Abstract
It has been well established that many species of Gram-negative bacteria release nanoscale outer membrane vesicles (OMVs) during normal growth. Furthermore, the roles of these structures in heterotrophic bacteria have been extensively characterized. However, little is known about the existence or function of OMVs in photoautotrophs. In the present study, we report for the first time the production of OMVs by the model photosynthetic organism Synechocystis sp. PCC 6803, a species of biotechnological importance. We detected extracellular proteins and lipids in cell-free supernatants derived from Synechocystis culture, yet the cytoplasmic and thylakoid membrane markers NADH oxidase and chlorophyll were absent. This indicated that the extracellular proteins and lipids derived from the outer membrane, and not from cell lysis. Furthermore, we identified spherical structures within the expected size range of OMVs in Synechocystis culture using scanning electron microscopy. Taken together, these results suggest that the repertoire of Gram-negative bacteria that are known to produce OMVs may be expanded to include Synechocystis PCC6803. Because of the considerable genetic characterization of Synechocystis in particular, our discovery has the potential to support novel biotechnological applications as well.
Keywords: cyanobacteria, photosynthesis, outer membrane vesicles
Secreted vesicles (outer membrane vesicles), which are known to exist in many heterotrophic bacteria, were detected in the photosynthetic bacterium Synechocystis for the first time.
Graphical Abstract Figure.
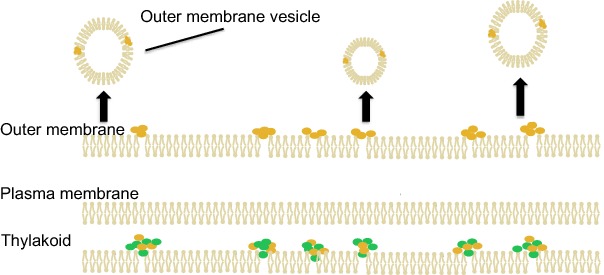
Secreted vesicles (outer membrane vesicles), which are known to exist in many heterotrophic bacteria, were detected in the photosynthetic bacterium Synechocystis for the first time.
INTRODUCTION
Outer membrane vesicle (OMV) production is ubiquitous among Gram-negative bacteria. OMVs are 20–300 nm spherical structures released from the outer membrane during normal growth and in response to foreign or self-produced small molecules (Kadurugamuwa and Beveridge 1995; Kobayashi et al. 2000; Mashburn and Whiteley 2005). Little is known about how OMVs are formed, but their similarity to the outer membrane in lipid and protein composition and relative lack of inner membrane or cytoplasmic components confirm their point of origin (Horstman and Kuehn 2000; Kato, Kowashi and Demuth 2002; Tashiro et al. 2011). Despite a lack of understanding about how they are produced, OMV functions have been extensively studied in heterotrophs. Important roles in bacterial threat avoidance, interspecies competition, virulence, horizontal gene transfer, cell–cell communication and biofilm formation have been well documented (Kulp and Kuehn 2010; Manning and Kuehn 2013; Schertzer and Whiteley 2013; Vella and Schertzer 2015). Biller et al. (2014) recently reported production of OMVs by a photoautotroph, describing their role in the cyanobacteria Prochlorococcus and Synechococcus. We were interested to know whether the model photosynthetic organism Synechocystis sp. PCC 6803 (referred to as Synechocystis in this paper) naturally produces OMVs. Synechocystis is naturally competent and its genome has been extensively studied, making it a species of biotechnological importance (Kaneko et al. 1996, 2003; Zang et al. 2007). In the work described here, we use microscopic and biochemical analyses to detect the production by Synechosystis of OMV-sized vesicles under standard growth conditions. Further, we demonstrate that the composition of these vesicles is consistent with an outer membrane origin, specifically excluding the presence of cytoplasmic and thylakoid membrane components. We therefore conclude that this model photosynthetic organism produces OMVs in a manner similar to other Gram-negative species, opening the possibilities for biotechnological applications in a well-studied and genetically tractable photoautotroph.
