This study compared human immunodeficiency virus (HIV) provider and hepatologist awareness of and adherence to the American Association for the Study of Liver Diseases guidelines for chronic hepatitis B virus management, specifically hepatocellular carcinoma (HCC) screening. HIV providers ordered significantly fewer HCC screenings than hepatologists.
Keywords: hepatitis B virus, HIV/HBV coinfection, management guidelines, hepatocellular carcinoma
Abstract
Background. In the era of combination therapy for human immunodeficiency virus (HIV), liver disease, and hepatocellular carcinoma (HCC) are major causes of death for patients coinfected with HIV and hepatitis B virus (HBV). This study compared HIV provider and hepatologist awareness of and adherence to the American Association for the Study of Liver Diseases (AASLD) practice guidelines for chronic HBV management. The primary endpoint of HIV provider adherence to HCC screening recommendations was compared to that of hepatologists at a large metropolitan academic medical center.
Methods. Medical record database searches by ICD-9 codes were used to identify HIV/HBV coinfected (n = 144) and HBV monoinfected (n = 225) patients who were seen at least twice over a 2-year period in outpatient clinics. Adherence to AASLD guidelines was assessed by chart review. Provider awareness was evaluated through a voluntary anonymous survey with knowledge-based questions.
Results. Over a 2-year period, only 36.0% of HIV/HBV coinfected patients seen in HIV practices completed HCC screening compared to 81.8% of HBV monoinfected patients in hepatology practices (P < .00001). Similarly, HIV providers less frequently monitored HBV viral load (P < .0001), HBeAg/anti-HBe (P < .00001), HBsAg/anti-HBs (P < .00001) than hepatologists but screened more often for hepatitis A immunity (P = .028). Self-reported adherence and knowledge scores were similar among 19 HIV providers and 16 hepatologists.
Conclusions. HIV providers ordered significantly fewer HCC screening and HBV monitoring tests than hepatologists within a single academic medical center. In the setting of increased reliance on quality indicators for care, both patients and providers will benefit from greater adherence to established guidelines.
An estimated 350 million people are chronically infected with hepatitis B virus (HBV) worldwide [1]. Approximately 80%–90% of patients infected with human immunodeficiency virus (HIV) have evidence of past or active infection with HBV [2, 3], and 5%–10% are chronically infected [4–7]. HIV/HBV coinfected patients have increased rates of HBV replication, higher rates of progression to cirrhosis, and higher rates of hepatitis B e antigen (HBeAg) positivity [8–10] than HBV monoinfected patients.
As HIV patients live longer in the era of highly active antiretroviral therapy (HAART), liver cirrhosis and hepatocellular carcinoma (HCC) are now major causes of death [11]. HIV/HBV coinfected patients are 8 times more likely to die from liver disease than those with HIV monoinfection, and almost 19 times more likely to die than those with HBV monoinfection [9]. The risk of HCC is seven times higher in HIV/HBV coinfected patients than in those with HBV monoinfection [11].
The American Association for the Study of Liver Diseases (AASLD) releases practice guidelines to assist providers in the care of patients with chronic HBV (CHB) [1]. These guidelines outline the proposed frequency of monitoring HBV viral load, liver enzyme tests, hepatitis B surface antigen (HBsAg) and antibody (anti-HBs), HBeAg and antibody (anti-HBe), and screening for HCC. The guidelines also recommend vaccination against hepatitis A virus (HAV) in patients not already immune. The AASLD guidelines for CHB management and for management of hepatocellular carcinoma both recommend HCC screening every 6–12 months for those at high risk: Asian males over 40 years or females over 50, Africans (with mention of North-American blacks as a potential risk group) over 20, patients with cirrhosis or family history of HCC, and patients with persistently high levels of HBV DNA, that is, over 2000 IU/mL (10 000 copies/mL) and age over 40 [1, 12].
Provider knowledge of and adherence to these guidelines is quite variable. Only 43% of primary care doctors in San Francisco reported familiarity with HBV management guidelines [13]. Forty-five percent of CHB patients seen by a diverse group of providers in a large Boston academic medical center did not have appropriate HCC screening, and 29% did not have appropriate lab testing per guidelines [14].
