Abstract
As the population of the world is rapidly ageing, the amount of surgery being performed in older patients is also increasing. Special attention is required for the anaesthetic and perioperative management of these patients. The clinical and non-clinical issues specific to older surgical patients are reviewed, with a special emphasis on areas of debate related to anaesthesia care in this group. These issues include the role of frailty and disability in preoperative assessment, choice of anaesthesia technique for hip fracture, postoperative delirium, and approaches to shared decision-making before surgical procedures.
Keywords: ageing, anaesthesia, frail elderly, geriatric anaesthesia, hip fracture
Editor's key points.
Measures of frailty can predict a range of adverse outcomes after surgery and might ultimately help guide care to minimize complications and accelerate recovery.
Substantial uncertainty remains regarding the advantages and disadvantages of neuraxial anaesthesia compared with general anaesthesia as the primary anaesthetic for hip fracture surgery in the elderly.
The aetiology and long-term outcomes of postoperative delirium remain to be described in high-quality studies.
Shared decision-making can make explicit the critical decisions required in the perioperative management of elderly patients with significant comorbidities.
The average age of the population of the world, particularly in Western countries, is increasing. According to the US Census, the population age 65 yr and older is expected to more than double between 2014 and 2060, increasing from 47.8 million (14.8% of the total population) to 98.1 million (23.6%). Those 85 and older are projected to more than triple from 6.3 million (2.0%) to 19.7 million (4.7%).1 The US National Hospital Discharge Survey showed that in 2010, patients age 65 yr and older constituted 33% of hospital discharges and 44% of days of inpatient care.2 Moreover, the amount of surgery performed in older patients is increasing at a rate greater than the aging of the population.3,4 As such, the care of older surgical patients is of increasing importance.
In the context of anaesthesia and perioperative care, the older adult population has been the focus of intense debate over the past decade with regard to optimal approaches to care, both from the perspective of clinical outcomes and regarding appropriate utilization of health-care resources.5 We review current work on the following four areas of active debate in the research literature related to geriatric anaesthesia: (i) the role of frailty in preoperative assessment; (ii) approaches to anaesthesia for hip fracture surgery; (iii) the effects of anaesthesia on the ageing brain; and (iv) shared decision-making in the perioperative setting (Table 1).
Table 1.
Guidelines and practice suggestions pertinent to the perioperative care of older adults
Preoperative assessment of older adults
|
Frailty
|
Management of hip fractures
|
Postoperative delirium
|
| Decision-making |
Frailty and disability in preoperative assessment for older adults
Concepts of preoperative risk assessment for older surgical patients have changed markedly over time.6 Before the late 1970s, assessments typically focused on general concepts of risk related to the overall health of the patient and physicians' judgements regarding survival prognosis. Beginning with the publication of Goldman's landmark Cardiac Risk Index in 1977, preoperative risk assessment took on a more quantitative and organ-specific focus, with subsequent proliferation of risk scoring systems for cardiac,7 pulmonary,8 renal,9 and neurological10 events. Alongside these risk-stratification systems, expert guidelines on risk assessment in the perioperative setting have largely focused on characterizing and mitigating the risk of specific organ-based complications, such as perioperative myocardial infarction.11
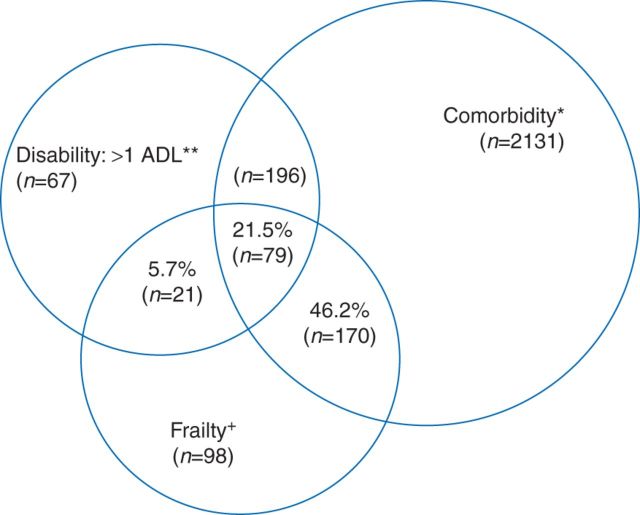
A growing integration of concepts drawn from geriatrics and gerontology into surgical and anaesthetic practice has led to a recognition of the role of progressive, systemic geriatric syndromes, such as frailty and baseline disability, in providing prognostic insights for older surgical patients not captured by organ-based risk scoring systems.12 In this context, ‘frailty’—defined as a syndrome of progressive multisystem decline leading to decreased physiological reserve and poor ability to respond to physiological stressors—has emerged as a central concept in research on surgical outcomes for older patients. In a general sense, frailty exists as a concept separate from both co-morbidity and disability and does not represent a consequence of normal or healthy ageing (Figure 1).13–15 In non-surgical populations, frailty is predictive of poor health outcomes, including falls, reduced mobility, hospitalizations, institutionalized discharge, and mortality.16,17
Fig 1.
