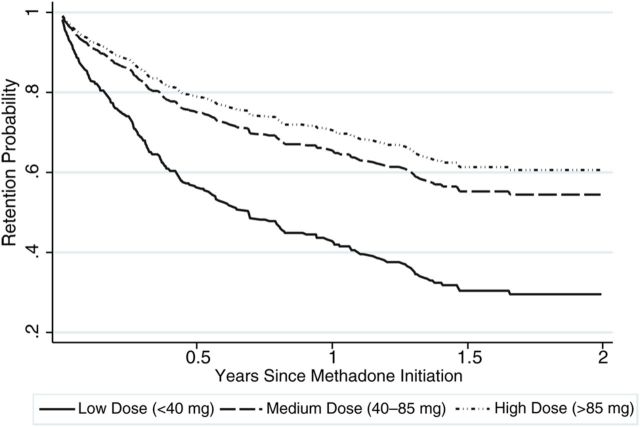
Table 2 shows the HRs for the associations of patient characteristics and attrition from methadone. In unadjusted analyses, patients who were older in age (P < .001) and who received a higher dose of methadone during their first quarter of treatment (P = .002) had a lower likelihood of attrition. In the adjusted analyses, clients receiving a medium (adjusted HR [aHR], 0.50 [95% CI, .37–.68]) or high (aHR, 0.41 [95% CI, .29–.59]) dose of methadone had a lower likelihood of attrition (P < .001), compared with those receiving a lower dose (Figure 1). Compared with those ≤25 years of age, clients aged 26–35 (aHR, 0.35 [95% CI, .23–.52]), 36–45 (aHR, 0.25 [95% CI, .16–.39]), and >45 (aHR, 0.11 [95% CI, .03–.38]) had a lower likelihood of attrition (P < .001). Compared with their male counterparts, female clients had a lower likelihood of attrition (aHR, 0.50 [95% CI, .28–.90]) from treatment (P = .021). In addition, clients with a history of sexual abuse (aHR, 2.84 [95% CI, 1.24–6.51]; P = .014) had a higher likelihood of attrition from methadone, and though not statistically significant, clients who had riskier sexual behavior (aHR, 1.29 [95% CI, .99–1.68]; P = .060) had a higher likelihood of attrition from methadone. Findings from forward stepwise regression yielded identical variables for the final model. In sensitivity analysis, we tested the proportional hazards assumption and found that there was no evidence that the proportional effect varied over time (P = .103).
Table 2.
Unadjusted and Adjusted Hazard Ratios for Attrition (N = 629)
| Characteristic | Unadjusted Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | ||||
| ≤25 | (ref) | <.001* | (ref) | <.001* |
| 26–35 | 0.61 (.38–.98) | 0.35 (.23–.52) | ||
| 36–45 | 0.48 (.29–.80) | 0.25 (.16–.39) | ||
| >45 | 0.20 (.07–.60) | 0.11 (.03–.38) | ||
| Female | 0.83 (.49–1.40) | .489 | 0.50 (.28–.90) | .021 |
| Primary schooling or less | 1.05 (.81–1.36) | .726 | ||
| At least 1 child | 0.90 (.68–1.19) | .464 | ||
| Married | 1.07 (.75–1.53) | .72 | ||
| Sexual risk factors | ||||
| Multiple sex partners in last 6 mo | 0.93 (.67–1.29) | .674 | ||
| Risky sexual behavior in last 6 mo | 1.16 (.90–1.50) | .245 | 1.29 (.99–1.68) | .060 |
| Injection risk factors | ||||
| Ever used flashblood | 1.06 (.68–1.66) | .797 | ||
| Shared needles at last injection | 1.26 (.89–1.78) | .192 | ||
| If shared needles, cleaned needles with bleach | 0.82 (.42–1.63) | .579 | ||
| Shared other equipment at last injection | 1.04 (.69–1.57) | .843 | ||
| Polysubstance use | 1.17 (.90–1.52) | .252 | ||
| Mental health history | ||||
| High substance dependence | 1.04 (.73–1.47) | .841 | ||
| Depression in last 30 d | 0.86 (.63–1.17) | .326 | ||
| Anxiety in last 30 d | 0.90 (.66–1.22) | .488 | ||
| Hopkins depression score | 1.01 (.83–1.22) | .943 | ||
| Hopkins overall score | 0.99 (.80–1.23) | .947 | ||
| History of abuse | ||||
| Any history of physical abuse | 1.06 (.72–1.56) | .759 | ||
| Any history of sexual abuse | 1.84 (.82–4.46) | .14 | 2.84 (1.24–6.51) | .014 |
| Criminal history | ||||
| Ever arrested | 1.10 (.85–1.43) | .449 | ||
| Methadone dose at initiation | ||||
| <40 mg | (ref) | <.001* | (ref) | <.001* |
| 40–85 mg | 0.60 (.45–.81) | 0.50 (.37–.68) | ||
| >85 mg | 0.47 (.34–.66) | 0.41 (.29–.59) | ||
Abbreviations: CI, confidence interval; ref, reference.