HIV providers generally manage both HIV and HBV in their coinfected patients. Although medical therapy has been simplified through the use of medications active against both HIV and HBV, several features of CHB management, as recommended by AASLD practice guidelines, are complex and provider adherence to these recommendations is inconsistent. Although 99% of HIV/HBV coinfected patients had HIV viral load checked prior to starting HAART, only 16% of patients followed by HIV providers in a large referral center in Dallas were assessed for HBV viral load and serology [15]. Furthermore, only 17% of these coinfected patients underwent imaging surveillance for HCC within 6 months of HIV/HBV coinfection diagnosis [15].
Although HIV coinfection is not an AASLD defined risk factor for HCC, data support its association with increased risk of HCC. This study sought to characterize HIV provider practices for HIV/HBV coinfection care, and HIV/HBV coinfected patient risk factors for HCC. The study tested the hypothesis that HIV providers have less awareness of and adherence to AASLD CHB guidelines, specifically in regards to HCC screening, than hepatologists at a large metropolitan academic medical center.
METHODS
Study Population
Subjects with HIV/HBV coinfection were identified retrospectively through Mount Sinai Data Warehouse medical record database searches for ICD 9 code 070.3 for HBV infection and ICD 9 codes 042 or V08 for HIV infection. For study inclusion, all patients had CHB defined as positive HBsAg for at least 6 months or a positive HBV viral load, were 18 years of age or older and had at least 2 outpatient visits at a Mount Sinai hepatology or HIV practice site during the time period of 1 January 2011 to 31 December 2012. The database search generated 217 records of patients seen in HIV practice sites and billed for both HIV and HBV care. Patients were excluded if they did not have evidence of CHB (71 excluded), were not seen at least twice over the designated period (31 excluded), or were deceased at the time of data abstraction (1 excluded). The final HIV/HBV coinfection cohort included 114 subjects.
As a comparison group, patients with CHB (defined as above) who were receiving care in a Mount Sinai hepatology practice were identified using the inclusion criteria described above yielding 895 records. Patients were excluded if they did not have evidence of CHB (131 excluded), were not seen at least twice over the designated period (113 excluded), were deceased at the time of data abstraction (22 excluded), or were only seen by hepatobiliary surgeons in the hepatology practice (86 excluded), yielding a group of 543 patients. SAS software was used to randomly select 2 patients from this HBV group for each HIV/HBV coinfected subject matched on sex, age above/ equal or below 40 years, and platelet value above/ equal or below 140 000 cells/µL. These criteria were selected to provide similar risk groups for liver disease and HCC with platelet count used as surrogate for advanced liver fibrosis/portal hypertension, and age cutoff of 40 as discriminator because this is considered the young age limit for HCC risk [1, 12]. After completion of data collection, 3 patients were excluded from the HBV monoinfection group due to HIV coinfection yielding a final HBV cohort of 225 subjects.
HIV/HBV coinfected subjects were seen in HIV practices for both HIV and HBV care; some were also seen by a referral-based HIV/hepatitis coinfection provider within their primary HIV practice. Subjects with HBV monoinfection were seen in hepatology practices.
Data Collection
Data collection was completed by a single author (B. H.) into a coded database with each subject only identified by study number. The following information was collected: age at first visit with CHB monitoring during study period, sex, ethnicity, clinic site, family history of HCC, HIV status, hepatitis C (HCV) status, hepatitis A virus (HAV) immune status, medications used to treat CHB and/or HIV, 2 separate visits with each visit's associated HBV viral load, platelet count, HBeAg and anti-HBe, HBsAg and anti-HBs, and if HCC screening was completed during the 2-year period including imaging modality and if HCC was found. All imaging studies were interpreted by radiologists. In addition, CD4 and HIV viral load values were recorded in HIV/HBV coinfected subjects. This study was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai and was conducted in compliance with the Helsinki accord.