Prevalences—and overlaps—of co-morbidity, disability, and frailty among community-dwelling men and women aged 65 yr and older participating in the Cardiovascular Health Study. Percentages listed indicate the proportion among those who were frail (n=368), who had co-morbidity, disability, or both, or neither. Total represented: 2762 participants who had co-morbidity, disability, frailty, or a combination of these. +n=368 frail participants overall. *n=2576 overall with two or more of the following nine conditions: myocardial infarction, angina, congestive heart failure, claudication, arthritis, cancer, diabetes, hypertension, and chronic obstructive pulmonary disease. Of these, 249 (total) were also frail. **n=363 overall with an activity of daily living (ADL) disability; of these, 100 (total) were also frail. Reprinted from Fried and colleagues, by permission from Oxford University Press on behalf of the British Journal of Anaesthesia.14
More recently, researchers have begun to translate frailty concepts from the medical literature to the perioperative setting, finding that available measures of frailty predict a range of adverse outcomes after surgery, including postoperative medical complications,18–20 increased length of stay,18 and short- and long-term mortality.21,22 As a result of the growing recognition of the potential importance of frailty as a marker of adverse postoperative outcomes, the American Geriatrics Society (AGS) and the National Institute on Aging (NIA) carried out a major consensus conference in 2015 on ‘Frailty for Specialists’, which defined an ultimate long-term goal of ‘incorporating frailty assessments into the preoperative flow’.23 Moreover, the American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP/AGS) 2012 Guidelines for the Optimal Preoperative Assessment of the Geriatric Surgical Patient specify a baseline frailty evaluation as a critical component of preoperative care for older adults.17
Despite these statements, the precise definition of frailty that should be used in the setting of preoperative evaluation, and precisely how the results of frailty evaluations should be used to guide further care, remain subjects of inquiry and debate. Importantly, conflicting definitions of ‘frailty’ exist. The phenotype model, developed by Fried and colleagues,15 is primarily a physical conceptualization of frailty encompassing weight loss of 4.5 kg in the preceding year, decreased grip strength, self-reported exhaustion, low physical activity, and slow walking. An alternative definition, the deficit accumulation model of the Canadian Study of Health and Aging, incorporates 70 variables in the domains of co-morbidities, daily activity, function, health attitude, and nutrition, each representing potential deficits, with the likelihood of being frail increasing with the accumulation of more deficits.24,25
Both the Fried phenotype model and the accumulating deficits model have been modified and studied in surgical populations and are predictive of poor outcomes, such as morbidity, prolonged hospital length of stay, and institutionalized discharge.18,26 While the ACS-NSQIP/AGS guidelines17 propose use of a frailty score based on the Fried model, subsequent authors have called for future work focused on the development of scales specific to surgical populations or development of approaches that potentially use more than one frailty assessment tool to capture distinct aspects of frailty that might affect outcomes for surgical patients.23
Defining the utility and application of frailty assessments in the perioperative setting remains an important challenge for both clinicians and researchers focused on surgery in the older population. Although the specific components of frailty vary according to the definition used, each concept includes potentially modifiable and non-modifiable factors. Research is ongoing as to whether presurgical ‘prehabilitation’ interventions focused on disease optimization, exercise training, and nutritional supplementation can reduce the risk of adverse outcomes for frail older adults.27 Preoperative frailty assessments might ultimately help to guide selection of regimens for intraoperative or postoperative care focused on minimizing complications and accelerating recovery. Beyond specific interventional strategies targeted at risk reduction, frailty measurements could be incorporated into prognostic assessments used to guide choices about surgical and postoperative care by older adults and their families.28
Functional disability among the elderly represents a related although distinct concept from frailty. Although a wide range of functional disability assessments exist, activities of daily living (ADLs) as defined by Katz and colleagues,29 include a range of necessary self-care activities frequently used to characterize degrees of disability among older adults. These activities include bathing, dressing, toileting, transferring from bed to chair, controlling bladder and bowel function, and feeding (Table 3).30 Additional information can also be gained from assessing performance in instrumental activities of daily living (IADLs), which are activities necessary to live independently, such as telephone use, walking, shopping, preparing meals, housework, doing laundry, doing handyman work, taking medications, and managing money (Table 4).31
Table 3.
Katz index of independence in activities of daily living (ADLs)30
| Activities (1 or 0 points) | Independence (1 point) No supervision, direction, or personal assistance |
Dependence (0 points) With supervision, direction, personal assistance, or total care |
|---|---|---|
| Bathing points: _________ | Bathes self completely or needs help in bathing only a single part of the body, such as the back, genital area, or disabled extremity | Needs help with bathing more than one part of the body, getting in or out of the bathtub or shower; requires total bathing |
| Dressing points: _________ | Gets clothes from closets and drawers and puts on clothes and outer garments complete with fasteners; may need help tying shoes | Needs help with dressing self or needs to be dressed completely |
| Toileting points: _________ | Goes to toilet, gets on and off, arranges clothes, cleans genital area without help | Needs help transferring to the toilet and cleaning self, or uses bedpan or commode |
| Transferring points: _________ | Moves in and out of bed or chair unassisted; mechanical transfer aids are acceptable | Needs help in moving from bed to chair or requires a complete transfer |
| Faecal and urinary continence points: _________ | Exercises complete self-control over urination and defecation | Is partly or totally incontinent of bowel or bladder |
| Feeding points: _________ | Gets food from plate into mouth without help; preparation of food may be done by another person | Needs partial or total help with feeding or requires parenteral feeding |
| Total points: ________ | ||
| Scoring: 6=High (patient independent) 0=Low (patient very dependent) |
Table 4.