* Test for trend from group-linear term.
Figure 1.
Retention curves disaggregated by methadone dose.
DISCUSSION
With data from 629 clients accessing the first publically funded methadone program on the mainland of sub-Saharan Africa, we assessed the retention of patients in methadone treatment and factors associated with attrition from the methadone program. Retention in the program is consistent with retention estimates from other programs throughout Europe [24, 25], Asia [26, 27], and North America [28–30]. Clients who were younger, were male, had a history of risky sexual behavior, and/or had a history of sexual abuse had a higher risk of attrition from the program. In contrast, patients receiving higher doses of methadone had a lower risk of attrition from methadone treatment. These are important findings as our results highlight an implementation strategy that can reduce the likelihood of attrition from the program and a group of patients in need of support to improve their continued engagement in methadone services.
Our experience in Tanzania indicates that delivery of MMT is feasible within an urban setting in sub-Saharan Africa and that favorable programmatic outcomes are achievable. Patient retention in MMT was comparable with that observed in other settings in the world, and given the demand and rapid initiation of clients into MMT, services should be scaled up in Dar es Salaam and throughout Tanzania.
At the patient level, research from other settings has shown that sex, age, injection and drug use history, and history of incarceration are associated with greater probability of dropping out of treatment [31–34]. Results from the multivariable model indicated that clients who were younger were less likely to remain on MMT, similar to programs from North America [30] and Asia [27]. Younger clients may have been more prone to risk taking and less motivated to stay in treatment over the long term. In addition, women were more likely to remain on MMT compared with their male counterparts, and this finding was similar to what has been observed in HIV treatment programs in sub-Saharan Africa, [35, 36] suggesting that females tend to be more adherent to care. However, to our knowledge, this is the first report of a history of sexual abuse being associated with attrition from methadone treatment. These clients may have specific psychological morbidities requiring special attention and highlight the need for additional efforts to understand the needs of improving retention among these clients.
This analysis built on our previous implementation science research with HIV prevention programs for drug users in Tanzania [37, 38]. In this study, the dose of methadone was strongly associated with remaining in treatment, similar to research from methadone programs in North America, Australia, Europe, and Asia [28, 39, 40]. Biologically, a higher dose of methadone is known to more effectively suppress withdrawal symptoms, primarily by assisting the patient to feel physiologically stabilized [41, 42]. Additionally, methadone competitively inhibits illicit opioids from accessing the opioid receptor and may be another factor that improves retention [43].
Methadone dosing has often been approached from an addiction perspective, not from a public health framework. In an area of the world with a generalized HIV epidemic and in the context of our client population with a high prevalence of HIV, retention in treatment is critical to prevent the spread of HIV [10]. Individuals who leave methadone programs abruptly are more likely to relapse to heroin, with 82% of heroin injectors returning to heroin injection within 12 months after leaving MMT [11]. In the context of the HIV epidemic among PWID in Tanzania, a relapse to heroin injection carries a significant risk of HIV acquisition and/or transmission. Additionally, the stability that methadone provides to drug users is critical not only through a reduction in injection drug use but also through an improvement in adherence to HIV treatment for those clients who are eligible [44]. Strategies that support engagement in antiretroviral therapy, and subsequently reduce HIV viremia in patients on methadone, will further reduce the risk of sexual transmission among serodiscordant sexual encounters [45]. Retention in treatment is, therefore, critical in Tanzania's HIV epidemic among heroin injectors, with clear public health considerations. An effective HIV prevention program for PWID is to retain them on treatment for as long as necessary to ensure that the relapse to heroin injection is minimal.