Study Endpoints
The primary endpoint was adherence to AASLD CHB management guidelines for HCC screening with imaging studies. Secondary endpoints included adherence to AASLD CHB management guidelines for monitoring of HBV viral loads, measurement of HBeAg and anti-HBe (or HBsAg in HBeAg negative patients), and HAV immune status. Adherence to these guidelines by HIV/hepatitis coinfection providers was also evaluated. Additionally, awareness of the existing CHB management guidelines, comparison of HIV and HBV viral loads with rates of HCC screening, and incidence of HCC occurrence were evaluated.
Statistical Analysis
A multivariable logistic model was used to investigate whether HIV or hepatology practice affected the HCC screening practices. Predictors that were considered included HIV/HBV or HBV infection status, age, sex, race (white, Asian, black, Hispanic, and other), HIV status, family history of HCC, HBV viral load, and HBe antigen status. First, the relationship between each individual predictor and the outcome was analyzed using t-test or Wilcoxon Sum Rank test for continuous predictors and χ2 or Fisher exact test for categorical predictors. All predictors significant at 0.2 level were included in the first model. Then, the least significant predictors were individually excluded, rerunning the model after each exclusion. Only covariates with P values less than .05 remained in the final model.
All analyses were conducted using R base version 3.1.0 (http://www.R-project.org).
Provider Survey
A voluntary, web-based survey was sent to 56 of our institution's HIV providers and hepatologists in November 2013 to assess their awareness of AASLD CHB management guidelines and collect demographic data. Most survey recipients were care providers during the chart review period of 2011–2012. Responses were kept anonymous. CHB knowledge was assessed by multiple choice questions based on AASLD guidelines with composite score derived from average percentage of correct answers. The survey was administered through the online SurveyMonkey (SurveyMonkey Inc, Palo Alto, California) tool configured to not capture IP addresses or names. A copy of the survey is provided in the Supplementary appendix.
RESULTS
Patient Characteristics
Table 1 includes the demographic and clinical characteristics of the subjects. There was a significant difference in ethnicity as the majority of patients seen in HIV clinics were black/African-American (53.5%), and the majority of patients seen in liver clinics were Asian (55.1%, P < .0001). Subjects seen in hepatology practices had significantly higher rates of both family and personal history of HCC compared with patients seen in HIV practices. Medications used to treat HBV significantly differed between groups with tenofovir-emtricitabine fixed dose combination and lamivudine more commonly used for HIV/HBV coinfected patients, and tenofovir, entecavir, and adefovir used more commonly in hepatology practices. There was no significant difference between FIB-4 scores suggestive of cirrhosis (FIB-4 > 3.25).
Table 1.
Subject Demographics, Clinical Characteristics and Prevalence of Hepatitis B Virus Testing
| HIV/HBV | HBV | P Value | |
|---|---|---|---|
| n = 114 (%) | n = 225 (%) | ||
| Age, mean (SD) | 45.5 (9.0) | 49.8 (12.6) | .001 |
| Female Sex | 20 (17.5) | 40 (17.8) | 1.000 |
| Ethnicity | <.00001 | ||
| Asian | 8 (7.0) | 124 (55.1) | |
| Black/African-American | 61 (53.5) | 20 (8.9) | |
| Hispanic/Latino | 23 (20.2) | 20 (8.9) | |
| Other | 6 (5.3) | 32 (14.2) | |
| White/Caucasian | 16 (14.0) | 29 (12.9) | |
| HCV coinfection | 16 (14.0) | 12 (5.3) | .129 |
| Family history of HCC | 0 (0.0) | 24 (10.7) | <.00001 |
| Tenofovir | 17 (14.9) | 102 (45.3) | <.00001 |
| Truvada | 77 (67.5) | 12 (5.3) | <.00001 |
| Lamivudine | 24 (21.1) | 11 (4.9) | <.00001 |
| Entecavir | 9 (7.9) | 75 (33.3) | <.00001 |
| Adefovir | 1 (0.9) | 21 (9.3) | .006 |
| FIB-4 Score, mean (SD) | 2.1 (1.7) | 2.9 (3.7) | .052 |
| FIB-4 Score, median | 1.7 [1.1–2.4] | 1.6 [1.0–2.8] | .643 |
| FIB-4 >3.25 | 15 (13.2) | 44 (19.6) | .188 |
| Undetectable HBV viral load, visit 1 | 34 (41.9)a | 82 (36.4) | .456 |
| Undetectable HBV viral load, visit 2 | 31 (38.3)a | 102 (45.3) | .333 |
| HBeAg Positivity, visit 1 | 19 (16.7) | 33 (14.7) | <.001 |
| HBeAg Positivity, visit 2 | 11 (9.6) | 28 (12.4) | .141 |
| Positive HCC | 2 (1.8) | 36 (16.0) | .037 |
| HCC Imaging Modality | .004 | ||
| CT scan | 16 (14.0) | 70 (31.1) | |
| MRI | 1 (0.9) | 44 (19.6) | |
| Ultrasound | 24 (21.1) | 69 (30.7) |
Abbreviations: CT, computed tomography; FIB-4, FIB-4 index score to approximate fibrosis of the liver, with score >3.25 suggestive of cirrhosis; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C Virus; HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; SD, standard deviation.