METHODS
OMV extraction
OMVs were extracted from stationary phase Synechocystis cultures (4–6 weeks of growth) using a method adapted from Schertzer, Brown and Whiteley (2010). Cells were pelleted at 6500 × g and the supernatant was filtered through a 0.2-μm syringe filter. The filtrate was concentrated 100 times by ultracentrifugation at ∼267 000 × g for 1 h (150 000 × g for SEM) in an Optima TLX (Beckman Coulter) tabletop ultracentrifuge and suspended in membrane vesicle (MV) buffer (50 mM Tris pH 7.4, 5 mM NaCl, 1 mM MgSO4). This cell-free culture extract is referred to as OMV extract throughout the remainder of this paper. For phospholipid detection assays, 100 kDa MW cutoff centrifugal protein concentrators (Pall Corporation, Port Washington, NY) were used to pre-concentrate the cell-free supernatant prior to ultracentrifugation.
Biomolecule detection
For general protein detection, 5 μL of sample was combined with 195 μL of Bradford reagent and were then read at 595 nm in a 96-well plate using a Biotek Synergy 2 (Winooski, VT) plate reader. A NanoDrop 2000 (Thermo Scientific, Waltham, MA) spectrophotometer was used to measure the absorbance of 2 μL samples from 250 to 700 nm in steps of 1 nm to detect photosynthetic pigments. NADH oxidase activity was measured using a protocol modified from the one described by Osborn et al. (1972). Samples were suspended in 80 μL MV buffer, and a plate reader (Biotek Synergy 2) was used to record the absorbance of the samples at 340 nm every 30 s for a period of 30 min. The presence of lipids in the OMV extract was detected as described by Schertzer, Brown and Whiteley (2010). All biomolecule detection assays were performed a minimum of three times.
Scanning electron microscopy
Samples were fixed in 2% glutaraldehyde, pushed onto a polycarbonate 0.1 μm membrane filter and then fixed in 2% glutaraldehyde, 3% paraformaldehyde and phosphate-buffered saline. The samples were dehydrated using an ethanol gradient (50, 75, 95, 100 and 100%), transferred to hexamethyl disilizane (HMDS) and dried overnight (1:2 HMDS: Ethanol, 2:1 HMDS: Ethanol, 100% HMDS). The filters were viewed with a Zeiss Supra 55 Scanning Electron Microscope (Oberkochen, Germany) at 5 keV. Scanning electron microscopy (SEM) images were analyzed using Image J (Schneider, Rasband and Eliceiri 2012).
RESULTS AND DISCUSSION
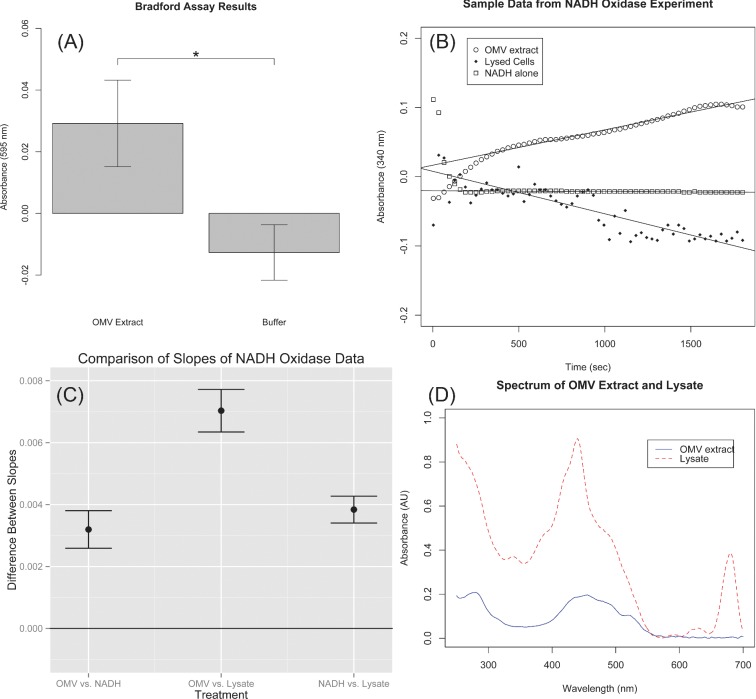