Strengths of the study included the standardization of protocols for care delivery, patient tracing, and data recording for the patients included in the study. Furthermore, the database had high levels of completeness with regard to clinical and pharmaceutical data. The principal limitation of our study was due to the observational nature of the research. Although we adjusted for a number of characteristics to address concerns of confounding, the potential for unmeasured or mismeasured factors to bias our results existed.
Another limitation was the self-report of behaviors for the baseline survey, and as a result, recall and social desirability were potential biases impacting the metrics collected as part of the baseline survey. In particular, specific metrics for sexual risk factors (eg, multiple sex partners), injection risk factors (eg, shared needles or cleaned shared needles with bleach), and history of abuse (eg, physical abuse or sexual abuse) were prone to social desirability bias. Due to the stigmatization of behaviors, these types of biases have been common when studying people who inject drugs [46]. However, the resulting misclassification would be nondifferential with regard to our primary exposure of interest and would bias our results toward the null, on average [47]. Last, we did not have substantial numbers of study participants to adequately assess for interactions between characteristics and attrition.
In conclusion, the government of Tanzania effectively established the first publically funded methadone program on the mainland of sub-Saharan Africa. Retention estimates were similar to what has been reported from other methadone programs in the world; however, opportunities remain regarding the quality and efficiency of care delivery and retention in methadone treatment. Our study found a lower risk of attrition among clients receiving a higher methadone dose. These results confirm what has been shown to be an effective implementation strategy in other settings and highlight the importance of higher doses of methadone to maximize HIV prevention benefits. Future research should focus on the impact of methadone dose on other quality-of-care measures as well as examining the impact of flexible dosing strategies on patient retention.
Notes
Acknowledgments. We thank the patients, providers, and program managers of the methadone clinic at Muhimbili National Hospital.
Author contributions. B. H. L., F. M., O. C., P. K., J. M., N. S., A. M., and R. D. B. assisted in drafting the manuscript. F. M., P. K., J. M., N. S., and A. M. assisted with the programmatic description. B. H. L. and O. C. developed the study design and conducted the data analysis. B. H. L. and R. D. B. interpreted the findings in light of other research. All authors have seen and approved the final version.
Financial support. This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the CDC under the terms of 5U2GPS000951.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.Ross MW, McCurdy SA, Kilonzo GP, Williams ML, Leshabari MT. Drug use careers and blood-borne pathogen risk behavior in male and female Tanzanian heroin injectors. Am J Trop Med Hyg. 2008;79:338–43. [PubMed] [Google Scholar]
- 2.United Nations on Drugs and Crime. The global Afghan opium trade: a threat assessment. Available at: http://www.unodc.org/documents/data-and-analysis/Studies/Global_Afghan_Opium_Trade_2011-web.pdf . Accessed 1 March 2013.
- 3.McCurdy SA, Ross MW, Kilonzo GP, Leshabari MT, Williams ML. HIV/AIDS and injection drug use in the neighborhoods of Dar es Salaam, Tanzania. Drug Alcohol Depend. 2006;82(suppl 1):S23–27. doi: 10.1016/s0376-8716(06)80004-9. [DOI] [PubMed] [Google Scholar]
- 4.McCurdy SA, Williams ML, Kilonzo GP, Ross MW, Leshabari MT. Heroin and HIV risk in Dar es Salaam, Tanzania: youth hangouts, mageto and injecting practices. AIDS Care. 2005;17(suppl 1):S65–76. doi: 10.1080/09540120500120930. [DOI] [PubMed] [Google Scholar]
- 5.Tanzania Drug Control Commission. The national strategic framework for HIV/AIDS prevention for injecting drug users (2011–2015) 2010. pp. 1–41. Dar es Salaam, Tanzania October.