a Sample size for HIV/HBV is 81 (33 subjects did not have HBV viral load checked).
Primary Endpoint
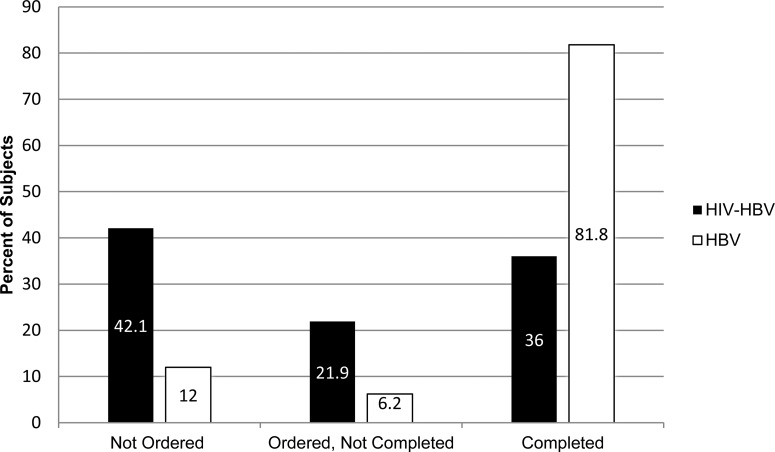
HIV providers screened less frequently for HCC (P < .00001) than hepatologists (Figure 1). Over a 2-year period, only 36.0% of patients seen in HIV practices completed HCC screening compared to 81.8% seen in hepatology practices. In those screened, 1.8% of HIV/HBV patients had evidence of HCC compared to 16% of those with HBV monoinfection.
Figure 1.
Comparing rates of HCC screening test ordering and completion over 2 years in HIV/HBV coinfected and HBV monoinfected patients (P < .00001). Abbreviations: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus.
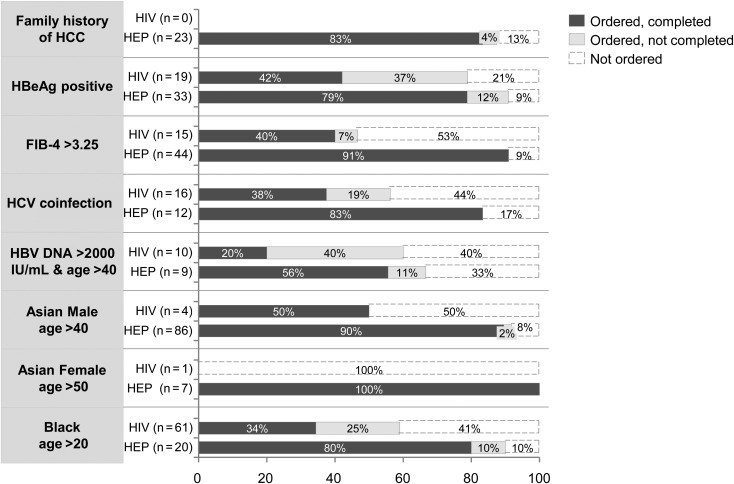
As HIV coinfection has not been identified as an independent risk factor for HCC, all subjects were examined by the same AASLD delineated high-risk factors for HCC (Table 2). There were significant differences in ethnicity and family history of HCC, but rates of other risk factors were not significantly different between groups. Based on ethnicity alone, many patients in both groups met AASLD criteria for routine HCC screening. In the HIV practices, there was no significant association between HCC screening and black or Asian ethnicity. Rates of HCC screening test order and completion were further evaluated by established HCC risk factors (Figure 2). Fewer than 50% of HIV/HBV subjects in HIV practices completed HCC screening despite having characteristics that placed them at high risk for HCC.