OMVs are formed from the outer membrane and are therefore expected to contain outer membrane proteins. Consequently, one convenient method for detecting OMVs is to detect the presence of protein in cell-free culture extract. Synechocystis cells were removed from stationary phase culture by centrifugation and filtering (see the section ‘Methods’) and the resulting culture extract was concentrated approximately 100 times by ultracentrifugation in MV buffer. A Bradford assay was performed to detect protein present in OMV extract. As shown in Fig. 1A, the absorbance at 595 nm (A595) of OMV extract was found to be 2.92 × 10−2 ± 1.40 × 10−2 while the A595 of buffer was found to be 1.27 × 10−2 ± 8.99 × 10−3. A two-sample t-test was used to compare the A595 of the buffer to the OMV extract, and a P value of 2.9 × 10−5 was obtained indicating that a significant quantity of protein was present in the OMV extract. However, Bradford assay does not discriminate between types of protein. Additional assays were performed to detect specific proteins and thereby establish that the protein in the OMV extract did not derive from lysed cells.
Figure 1.
Results from assays analyzing the composition of acellular extract (OMV extract) derived from Synechocystis cultures. (A) A Bradford assay demonstrated that a significant quantity of extracellular protein was present in Synechocystis cultures. (B) In order to demonstrate that the cytoplasmic membrane enzyme NADH oxidase is not present in OMV extract, the NADH oxidase activity in three treatment groups was compared by measuring the decrease in NADH concentration over time. (C) Confidence intervals for the differences between slopes of the three treatments demonstrate that OMV extract has significantly lower NADH oxidase activity than cell lysate. (D) The absorbance spectrum of OMV extract is indicative of the presence of carotenoids, but not chlorophyll, supporting the conclusion that the OMV extract is derived exclusively from the outer membrane of Synechocystis.
To confirm that the proteins present in the OMV extract were different from those in cell lysate, samples of lysate and OMV extract were run on a 12% SDS-PAGE (Fig. 2). The clear visual difference between the protein profiles in these two samples illustrates on a qualitative level that OMV extract is not equivalent to lysate.
Figure 2.
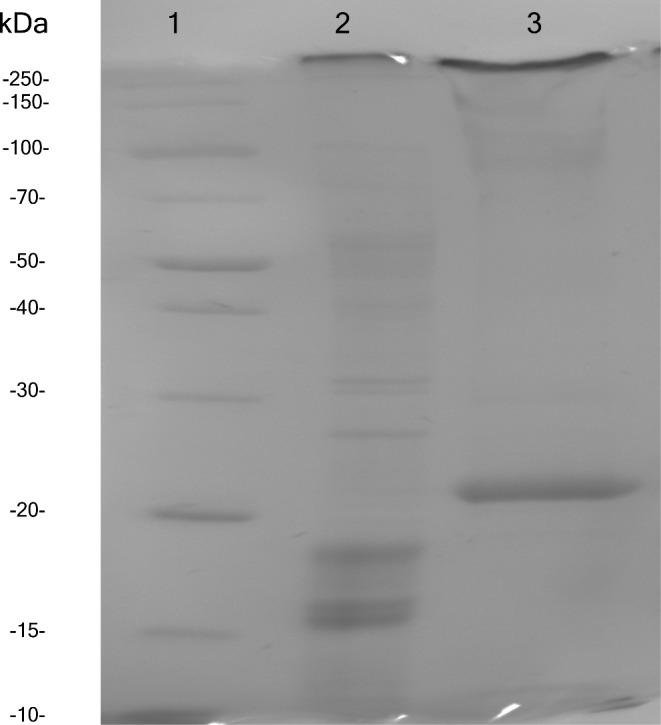
SDS-PAGE comparing protein composition of OMV extract to that of cell lysate. Lane 1 contains a protein ladder, Lane 2 contains cell lysate and Lane 3 contains OMV extract.