The Lawton instrumental activities of daily living scale39
| A. Ability to use telephone | |
| 1. Operates telephone on own initiative; looks up and dials numbers | 1 |
| 2. Dials a few well-known numbers | 1 |
| 3. Answers telephone but does not dial | 1 |
| 4. Does not use telephone at all | 0 |
| B. Shopping | |
| 1. Takes care of all shopping needs independently | 1 |
| 2. Shops independently for small purchases | 0 |
| 3. Needs to be accompanied on any shopping trip | 0 |
| 4. Completely unable to shop | 0 |
| C. Food preparation | |
| 1. Plans, prepares, and serves adequate meals independently | 1 |
| 2. Prepares adequate meals if supplied with ingredients | 0 |
| 3. Heats, serves, and prepares meals, or prepares meals, or prepares meals but does not maintain adequate diet | 0 |
| 4. Needs to have meals prepared and served | 0 |
| D. Housekeeping | |
| 1. Maintains house alone or with occasional assistance (e.g. ‘heavy work domestic help’) | 1 |
| 2. Performs light daily tasks, such as dish-washing, bed-making | 1 |
| 3. Performs light daily tasks but cannot maintain acceptable level of cleanliness | 1 |
| 4. Needs help with all home maintenance tasks | 1 |
| 5. Does not participate in any housekeeping tasks | 0 |
| E. Laundry | |
| 1. Does personal laundry completely | 1 |
| 2. Launders small items; rinses stockings, etc. | 1 |
| 3. All laundry must be done by others | 0 |
| F. Mode of transportation | |
| 1. Travels independently on public transportation or drives own car | 1 |
| 2. Arranges own travel via taxi, but does not otherwise use public transportation | 1 |
| 3. Travels on public transportation when accompanied by another | 1 |
| 4. Travel limited to taxi or automobile with assistance of another | 0 |
| 5. Does not travel at all | 0 |
| G. Responsibility for own medications | |
| 1. Is responsible for taking medication in correct dosages at correct time | 1 |
| 2. Takes responsibility if medication is prepared in advance in separate dosage | 0 |
| 3. Is not capable of dispensing own medication | 0 |
| H. Ability to handle finances | |
| 1. Manages financial matters independently (budgets, writes checks, pays rent, bills, goes to bank), collects and keeps track of income | 1 |
| 2. Manages day-to-day purchases, but needs help with banking, major purchases, etc. | 1 |
| 3. Incapable of handling money | 0 |
| Scoring: for each category, circle the item description that most closely resembles the patient's highest functional level (either 0 or 1) | |
In the context of surgery and anaesthesia, the extent and determinants of postoperative disability among older adults have emerged as important themes in research related to a range of surgical procedures. In particular, recent work focusing specifically on nursing-home patients undergoing vascular surgery, colorectal surgery, and orthopaedic surgery has documented high rates of new postoperative functional disability developing over the months to years after surgery.32–34
Assessments of functional status have increasingly been advocated as a critical component of patient evaluation before surgery. The 2012 ACS-NSQIP/AGS guidelines recommend screening all patients for independence in getting out of bed or a chair; bathing and dressing; preparing meals; and shopping.17 As with preoperative frailty status, future work should focus on defining the value of routine preoperative functional assessments for older adults, either as a foundation for targeted preoperative, intraoperative, or postoperative interventions to improve outcomes, or as a basis for improved efforts at prognostication to inform patient decision-making (Table 5).
Table 5.
Frailty screening instruments
| Instrument | Description | Strengths | Weaknesses |
|---|---|---|---|
| Fried Frailty Phenotype18 | Assesses presence of five frailty characteristics (weight loss, grip strength, exhaustion, physical activity, gait speed) | Validated in surgical population. Stratifies patients as robust, pre-frail, and frail | Does not account for changes in cognition or mood |
| Canadian Study of Health and Aging Frailty Index (CSHA-FI)24 | A count of 70 factors, including co-morbidities, mood disorders, cognition, functional status, and nutrition. Frailty assessed as a percentage difference from average scores | Modified versions have been validated in surgical populations. Incorporates many aspects of the frailty syndrome26 | Large number of components. Can be complicated to administer |
| Frailty Index/Comprehensive Geriatric Assessment (FI-CGA)107 | Assesses 10 frailty domains based on co-morbidity and clinical judgment. Based on frailty assessment in the CGA | Stratifies frailty as mild, moderate, and severe. Good inter-rater reliability | Requires specialized training and significant time to perform |
| Edmonton Frail Scale (EFS)108 | Patient and clinician review 10 domains, including medication use, cognitive impairment, balance, and mobility | Validated for use among non-geriatricians. Less than 5 min to perform | Less comprehensive than other frailty assessments |
Anaesthesia for hip fracture surgery
Hip fracture represents a major global public health problem, occurring 250 000 times each year in the USA and more than 1.6 million times worldwide.35,36 Hip fracture, including fractures of the femoral neck and the intertrochanteric and subtrochanteric portions of the femur, is associated with excess disability and mortality.37 For example, of patients living independently in the community before fracture, approximately half will have died or required placement in a nursing home at 1 yr after fracture.38 Hip fractures carry major cost implications for the individual and society; they are being associated with high rates of new financial insecurity among affected individuals and costs to society as a whole that are anticipated to exceed $40 billion by 2040.38,39
Hip fracture is the quintessential geriatric illness; it is typically associated with osteoporosis and other progressive geriatric syndromes, such as frailty, disability, and gait disorders.40,41 Hip fracture is a surgical disease, with more than 95% of all hip fracture patients receiving some form of operative treatment as a means of improving functional recovery and treating pain.42,43 Recent work in the anaesthesia and orthopaedics literature has placed a renewed emphasis on longstanding debates regarding the optimal primary anaesthetic modality for common hip fracture surgeries, which include hemiarthroplasty and total hip arthroplasty for femoral neck fractures, and internal fixation procedures for femoral neck, intertrochanteric, and subtrochanteric fractures.