Table 2.
Percentage of Subjects With American Association for the Study of Liver Diseases High-Risk Factors for Hepatocellular Carcinoma
| HIV/HBV | HBV | P Value | |
|---|---|---|---|
| n = 114 (%) | n = 225 (%) | ||
| Family history of HCC | 0 | 24 (10.7) | .0003 |
| HBeAg Positive | 19 (16.7) | 33 (14.7) | .63 |
| Cirrhosis by FIB-4>3.25 | 15 (13.2) | 44 (19.6) | .14 |
| HCV coinfection | 16 (14.0) | 12 (5.3) | .129 |
| HBV DNA > 2000 IU/mL and age >40a | 10 (8.8) | 9 (4.0) | .071 |
| Asian Male age >40 | 4 (3.5) | 86 (38.2) | <.0001 |
| Asian Female age >50 | 1 (0.9) | 7 (3.1) | .37 |
| Black age >20 | 61 (53.5) | 20 (8.9) | <.0001 |
Abbreviations: FIB-4, FIB-4 index score to approximate fibrosis of the liver, with score >3.25 suggestive of cirrhosis; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
a HBV DNA > 2000 IU/mL and age >40 = patients above age 40 who have persistently elevated hepatitis B DNA viral load levels above 2000 IU/mL
Figure 2.
Rates of HCC screening delineated by risk factors. Abbreviations: FIB-4>3.25, FIB-4 index score to approximate fibrosis of the liver, with score >3.25 suggestive of cirrhosis; HBV DNA >2000 IU/mL and age >40, patients above age 40 who have persistently elevated hepatitis B DNA viral load levels above 2000 IU/mL; HBeAg, hepatitis B e antigen; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HEP, hepatitis; HIV, human immunodeficiency virus.
In multivariable analysis, practice location (HIV vs hepatology) and subject age were significantly associated with rates of HCC screening. Subjects in the HBV cohort (seen in hepatology practices) had 5.5 (95% CI, [2.7, 11.5], P value <.001) times higher odds for HCC screening to be ordered than HIV/HBV coinfected subjects (seen in HIV practices), adjusted for age. Furthermore, for every 1 year increase in age, the odds of HCC screening were 0.97 (95% CI, [.95, .99], P value = .05) times lower. Differences in ethnicity were not statistically significant when adjusted for group. There was no significant association between HIV or HBV viral load, and the frequency of HCC screening test completion or order.
Secondary Endpoints
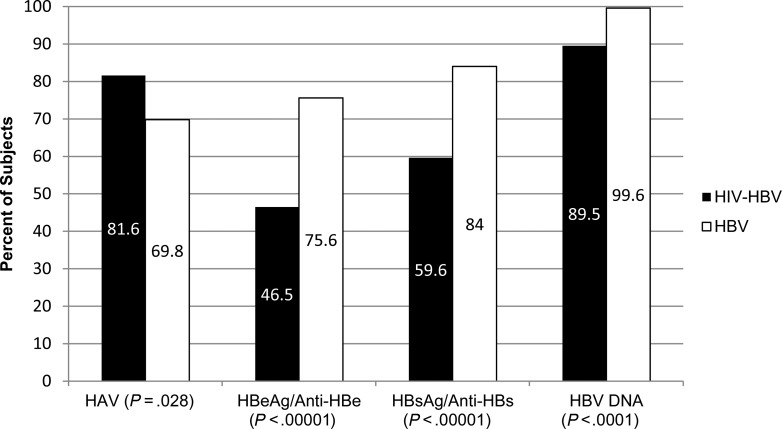
HIV providers screened more often for HAV immunity (P = .028) but less frequently monitored HBV viral load (P < .0001), HBeAg and anti-HBe (P < .00001), HBsAg and anti-HBs (P < .00001) compared with hepatologists (Figure 3).