We supported these qualitative results with quantitative assays for detecting non-outer membrane proteins. It has been demonstrated that the enzyme NADH oxidase is localized to the cytoplasmic membrane of the Gram-negative bacterium Salmonella typhimurium (Osborn et al. 1972). In previous studies of OMVs, the presence of NADH oxidase has been used as a marker for the presence of cytoplasmic membrane components (Horstman and Kuehn 2000; Renelli et al. 2004). We hypothesized that the absence of NADH oxidase in the OMV extract would support the conclusion that the OMV extract derived from the outer membrane rather than cell lysate.
The NADH oxidase activity of the OMV extract was compared with that of cells that had been intentionally lysed through sonication. Reduction in NADH concentration over time was measured by recording the loss of absorbance at 340 nm over time for three treatment groups: OMV extract, lysed cells and NADH in buffer without the presence of enzyme. The slope of the three curves was compared between treatment groups.
The level of the response variable was found to be different for the different treatments. The data were pre-processed by subtracting the intercepts of each replicate of each treatment from corresponding response data. In this way, the data were normalized and the slopes could be compared without regard for the level of the response. A dummy variable linear regression (Draper and Smith 1998) (see Supplementary Methods) was performed to allow the three slopes to be calculated in a single model and compared at the P = 0.05 significance level. Figure 1B presents a sample of the normalized data obtained from the NADH oxidase assay. In order to avoid overcrowding the plot, only one replicate of each treatment is shown.
The slopes for each treatment (coefficients for the dummy variable interacting with time terms) along with P values indicating that each coefficient is significantly different from zero are presented in Table 1. The slope of the cell lysate is clearly negative, indicative of active NADH oxidase oxidizing NADH to NAD+. While the slope of the NADH without extract is negative as well, it is nearly an order of magnitude closer to zero, suggesting that in the absence of NADH oxidase the NADH was stable over time. The slope of the OMV extract was consistently found to be positive. We hypothesized that this slight increase in absorbance over time was due to settling of vesicles in the OMV extract and that there was no NADH oxidase activity in the OMV extract. Since absorbance was measured through the top and bottom of each well, as cellular material settled, the sample appeared to become more concentrated.
Table 1.
Slopes for each treatment in the NADH oxidase assay and associated P values calculated via dummy variable regression.
| Treatment | Slope (s-1) | P value |
|---|---|---|
| OMV extract | 4.208 × 10-5 | <2e-16 |
| Lysate | −7.511 × 10-5 | <2e-16 |
| NADH | −1.116 × 10-5 | 3.86e-10 |
In order to compare the three treatment groups, 95% confidence intervals were calculated for the differences between pairs of slopes for each treatment. If the confidence interval contained zero, it could not be concluded that a significant difference existed between the slopes. Figure 1C shows the confidence intervals for the differences between slopes. As is evident in the figure, none of the confidence intervals overlapped with the zero line indicating that all treatment groups were significantly different from each other, i.e. the NADH activity in lysate was significantly greater than the activity in OMV extract.
Unlike the majority of other organisms in which OMVs have been studied, Synechocystis contains a separate thylakoid membrane in addition to the inner and outer membranes (Liberton et al. 2006). Therefore, in order to be fully confident that proteins detected in OMV extract were derived from the outer membrane and not the thylakoid membranes, OMV extract was assayed to detect the presence of photosynthetic pigments. Figure 1D displays the absorbance spectrum of cell lysate overlaid on the spectrum of the OMV extract. As with Fig. 1B, in order to avoid overcrowding the plot, only one replicate measurement of each spectrum is shown. The lysate spectrum contains a peak at 680 nm that is clearly missing from the OMV extract. This peak is due to the presence of chlorophyll in the lysate.