Spinal anaesthesia, often paired with intraoperative sedation, and general anaesthesia currently represent the two most common primary anaesthetic modalities for hip fracture. A recent multicentre audit conducted as a part of the UK National Hip Fracture Database project found that 50.7% of all patients treated in 182 UK hospitals between May and July 2013 received general anaesthesia, with or without additional nerve blocks for pain relief, and 44% received spinal anaesthesia, with or without additional nerve blocks.44 Although national data are not available for the USA, findings from the ACS-NSQIP database, an elective clinical registry including ∼650 US hospitals, suggests a rate of neuraxial techniques for hip fracture in the USA approximately half that seen in the UK.45–47
This variation in practice reflects the substantial uncertainty that remains regarding the relative advantages and disadvantages of neuraxial anaesthesia compared with general anaesthesia as the primary anaesthetic for hip fracture surgery in the elderly. The available base of clinical trial data is inconclusive regarding the optimal approach to anaesthesia for hip fracture care. A 2004 Cochrane review of 22 trials published between 1977 and 2003 found that spinal anaesthesia was associated with lower rates of postoperative confusion, although insufficient evidence existed to rule out important differences in outcomes between techniques.48 A 2011 review conducted by the UK National Clinical Guideline Centre examined the same group of studies and concluded that ‘no recent randomised controlled trials were identified that fully address [the comparative effectiveness of neuraxial versus general anesthesia for hip fractures]… The evidence is old and does not reflect current practice.’49
In this context, retrospective comparisons of outcomes among patients receiving neuraxial or general anaesthesia for hip fracture surgery have proliferated during the past 3 yr. A 2012 retrospective cohort by Neuman and colleagues50 of administrative data on 18,000 patients undergoing surgery for hip fracture at hospitals in New York State found a markedly lower odds of both in-hospital mortality and pulmonary complications in patients treated with regional anaesthesia. Subsequent retrospective analyses have produced conflicting results regarding the relationship between anaesthesia technique and outcomes for this condition. Although one large analysis of administrative claims from Taiwan found a marked reduction in mortality and major complications with the use of spinal compared with general anaesthesia,51 such differences have not been found in recent retrospective analyses from the USA and the UK.52–54 Studies using the US ACS-NSQIP database in different years have alternately suggested both a higher46 and a lower rate of complications45,47 associated with spinal compared with general anaesthesia.
The divergence between available studies in this area is mirrored in differences across guidance put forward by major organizations regarding anaesthetic care for hip fracture patients. For example, the 2014 Anaesthesia Sprint Audit of Practice of the Association of Anaesthetists of Great Britain and Ireland proposed as a quality standard that ‘Spinal/epidural anaesthesia should be considered for all patients;’44 in contrast, the 2014 American Association of Orthopedic Surgeons' (AAOS) Guidelines on the Management of Hip Fractures in the Elderly states that ‘strong evidence supports similar outcomes for general or spinal anesthesia for patients undergoing hip fracture surgery.’55
Such disagreements in the original literature and in expert guidelines highlight a need for additional research to provide a clearer definition of the risks and benefits of alternative anaesthesia techniques. Importantly, available retrospective studies of anaesthesia care for hip fracture are limited by an inability to account fully for selection bias. Given that patients chosen to receive one or another type of anaesthesia for hip fracture surgery could differ in terms of baseline illness, such analyses may either underestimate or overestimate associations between a given type of anaesthesia and patient outcomes. Likewise, given that hospitals vary markedly in their use of one or another technique, broader differences in quality of care across hospitals may represent an additional source of confounding in retrospective comparisons of outcomes among patients receiving spinal or general anaesthesia for hip fracture.
Available retrospective studies in this area are often limited in terms of the information they can provide regarding the anaesthetic regimens that are being compared, because large-scale clinical and administrative databases rarely contain specific information regarding the type of block used, the amount of sedation (if any) provided to patients undergoing neuraxial anaesthesia, or the medications delivered to maintain general anaesthesia. Moreover, the outcomes available for study have been limited to mortality, major morbidity, and utilization measures, such as hospital length of stay. While patient-centred outcomes, such as pain, satisfaction, and recovery of functional independence may be useful for informing patients' decisions about anaesthesia care, these data are not available in most retrospective studies in this area.
Ongoing and planned work promises to begin to fill some of these gaps. In addition to the ongoing National Hip Fracture Database project,44 the AAOS has recently completed the pilot data collection phase of a new multicentre hip fracture registry developed in partnership with ACS-NSQIP. These two important registry projects promise to provide increasingly granular data on the anaesthetic and surgical care received by hip fracture patients, while also recording important new data on key patient-centred outcomes. The REGAIN trial (Regional vs General Anesthesia for Promoting Independence after Hip Fracture Surgery; ClinicalTrials.gov number: NCT02507505) is anticipated to begin patient enrolment in spring 2016. This project is a 1600 patient multicentre randomized trial that will compare a range of patient-centred outcomes, including recovery of functional independence, among patients receiving spinal or general anaesthesia for hip fracture surgery at hospitals in the USA and Canada. Ultimately, REGAIN will seek to increase the base of high-quality evidence available to inform decisions about anaesthesia for hip fracture surgery while also potentially serving as a model for further randomized comparisons in this area.