Figure 3.
Comparing rates of HBV and HAV laboratory monitoring over 2 years in HIV/HBV coinfected and HBV monoinfected patients. Abbreviations: HAV, hepatitis A virus; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus.
The utilization of an HIV/hepatitis coinfection provider within HIV practices was examined for significant differences in screening and monitoring. Patients who were seen by an HIV/hepatitis coinfection provider in addition to their primary HIV provider had significantly higher rates of HAV screening (P = .039), HBeAg and anti-HBe monitoring (P = .015); how-ever, there was only a positive trend (P = .084) toward higher rates of HCC screening.
HIV clinic subjects who had poorly controlled HIV with detectable HIV viral loads also had detectable HBV viral loads (P < .0001). Rates of HAV screening (P < .00001), HBeAg and anti-HBe monitoring (P < .00001), and HBsAg and anti-HBs monitoring (P < .00001) were significantly higher in HIV clinic patients with undetectable HIV viral loads.
Survey Results
Of the 56 providers who received the electronic survey, 35 (63%) responded with 19/34 (56%) of HIV providers and 16/22 (68%) of hepatologists. Average scores on the knowledge-based questions were similar between HIV providers (60%) and hepatologists (58%). The Supplementary Appendix contains a list of average correct scores per question by group. Hepatologists reported feeling more comfortable caring for HBV patients than HIV providers, with 20% of HIV providers reporting at least some discomfort compared to 13% of hepatologists. Conversely, HIV providers felt more comfortable caring for HIV/HBV coinfected patients than hepatologists with 35% of hepatologists reporting at least some discomfort compared to 11% of HIV providers. Provider reported nonadherence to AASLD guidelines for CHB was similar across groups with 5%–6% of both groups reporting hardly ever or never following guidelines. The majority of hepatologists (73%) reported following AASLD guidelines most of the time, and 47% of HIV providers reported following AASLD guidelines sometimes.
DISCUSSION
HIV providers placed significantly fewer HCC screening test orders than hepatologists. Our study showed suboptimal adherence in HIV practices with only 36% of HIV/HBV coinfected subjects completing screening over a 2-year period. Our data are consistent with the results reported by Jain et al who found that only 36% of their HIV/HBV coinfected patients completed at least one HCC screening imaging test from 1999 to 2003 in their HIV clinics [15]. Given the 8-fold increased risk of death related to liver disease in HIV/HBV coinfection [9] and a 7-fold increased risk for HCC [11] compared to HBV monoinfection, this study and published findings strongly indicate that improving HCC screening practices should be a priority for HIV clinicians caring for HIV/HBV coinfected patients.
Reasons for poor adherence to AASLD HCC screening guidelines in HIV practices are likely multifactorial. We found evidence that providers failed to order tests, and that patients did not complete all tests that were ordered. Of interest, we found higher rates of HBV clinical laboratory-based monitoring in patients with well controlled HIV suggesting that HIV providers may be focusing on HIV care (rather than HBV care) in patients with poorly controlled HIV infection. However, there was no significant association between rates of HCC screening and HIV viral load indicating that imaging tests were not consistently ordered even in patients with well-controlled HIV. There was also a higher rate of HCC test order without completion in HIV practices which may indicate a less adherent patient population or greater barriers to obtaining care. HIV providers may have had a gap in knowledge regarding a coinfected patient's risk factors for HCC given the low rates of personal and family history of HCC in our HIV/HBV coinfection cohort, but our study shows this population has other risk factors for HCC that warrant routine screening. Furthermore, although results from our survey demonstrated that HIV providers had similar knowledge scores compared to hepatologists, there may be a discrepancy between knowledge and how this translates to care delivered in clinical practice. It is possible that HIV providers acquired additional HBV care knowledge between January 2011 and November 2013; however, there was no formal training. Finally, the current AASLD CHB management guidelines do not directly address care for HIV/HBV coinfected patients, and this should be characterized in the next guideline update.