The broad absorbance peak in the range 400–500 nm of the OMV extract is likely due to the presence of various carotenoids. Although functional photosystems containing chlorophyll are only expected to be present in the thylakoid membranes, the related cyanobacterium Synechocystis sp. PCC 6714 has been shown to contain carotenoids in its outer membrane (Jurgens and Weckesser 1985). Jürgens and Weckesser (1985) compared the absorbance spectrum of isolated outer membrane of Synechocystis sp. PCC 6714 with that of plasma and thylakoid membrane. The spectrum we obtained of the OMV extract in Fig. 1D closely matches with the spectrum of the outer membrane obtained by Jürgens and Weckesser, while our spectrum of the cell lysate closely matches their spectrum of the plasma and thylakoid membrane. Thus, the absorbance spectrum in Fig. 1D not only provides evidence that thylakoid membrane is absent from the OMV extract, but also provides direct evidence that the OMV extract is derived from the outer membrane.
A phospholipid assay was used to detect the presence of lipid membranes in the OMV extract. The mean absorbance of the buffer samples was found to be 7.97 × 10−3 with a standard deviation of 3.06 × 10−4. However, the mean absorbance of the OMV extract was found to be 9.48 × 10−2 with a standard deviation of 6.02 × 10−2. We hypothesized that the increased variation in the OMV extract sample was due to variation in the percentage of the total lipid that was dissolved in chloroform during the initial stage of the assay.
Because of the large discrepancy in standard deviation between the two treatments and the relatively small sample size (three replicates of each treatment), a t-test was not the most appropriate method by which the data could be analyzed. Therefore, a permutation test (Good 1994) (see Supplementary Methods) was applied to determine if the absorbance of the OMV extract at 470 nm was significantly greater than the absorbance of the buffer. The 95th percentile of the null distribution was found to be 7.08 × 10−2 while the true difference in mean absorbance was found to be 8.68 × 10−2. This result indicates that there is less than a 5% probability of obtaining the observed difference in mean absorbance by random chance.
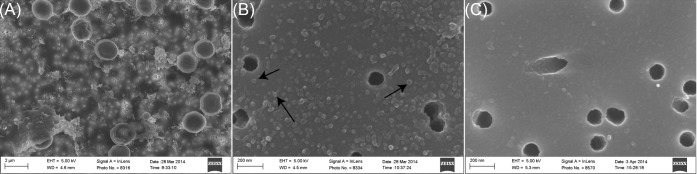
The most direct way to confirm that vesicle structures were present in the OMV extract was microscopic visualization. SEM was used to observe samples of Synechocystis cells and OMV extract bound to polycarbonate membrane filters. The cell samples were primarily used as a means to confirm that the SEM preparation procedure was successful. The filters were evenly coated with spherical structures 30.0 ± 2.59 nm in diameter. It should be noted that since the OMV extract was passed through a 0.2-μm filter, large OMVs, if present, would have been excluded. In order to preclude the possibility that these structures arose as a result of the preparation procedure, a control filter was prepared in which buffer was substituted for OMV extract. Figure 3 presents SEM images of Synechocystis cells (A), OMV extract (B) and the buffer control (C). The OMV extract sample has a much higher concentration of the spherical structures indicating that they originated from the OMV extract and not the preparation procedure. The shape, size and consistency with which the spherical particles were observed lead us to identify the particles as MVs.
Figure 3.
SEM was used to demonstrate that particles within the expected size range of OMVs (20–500 nm) are present in Synechocystis cultures. (A) SEM of Synechocystis cells. (B) SEM of acellular culture extract showing the presence of particles ∼30 nm in diameter. (C) SEM of buffer confirming that the particles in (B) originate from Synechocystis culture and are not a byproduct of the SEM preparation procedure.
High magnification SEM images of Synechocystis cells showed spherical particles on the membrane surface (see Fig. S1, Supporting Information). The similarity of these particles with those observed in OMV extract samples suggested that the particles might be OMVs in the process of budding from the outer membrane.