Effects of anaesthesia on the ageing brain: postoperative delirium
Older patients often display reduced cognitive reserve. Brain mass decreases with age, particularly in the frontal and temporal lobes.56 As patients age, they are more prone to neurodegenerative disorders, the most common of which is Alzheimer's disease, with a 50% incidence in persons aged ≥85 yr.57,58 The prevalence of Parkinson's disease, Huntington disease, vascular dementia, and prion diseases also increases with age; all of these have a clinical presentation late in the disease process.59 Cognitive impairment without dementia is common in older adults, with an estimated prevalence of 22% in people >70 yr old in the USA.60
Delirium is among the most common complications after surgery and is more common among older patients. In their 2014 systematic review, Inouye and colleagues61 reported that 13–50% of older patients undergoing non-cardiac surgery develop postoperative delirium. Delirium is characterized by an acute onset and fluctuating course of inattention, disorganized thinking, and altered mental status.62 Postoperative cognitive dysfunction (POCD) is distinct from delirium and usually describes the significant change in cognition many patients experience after surgery and anaesthesia, although at this time there is no consensus definition of POCD.63 Compared with postoperative delirium, POCD is thought to be a more persistent change in cognitive performance, particularly memory and attention.59,64
Postoperative delirium typically lasts for hours to days, but can persist for weeks; risk factors include age 70 yr or older, pre-existing cognitive dysfunction or dementia, history of delirium, history of alcohol abuse, and preoperative use of opioid analgesics.65,66 Studies in cardiac and non-cardiac surgical patients have also shown that low preoperative executive scores and depressive symptoms independently predict postoperative delirium in older patients.67,68 Among patients undergoing non-cardiac surgery, the incidence of delirium also appears to mirror the severity of surgery.61 Like many surgical complications, postoperative delirium appears to be correlated both with the severity and type of surgery and with the number and severity of preoperative patient co-morbidities. In patients with a greater number of preoperative risk factors, a smaller surgical stressor is required to result in delirium than in a healthier patient with more cognitive reserve.69 Given that advanced age is associated with reductions in cognitive reserve and is a known risk factor for postoperative delirium, the American Geriatrics Society issued ‘Postoperative delirium in older adults: best practice statement from the American Geriatrics Society’ in 2015, regarding the prevention, diagnosis, and treatment of postoperative delirium.65
The presence of delirium is formally assessed by applying the standards of the Diagnostic and Stasticical Manual of Mental Disorders (DSM-5), which focus on delirium's acute onset, change from baseline attention and awareness, and fluctuating course.70 One useful screening tool for postoperative delirium is the Confusion Assessment Method (CAM), which is simple to administer and has been validated in multiple settings.71,72 The CAM systematically guides the clinician through assessing the acute onset of symptoms, fluctuating course, level of consciousness, and disorganized thinking. Additional validated delirium screening tools include the CAM-ICU (designed for critically ill patients), 3D-CAM (an abbreviated version of CAM), 4AT Rapid Assessment Test for Delirium, and Delirium Rating Scale Revised-98 (DRS-Revised-98) (Table 2).72–77 Although these screening tools have been helpful in diagnosing the presence of delirium, they do not assess severity or subtypes of delirium, which might be helpful in guiding management of delirious patients. For example, the 2015 AGS Best Practice Report recommends low-dose pharmacological treatment of severely agitated delirious patients (perhaps with antipsychotics, but the evidence is heterogeneous) but cautions against pharmacological treatment of hypoactive delirious patients who are not agitated.65
Table 2.