As many HIV providers care for coinfected patients, it is important for HIV providers to follow nationally established guidelines to provide effective CHB care, particularly for HCC screening. Educational sessions for HIV providers focused on hepatitis care and clinical reminders may be beneficial for increasing rates of compliance. Prepopulated electronic medical record order sets and progress note templates for CHB care including lab tests and HCC screening could be considered to enhance provider adherence. Adjunct use of referral based HIV/hepatitis coinfection providers at HIV practices may also improve adherence to AASLD recommended management; however, our study shows that utilization of this specialty service was quite limited, and their performance lagged behind that of hepatologists.
Our study of CHB management practices was limited by its retrospective nature, relatively small sample size, and single institution focus. It is possible that the rate of family history of HCC in both patient groups is incorrectly represented due to incomplete medical record charting. Serologic tests may have been ordered but not performed; such performance failures were not recorded during data collection.
In conclusion, HIV providers ordered significantly fewer HCC screening tests than hepatologists. Educational interventions focused on CHB care for HIV providers and clinical tools such as electronic medical record prompted screening reminders, order sets, and progress note templates may all improve adherence, but further studies are needed. In the setting of increased reliance on quality indicators for care, both patients and their providers will benefit from greater adherence to established guidelines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors would like to thank Demetre Daskalakis, MD, MPH, and Sanders Chang, MS, for their contributions to this work.
Financial support. The work was supported in part from DK090317, DA031095, and CA152514 (to A. D. B.).
Potential conflicts of interest. D. T. D. receives financial compensation as a consultant, paid lecturer, and for his service on scientific advisory boards of companies which either develop or assess medicines used for the treatment of viral hepatitis. These companies include Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Vertex Pharmaceuticals, Merck & Co., Inc., and Hoffman La Roche, Inc. (the parent company of Genentech). He also receives compensation from Tibotec, Novartis, Achillion, Idenix, Pfizer, and Kadmon. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–2. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Mendez ML, Gonzalez-Quintela A, Aguilera A, Barrio E. Prevalence, patterns, and course of past hepatitis B virus infection in intravenous drug users with HIV-1 infection. Am J Gastroenterol 2000; 95:1316–22. [DOI] [PubMed] [Google Scholar]
- 3.Scharschmidt BF, Held MJ, Hollander HH et al. . Hepatitis B in patients with HIV infection: relationship to AIDS and patient survival. Ann Intern Med 1992; 117:837–8. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald D-C. Hepatocellular carcinoma, human immunodeficiency virus and viral hepatitis in the HAART era. World J Gastroenterol 2008; 14:1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann CJ, Seaberg EC, Young S et al. . Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS 2009; 23:1881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaglio PJ, Sterling R, Daniels E, Tedaldi E, Terry Beirn Community Programs for Clinical Research on AHWG. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007; 45:618–23. [DOI] [PubMed] [Google Scholar]
- 7.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis 2003; 188:571–7. [DOI] [PubMed] [Google Scholar]
- 8.Chun HM, Mesner O, Thio CL et al. . HIV outcomes in Hepatitis B virus coinfected individuals on HAART. J Acquir Immune Defic Syndr 2014; 66:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thio CL, Seaberg EC, Skolasky R Jr et al. . HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–6. [DOI] [PubMed] [Google Scholar]
- 10.Thio CL, Smeaton L, Saulynas M et al. . Characterization of HIV-HBV coinfection in a multinational HIV-infected cohort. AIDS 2013; 27:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berretta M, Garlassi E, Cacopardo B et al. . Hepatocellular carcinoma in HIV-infected patients: check early, treat hard. Oncologist 2011; 16:1258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burman BE, Mukhtar NA, Toy BC et al. . Hepatitis B management in vulnerable populations: gaps in disease monitoring and opportunities for improved care. Dig Dis Sci 2014; 59:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Johnson KB, Roccaro G et al. . Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol 2014; 109:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain MK, Opio CK, Osuagwu CC, Pillai R, Keiser P, Lee WM. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007; 44:996–1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.