The results of the Bradford and phospholipid assays collectively demonstrate that the principle components of cellular membranes, i.e. proteins and lipids, are present in OMV extract. Extracellular membrane components are most likely to have derived either from vesicles released from the cell surface or from lysed cells. While vesicles released from the outer membrane predominantly contain outer membrane and periplasmic components, cell lysate is non-specific in its composition, containing components from all cell membranes (three in the case of Synechocystis) and the cytoplasm. We have demonstrated that key biomolecules (NADH oxidase and photosynthetic pigments) that are present in Synechocystis cell lysate are absent from the OMV extract indicating that the membrane components did not derive from cell lysis. Furthermore, the spectrum of the OMV extract corresponds closely with that of the outer membrane of a related species of Synechocystis supporting the conclusion that the proteins and lipids in the OMV extract originated from OMVs. Combined with the SEM data, we are confident in concluding that Synechocystis naturally releases OMVs.
OMVs in other bacterial species have been utilized as delivery vehicles for both endogenous antigens and non-endogenous proteins (Holst et al. 2005; Kim et al. 2008). Proteins, lipopolysaccharides and lipoproteins contained in OMVs elicit immune response in mammals making OMVs convenient vehicles to deliver these endogenous antigens as vaccines (Holst et al. 2005). Researchers have demonstrated that OMVs can be used as delivery vehicles for non-endogenous proteins as well by targeting those proteins to the outer membrane. For example, Kim et al. (2008) found that by creating chimeras of ClyA and proteins such as GFP and β-lactamase, the chimera would be localized to OMVs. Given our detection of OMVs in Synechocystis, we propose a novel application for OMVs as extraction vehicles for photosynthetic reaction centers, potentially representing a significant technological advancement in photovoltaic cell production.
Previous research has demonstrated that extracted photosystems may be manipulated to develop photosynthetic photovoltaic cells capable of generating hydrogen and electricity in vitro (Greenbaum 1982; Lee, Lee and Greenbaum 1997; Faulkner et al. 2008). However, these approaches involve the extraction of functional photosystems from thylakoid membranes. Thus, the size of the culture from which the photosystems are extracted directly limits the amount of photovoltaic cells that can be constructed. By using OMVs as vehicles to export genetically modified reaction centers from living cyanobacteria, photosystems could potentially be harvested continuously and in much larger quantities at reduced expense.
We hypothesize that photosystems may be targeted to the outer membrane and subsequently released in OMVs through a chimeric association with an outer membrane protein or the addition of an N-terminal targeting sequence. However, as noted above, we are aware of only one published account of the production of OMVs by cyanobacteria. In addition, that research focused on the ecological role of OMVs, and did not address biotechnological applications of OMVs. A need therefore exists for studies such as that presented here of OMVs in photosynthetic organisms, particularly species that may be manipulated through modern molecular genetic methods. Synechocystis is a naturally competent species and has had its entire genome and four of its plasmids sequenced by Kaneko et al. (1996, 2003) and Zang et al. (2007). Synechocystis is ideally suited to the extensive genetic manipulations needed to localize functional photosystems to OMVs. By demonstrating that OMVs are naturally produced by Synechocystis, the present work potentially represents a significant advancement in photosynthetic solar technology.
Supplementary Material
Acknowledgments
We gratefully acknowledge the aid and guidance of Dr. Scott Pardo with statistical analyses. We also thank the Analytical Diagnostics Laboratory and Small Scale Systems Integration and Packaging Center at Binghamton University for providing access to and training on their scanning electron microscope.
FUNDING
This work was supported by the National Institutes of Health (1R15ES022828) and the Michael Connolly Endowment Fund.
SUPPLEMENTARY DATA
Conflict of interest. The authors certify that they have no conflict of interest in the subject matter or materials discussed in this manuscript.