Commonly used delirium screening tools
| Tool | Description | Strengths | Weaknesses |
|---|---|---|---|
| Confusion Assessment Method (CAM)71 | Assesses the presence, severity, and fluctuation of nine delirium features. Based on the Diagnostic and Statistical Manual of Mental Disorders III criteria for delirium | Simple to administer. Validated in many clinical settings and translated into multiple languages | Some heterogeneity of delirium detection across different studies72 |
| CAM-ICU73 | CAM adapted for non-verbal, mechanically ventilated patients | Simple to administer. Excellent inter-rater reliability | Good specificity but modest sensitivity depending on how providers are trained74 |
| 4AT Rapid Assessment Test for Delirium76 | Four items that assess attention, cognition, and alertness | Quick and simple to administer, allows for testing of agitated or drowsy patients, incorporates general cognitive screening | Overall score more sensitive than specific |
| Delirium Rating Scale-Revised-98 (DRS-Revised-98)77 | Three diagnostic items for initial ratings and a 13-item scale for repeated measures (range, 0–46) | High sensitivity and specificity | Requires special training |
In patients at risk for postoperative delirium, the 2015 AGS Best Practice Report recommends avoiding benzodiazapenes when possible, minimizing the use of H1 antagonists and other medications with strong anticholinergic side-effects, and avoiding meperidine for treatment of pain.17,65 It is important to achieve adequate pain control by other means because untreated pain can contribute to the development of delirium.78 The AGS recommends use of postoperative regional analgesia when possible to minimize administration of opioids, although this is still an area of investigation.65,79,80 The AGS also suggests that maintaining a lighter intraoperative plane of anaesthesia with the aid of a processed EEG might lower the risk of postoperative delirium.65 Trials with patients undergoing general anaesthesia found that the incidence of postoperative delirium was lower among patients whose anaesthetist was randomized to use a processed EEG to guide anaesthetic depth.81,82 However, the benefits of lighter anaesthesia must be weighed against its risks, which include exposing the patient to intraoperative recall, sympathetic stimulation, and movement during surgery. Additional measures to prevent postoperative delirium can be implemented after surgery and include structured protocols that target the management of cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration after surgery.83,84
While the importance of delirium to the immediate postoperative course is largely recognized, the aetiology and long-term outcomes of postoperative delirium remain to be described in high-quality studies. The landmark multi-institutional SAGES (Successful Aging after Elective Surgery) trial is ongoing and seeks to use biomarkers, neuroimaging, cognitive reserve markers, and serial neuropsychological testing to gain a better understanding of the contribution of delirium to long-term cognitive and functional decline.85 This will hopefully inform future high-quality trials that study novel techniques to prevent and treat postoperative delirium. Recent evidence from the SAGES trial has examined the interplay between postoperative delirium and other postoperative outcomes, and suggests that postoperative delirium is a distinct predictor of adverse events. The combination of delirium and other postoperative complications leads to the poorest long-term outcomes, adversely affecting hospital length of stay, rates of institutionalized discharge, and 30 day readmission rates.86
Shared decision-making with older patients
Given that they often present with complex co-morbidities, decreased physiological reserve, and unique life experiences, there is often a greater need to consider the values, goals, and concerns of older patients.87 Seriously ill older patients may have treatment preferences influenced not only by the risk of mortality and likelihood of potential benefits from the procedure, but also by the burden of treatment and risk of postoperative cognitive and functional impairment.88 In addition, older adults may lack a strong social support network and may be underinsured, resulting in limited access to home care or rehabilitation.89 With these considerations, the decision-making regarding surgery is often complex and may require a redesigned approach to framing discussions.5 Cooper and colleagues90 have developed a conceptual framework whereby an appropriate decision about whether to pursue surgery requires the best clinical evidence, qualified health-care providers, a health-care facility capable of managing the operation and postoperative care, and patients who are well-informed and meaningfully involved in surgical decision-making.
Surgeons often assume patients' preoperative commitment to postoperative life-supporting care, also known as ‘surgical buy-in’, when planning for complex surgeries.91,92 When surveying 912 surgeons across the USA, Schwarze and colleagues93 found that 60% of surgeons endorsed sometimes or always refusing to operate on patients with preferences to limit life support, 62% of surgeons reported that they would create an informal contract with patients describing agreed upon limitations of aggressive therapy, and 20% reported that they would formally document this contractual agreement. However, surgical patients often display suboptimal understanding of the risks and benefits of their upcoming surgery.94 Moreover, they often do not engage in thorough discussion of their treatment preferences about advanced care planning, particularly preferences about how aggressively care should proceed in the wake of complications.95 One way of learning about patients' goals, values, and social situations is shared decision-making, a process that makes explicit the communication that has always been the cornerstone of the doctor–patient relationship.
Before any decision-making process, the decision-making capacity of the patient must be established. Mental capacity varies on a continuum and depends upon the complexity of the decision in question.96 The 2012 ACS-NSQUIP/AGS guidelines state that at minimum, the patient should be able to explain in his or her own words the nature of the medical problem, the procedure about to be performed, the attendant risks and benefits, and alternative treatments considered.17 If the patient does not have decision-making capacity, a surrogate decision-maker should be clearly identified and present for all major discussions.
Shared decision-making involves three basic steps. First, patients must be informed. Specifically, they must be given an objective presentation of reasonable options and be told the risks and benefits of those options. Once informed, patients should consider their own values, goals, and fears and consider how each option presented will be likely to unfold in terms of these values and goals. Finally, patients must communicate their goals and concerns to their health-care provider, and these goals and concerns should be incorporated through a shared process into the decision at hand.97 Shared decision-making allows both the clinician and the patient to have input in the decision-making process.
Shared decision-making can be a nuanced process, but there are a number of topics that should be explicitly discussed, particularly when approaching elective high-risk surgery in older patients. Resuscitation status and the presence of advanced directives should be discussed and documented.98 There should also be a discussion about whether the patient prefers maintenance or de-escalation of care if severe complications occur. Some providers have advocated the strategy of a time-limited trial in the setting of highly uncertain outcomes. With a time-limited trial, the current health status and treatment of the patient is clearly presented and a specific window of time is defined, for example a few days to 1 month, to re-evaluate treatment efficacy and goals of care.99 Finally, a health-care proxy should be identified and informed about their role and the patient's goals and values. This person will act as a surrogate to make decisions, if necessary, while the patient is sedated or under anaesthesia.