- 6.Williams ML, McCurdy SA, Bowen AM, et al. HIV seroprevalence in a sample of Tanzanian intravenous drug users. AIDS Educ Prev. 2009;21:474–83. doi: 10.1521/aeap.2009.21.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams ML, McCurdy SA, Atkinson JS, Kilonzo GP, Leshabari MT, Ross MW. Differences in HIV risk behaviors by gender in a sample of Tanzanian injection drug users. AIDS Behav. 2007;11:137–44. doi: 10.1007/s10461-006-9102-x. [DOI] [PubMed] [Google Scholar]
- 8.Tanzania Commission for AIDS, Zanzibar AIDS Commission, National Bureau of Statistics, Office of Chief Government Statistician Zanzibar, ICF International. Third Tanzania HIV/AIDS and malaria indicator survey 2011–2012. 2013. Available at: http://www.tacaids.go.tz/index.php?option=com_content&view=article&id=138:about-hiv-and-aids&catid=31:hiv-and-aids-information-&Itemid=158 . Accessed 12 August 2013.
- 9.Nyandindi C, Mbwambo J, McCurdy S, Lambdin B, Copenhaver M, Bruce R. Prevalence of HIV, hepatitis C and depression among people who inject drugs in the Kinondoni Municipality in Dar es Salaam, Tanzania. College on Problems of Drug Dependence,; San Diego, CA. 2013. [Google Scholar]
- 10.Bruce RD. Methadone as HIV prevention: high volume methadone sites to decrease HIV incidence rates in resource limited settings. Int J Drug Policy. 2010;21:122–4. doi: 10.1016/j.drugpo.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ball JC, Ross A, Dole VP. The effectiveness of methadone maintenance treatment: patients, programs, services and outcome. Vol xiv. New York: Springer-Verlag; 1991. [Google Scholar]
- 12.Metzger DS, Woody GE, McLellan AT, et al. Human immunodeficiency virus seroconversion among intravenous drug users in-and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6:1049–56. [PubMed] [Google Scholar]
- 13.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171. doi: 10.3310/hta11090. iii–iv. [DOI] [PubMed] [Google Scholar]
- 14.Gibson DR, Flynn NM, McCarthy JJ. Effectiveness of methadone treatment in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS. 1999;13:1807–18. doi: 10.1097/00002030-199910010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Uhlmann S, Milloy MJ, Kerr T, et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105:907–13. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood E, Hogg RS, Kerr T, Palepu A, Zhang R, Montaner JS. Impact of accessing methadone on the time to initiating HIV treatment among antiretroviral-naive HIV-infected injection drug users. AIDS. 2005;19:837–9. doi: 10.1097/01.aids.0000168982.20456.eb. [DOI] [PubMed] [Google Scholar]
- 17.McCurdy SA, Ross MW, Williams ML, Kilonzo GP, Leshabari MT. Flashblood: blood sharing among female injecting drug users in Tanzania. Addiction. 2010;105:1062–70. doi: 10.1111/j.1360-0443.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: APA; 2000. Text revision (DSM-IV-TR). Available at: http://buprenorphine.samhsa.gov/Bup_Guidelines.pdf . Accessed 15 August 2013. [Google Scholar]
- 19.Substance Abuse and Mental Health Services Administration (SAMHSA) Treatment improvement protocol (TIP) series, No. 40. Rockville, MD: SAMHA; 2004. Available at: http://www.ncbi.nlm.nih.gov/books/NBK64247/ . Accessed 15 August 2013. [Google Scholar]
- 20.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins symptom checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatr. 1974;7:79–110. doi: 10.1159/000395070. [DOI] [PubMed] [Google Scholar]
- 21.Lee B, Kaaya SF, Mbwambo JK, Smith-Fawzi MC, Leshabari MT. Detecting depressive disorder with the Hopkins Symptom Checklist-25 in Tanzania. Int J Soc Psychiatry. 2008;54:7–20. doi: 10.1177/0020764006074995. [DOI] [PubMed] [Google Scholar]
- 22.Kaaya SF, Fawzi MCS, Mbwambo JK, Lee B, Msamanga GI, Fawzi W. Validity of the Hopkins Symptom Checklist-25 amongst HIV-positive pregnant women in Tanzania. Acta Psychiatrica Scandinavica. 2002;106:9–19. doi: 10.1034/j.1600-0447.2002.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2 doi: 10.1002/14651858.CD002207.pub4. CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gossop M, Marsden J, Stewart D, Treacy S. Outcomes after methadone maintenance and methadone reduction treatments: two-year follow-up results from the National Treatment Outcome Research Study. Drug Alcohol Depend. 2001;62:255–64. doi: 10.1016/s0376-8716(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 25.Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641–53. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- 26.Liu E, Liang T, Shen L, et al. Correlates of methadone client retention: a prospective cohort study in Guizhou province, China. Int J Drug Policy. 2009;20:304–8. doi: 10.1016/j.drugpo.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarasvita R, Tonkin A, Utomo B, Ali R. Predictive factors for treatment retention in methadone programs in Indonesia. J Subst Abuse Treat. 2012;42:239–46. doi: 10.1016/j.jsat.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JF, Warren LD. Client retention in the British Columbia methadone program, 1996–1999. Can J Public Health. 2004;95:104–9. doi: 10.1007/BF03405776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth RE, Corsi KF, Mikulich-Gilbertson SK. Factors associated with methadone maintenance treatment retention among street-recruited injection drug users. Drug Alcohol Depend. 2004;74:177–85. doi: 10.1016/j.drugalcdep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93:51–60. doi: 10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- 31.Beynon CM, McMinn AM, Marr AJ. Factors predicting drop out from, and retention in, specialist drug treatment services: a case control study in the north west of England. BMC Public Health. 2008;8:1–11. doi: 10.1186/1471-2458-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Encrenaz G, Rondeau V, Messiah A, Auriacombe M. Examining the influence of drop-outs in a follow-up of maintained opiate users. Drug Alcohol Depend. 2005;79:303–10. doi: 10.1016/j.drugalcdep.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Gryczynski J, Kinlock TW, Kelly SM, O'Grady KE, Gordon MS, Schwartz RP. Opioid agonist maintenance for probationers: patient-level predictors of treatment retention, drug use, and crime. Subst Abus. 2012;33:30–9. doi: 10.1080/08897077.2011.616816. [DOI] [PubMed] [Google Scholar]
- 34.Craig RJ, Rogalski C, Veltri D. Predicting treatment dropouts from a drug abuse rehabilitation program. Int J Addict. 1982;17:641–53. doi: 10.3109/10826088209053008. [DOI] [PubMed] [Google Scholar]
- 35.Toure S, Kouadio B, Seyler C, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wools-Kaloustian K, Kimaiyo S, Diero L, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS. 2006;20:41–8. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 37.Lambdin B, Bruce D, Chang O, Masao F, Nyandindi C, Ivo Y. Program and patient characteristics associated with people who inject drugs defaulting from medication assisted treatment in Dar es Salaam, Tanzania. International AIDS Society,; Kuala Lumpur, Malaysia. 2013. [Google Scholar]
- 38.Lambdin BH, Bruce RD, Chang O, et al. Identifying programmatic gaps: inequities in harm reduction service utilization among male and female drug users in Dar es Salaam, Tanzania. PLoS One. 2013;8:e67062 doi: 10.1371/journal.pone.0067062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao YP, Liu ZM, Epstein DH, Du C, Shi J, Lu L. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35:28–33. doi: 10.1080/00952990802342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang PW, Wu HC, Yen CN, et al. Change in quality of life and its predictors in heroin users receiving methadone maintenance treatment in Taiwan: an 18-month follow-up study. Am J Drug Alcohol Abuse. 2012;38:213–9. doi: 10.3109/00952990.2011.649222. [DOI] [PubMed] [Google Scholar]
- 41.Donny EC, Walsh SL, Bigelow GE, Eissenberg T, Stitzer ML. High-dose methadone produces superior opioid blockade and comparable withdrawal suppression to lower doses in opioid-dependent humans. Psychopharmacology (Berl) 2002;161:202–12. doi: 10.1007/s00213-002-1027-0. [DOI] [PubMed] [Google Scholar]
- 42.Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction. 2005;100:1496–509. doi: 10.1111/j.1360-0443.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- 43.Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: a randomized trial. JAMA. 1999;281:1000–5. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- 44.Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 2011;113:192–9. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perlis TE, Des Jarlais DC, Friedman SR, Arasteh K, Turner CF. Audio-computerized self-interviewing versus face-to-face interviewing for research data collection at drug abuse treatment programs. Addiction. 2004;99:1–7. doi: 10.1111/j.1360-0443.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- 47.Koepsell TD, Weiss NS. Epidemiologic methods: studying the occurrence of illness. New York: Oxford University Press; 2003. [Google Scholar]