REFERENCES
- Biller SJ, Schubotz F, Roggensack SE, et al. Bacterial vesicles in marine ecosystems. Science. 2014;343:183–6. doi: 10.1126/science.1243457. [DOI] [PubMed] [Google Scholar]
- Draper NRS, Smith H. Applied Regression Analysis. New York: John Wiley & Sons; 1998. [Google Scholar]
- Faulkner CJ, Lees S, Ciesielski PN, et al. Rapid assembly of photosystem I monolayers on gold electrodes. Langmuir. 2008;24:8409–12. doi: 10.1021/la800670b. [DOI] [PubMed] [Google Scholar]
- Good P. Permutation Tests (A Practical Guide to Resampling Methods for Testing Hypotheses) New York: Springer; 1994. [Google Scholar]
- Greenbaum E. Photosynthetic hydrogen and oxygen production: kinetic studies. Science. 1982;215:291–3. doi: 10.1126/science.215.4530.291. [DOI] [PubMed] [Google Scholar]
- Holst J, Feiring B, Naess LM, et al. The concept of ‘tailor-made’, protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine. 2005;23:2202–5. doi: 10.1016/j.vaccine.2005.01.058. [DOI] [PubMed] [Google Scholar]
- Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem. 2000;275:12489–96. doi: 10.1074/jbc.275.17.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens UJ, Weckesser J. Carotenoid-containing outer membrane of Synechocystis sp. strain PCC6714. J Bacteriol. 1985;164:384–9. doi: 10.1128/jb.164.1.384-389.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Nakamura Y, Sasamoto S, et al. Structural analysis of four large plasmids harboring in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. DNA Res. 2003;10:221–8. doi: 10.1093/dnares/10.5.221. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Sato S, Kotani H, et al. Sequence analysis of the genome of the unicellular Cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–36. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32:1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- Kim JY, Doody AM, Chen DJ, et al. Engineered bacterial outer membrane vesicles with enhanced functionality. J Mol Biol. 2008;380:51–66. doi: 10.1016/j.jmb.2008.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Uematsu K, Hirayama H, et al. Novel toluene elimination system in a toluene-tolerant microorganism. J Bacteriol. 2000;182:6451–5. doi: 10.1128/jb.182.22.6451-6455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IL, Lee JW, Greenbaum E. Biomolecular electronics: vectorial arrays of photosynthetic reaction centers. Phys Rev Lett. 1997;79:3294–7. [Google Scholar]
- Liberton M, Howard Berg R, Heuser J, et al. Ultrastructure of the membrane systems in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Protoplasma. 2006;227:129–38. doi: 10.1007/s00709-006-0145-7. [DOI] [PubMed] [Google Scholar]
- Manning AJ, Kuehn MJ. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J Mol Microb Biotech. 2013;23:131–41. doi: 10.1159/000346548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–5. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- Osborn MJ, Gardener JE, Parisi E, et al. Mechanism of assembly of the outer membrane of Salmonella typhimurium: isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972;247:3962–72. [PubMed] [Google Scholar]
- Renelli M, Matias V, Lo RY, et al. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology. 2004;150:2161–9. doi: 10.1099/mic.0.26841-0. [DOI] [PubMed] [Google Scholar]
- Schertzer JW, Brown SA, Whiteley M. Oxygen levels rapidly modulate Pseudomonas aeruginosa social behaviours via substrate limitation of PqsH. Mol Microbiol. 2010;77:1527–38. doi: 10.1111/j.1365-2958.2010.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertzer JW, Whiteley M. Bacterial outer membrane vesicles in trafficking, communication and the host-pathogen interaction. J Mol Microb Biotech. 2013;23:118–30. doi: 10.1159/000346770. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro Y, Inagaki A, Shimizu M, et al. Characterization of phospholipids in membrane vesicles derived from Pseudomonas aeruginosa. Biosci Biotech Bioch. 2011;75:605–7. doi: 10.1271/bbb.100754. [DOI] [PubMed] [Google Scholar]
- Vella BD, Schertzer JW. Understanding and exploiting bacterial outer membrane vesicles. In: Ramos JL, Goldberg JB, Filloux A, editors. Pseudomonas VII: New Aspects of Pseudomonas Biology. New York: Springer; 2015. pp. 217–50. [Google Scholar]
- Zang X, Liu B, Liu S, et al. Optimum conditions for transformation of Synechocystis sp. PCC 6803. J Microbiol. 2007;45:241–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.