Shared decision-making has received much recent attention, particularly with the development of decision aids for certain discrete scenarios (treatment of hyperlipidaemia, breast cancer treatment, and decisions about knee replacements). Decision aids have been shown to improve patients' knowledge about their options and reduce their decisional conflict related to feeling unclear about their values.97,100 However, some have argued that shared decision-making serves only to confuse patients, particularly in the setting of complex medical decisions that can be overwhelming.101 In these scenarios, rather than discussing the technical aspects of the treatment at hand, it may be useful to focus the conversation on outcomes that matter to patients, such as long-term functional status, ability to eat, and postoperative pain.5,102 Decision-making around surgery is further complicated because the patient has multiple providers (at minimum a surgeon and anaesthetist, often more) and must negotiate different discussions with each. Older surgical patients may benefit from a multidisciplinary approach to care that involves not only their anaesthetists and surgeons, but also consultation from cardiology, oncology, nephrology, rehabilitation medicine, nutrition, social work, and their longitudinal primary care provider.103 Geriatric and palliative care specialists can be of particular help in managing older patients.104–106 Work remains to be done to optimize the role of each provider in assessment of risk, communication of risk, and decision-making with patients.
Conclusion
Ongoing studies will guide future clinical practice recommendations and areas of future research on anaesthesia and surgery in older adults that will allow us to refine our assessment of these patients and improve our care.
Authors' contributions
Equal contribution to the development, drafting, and final review of the manuscript: S.M., D.L.H., Z.C., A.M.B., M.D.N.
Declaration of interest
None declared.
References
- 1.US Census Bureau. 2014 National Population Projections: Summary Tables 2014. Available from https://www.census.gov/population/projections/data/national/2014/summarytables.html (accessed 31 July 2015)
- 2.CDC/NCHS National Hospital Discharge Survey. Number, percent distribution, rate, days of care with average length of stay, and standard error of discharges from short-stay hospitals, by sex and age: United States, 2010. Available from http://www.cdc.gov/nchs/data/nhds/2average/2010ave2_ratesexage.pdf (accessed 31 July 2015)
- 3.Klopfenstein CE, Herrmann FR, Michel JP, Clergue F, Forster A. The influence of an aging surgical population on the anesthesia workload: a ten-year survey. Anesth Analg 1998; 86: 1165–70 [DOI] [PubMed] [Google Scholar]
- 4.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg 2003; 238: 170–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glance LG, Osler TM, Neuman MD. Redesigning surgical decision making for high-risk patients. N Engl J Med 2014; 370: 1379–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuman MD, Bosk CL. What we talk about when we talk about risk: refining surgery's hazards in medical thought. Milbank Q 2012; 90: 135–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043–9 [DOI] [PubMed] [Google Scholar]
- 8.Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg 2000; 232: 242–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119: 495–502 [DOI] [PubMed] [Google Scholar]
- 10.Gupta N, Aggarwal S, Murugiah K, Slawski B, Cinquegrani M. Chads2 score predicts perioperative stroke risk in patients without atrial fibrillation. J Am Coll Cardiol 2013; 61: doi:10.1016/S0735-1097(13)60341-1 [Google Scholar]
- 11.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64: e77–137 [DOI] [PubMed] [Google Scholar]
- 12.Neuman MD, Bosk CL. The redefinition of aging in American surgery. Milbank Q 2013; 91: 288–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004; 59: 255–63 [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56 [DOI] [PubMed] [Google Scholar]
- 16.Partridge JS, Harari D, Dhesi JK. Frailty in the older surgical patient: a review. Age Ageing 2012; 41: 142–7 [DOI] [PubMed] [Google Scholar]
- 17.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg 2012; 215: 453–66 [DOI] [PubMed] [Google Scholar]
- 18.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210: 901–8 [DOI] [PubMed] [Google Scholar]
- 19.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 2013; 206: 544–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr 2009; 48: 78–83 [DOI] [PubMed] [Google Scholar]
- 21.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 2009; 250: 449–55 [DOI] [PubMed] [Google Scholar]
- 22.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010; 121: 973–8 [DOI] [PubMed] [Google Scholar]
- 23.Robinson TN, Walston JD, Brummel NE, et al. Frailty for surgeons: review of a National Institute on Aging conference on frailty for specialists. J Am Coll Surg. Advance Access published on September 11, 2015, doi:10.1016/j.jamcollsurg.2015.08.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722–7 [DOI] [PubMed] [Google Scholar]
- 26.Joseph B, Pandit V, Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg 2014; 149: 766–72 [DOI] [PubMed] [Google Scholar]
- 27.Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013; 27: 1072–82 [DOI] [PubMed] [Google Scholar]
- 28.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA 2014; 311: 2110–20 [DOI] [PubMed] [Google Scholar]
- 29.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963; 185: 914–9 [DOI] [PubMed] [Google Scholar]
- 30.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970; 10: 20–30 [DOI] [PubMed] [Google Scholar]
- 31.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–86 [PubMed] [Google Scholar]
- 32.Oresanya L, Zhao S, Gan S, et al. Functional outcomes after lower extremity revascularization in nursing home residents: a national cohort study. JAMA Intern Med 2015; 175: 951–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finlayson E, Zhao S, Boscardin WJ, Fries BE, Landefeld CS, Dudley RA. Functional status after colon cancer surgery in elderly nursing home residents. J Am Geriatr Soc 2012; 60: 967–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med 2014; 174: 1273–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA 2009; 302: 1573–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int 2004; 15: 897–902 [DOI] [PubMed] [Google Scholar]
- 37.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci 2000; 55: M498–507 [DOI] [PubMed] [Google Scholar]
- 38.Tajeu GS, Delzell E, Smith W, et al. Death, debility, and destitution following hip fracture. J Gerontol A Biol Sci Med Sci 2014; 69: 346–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc 2003; 51: 364–70 [DOI] [PubMed] [Google Scholar]
- 40.Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA 2012; 307: 2185–94 [DOI] [PubMed] [Google Scholar]
- 41.Hung WW, Morrison RS. Hip fracture: a complex illness among complex patients. Ann Intern Med 2011; 155: 267–8 [DOI] [PubMed] [Google Scholar]
- 42.Neuman MD, Fleisher LA, Even-Shoshan O, Mi L, Silber JH. Nonoperative care for hip fracture in the elderly: the influence of race, income, and comorbidities. Med Care 2010; 48: 314–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker MJ, Handoll HH. Conservative versus operative treatment for extracapsular hip fractures. Cochrane Database Syst Rev 2000; CD000337. [DOI] [PubMed] [Google Scholar]
- 44.Boulton C, Currie C, Griffiths R, et al. Falls and Fragility Fracture Audit Programme National Hip Fracture Database: Anaesthesia Sprint Audit of Practice 2014 2014. Available from https://www.aagbi.org/sites/default/files/NHFDanaesthesticreport.pdf (accessed 5 October 2015)
- 45.Basques BA, Bohl DD, Golinvaux NS, Samuel AM, Grauer JG. General versus spinal anaesthesia for patients aged 70 years and older with a fracture of the hip. Bone Joint J 2015; 97-B: 689–95 [DOI] [PubMed] [Google Scholar]
- 46.Whiting PS, Molina CS, Greenberg SE, Thakore RV, Obremskey WT, Sethi MK. Regional anaesthesia for hip fracture surgery is associated with significantly more peri-operative complications compared with general anaesthesia. Int Orthop 2015; 39: 1321–7 [DOI] [PubMed] [Google Scholar]
- 47.Fields AC, Dieterich JD, Buterbaugh K, Moucha CS. Short-term complications in hip fracture surgery using spinal versus general anaesthesia. Injury 2015; 46: 719–23 [DOI] [PubMed] [Google Scholar]
- 48.Parker MJ, Handoll HH, Griffiths R. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev 2004; 4: CD000521. [DOI] [PubMed] [Google Scholar]
- 49. National Clinical Guideline Centre (UK). The Management of Hip Fracture in Adults [Internet]. London: Royal College of Physicians (UK); 2011. (NICE Clinical Guidelines, No. 124.) Available from: http://www.ncbi.nlm.nih.gov/books/NBK83014/ (accessed 2 November 2015) [Google Scholar]
- 50.Neuman MD, Silber JH, Elkassabany NM, Ludwig JM, Fleisher LA. Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology 2012; 117: 72–92 [DOI] [PubMed] [Google Scholar]
- 51.Chu CC, Weng SF, Chen KT, et al. Propensity score-matched comparison of postoperative adverse outcomes between geriatric patients given a general or a neuraxial anesthetic for hip surgery: a population-based study. Anesthesiology 2015; 123: 136–47 [DOI] [PubMed] [Google Scholar]
- 52.Neuman MD, Rosenbaum PR, Ludwig JM, Zubizarreta JR, Silber JH. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA 2014; 311: 2508–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patorno E, Neuman MD, Schneeweiss S, Mogun H, Bateman BT. Comparative safety of anesthetic type for hip fracture surgery in adults: retrospective cohort study. Br Med J 2014; 348: g4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia 2014; 69: 224–30 [DOI] [PubMed] [Google Scholar]
- 55.Brox WT, Roberts KC, Taksali S, et al. The American Academy of Orthopaedic Surgeons Evidence-Based Guideline on Management of Hip Fractures in the Elderly. J Bone Joint Surg Am 2015; 97: 1196–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lockhart SN, DeCarli C. Structural imaging measures of brain aging. Neuropsychol Rev 2014; 24: 271–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 2007; 3: 186–91 [DOI] [PubMed] [Google Scholar]
- 58.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366: 2112–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang J, Eckenhoff MF, Eckenhoff RG. Anesthesia and the old brain. Anesth Analg 2010; 110: 421–6 [DOI] [PubMed] [Google Scholar]
- 60.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med 2008; 148: 427–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014; 383: 911–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inouye SK. Delirium in older persons. N Engl J Med 2006; 354: 1157–65 [DOI] [PubMed] [Google Scholar]
- 63.Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth 2014; 112: 440–51 [DOI] [PubMed] [Google Scholar]
- 64.Ballard C, Jones E, Gauge N, et al. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS ONE 2012; 7: e37410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg 2015; 220: 136–48.e1 [DOI] [PubMed] [Google Scholar]
- 66.Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology 2007; 106: 622–8 [DOI] [PubMed] [Google Scholar]
- 67.Greene NH, Attix DK, Weldon BC, Smith PJ, McDonagh DL, Monk TG. Measures of executive function and depression identify patients at risk for postoperative delirium. Anesthesiology 2009; 110: 788–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367: 30–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sieber FE. Postoperative delirium in the elderly surgical patient. Anesthesiol Clin 2009; 27: 451–64, table of contents [DOI] [PubMed] [Google Scholar]
- 70.The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med 2014; 12: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–8 [DOI] [PubMed] [Google Scholar]
- 72.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA 2010; 304: 779–86 [DOI] [PubMed] [Google Scholar]
- 73.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286: 2703–10 [DOI] [PubMed] [Google Scholar]
- 74.van Eijk MM, van den Boogaard M, van Marum RJ, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med 2011; 184: 340–4 [DOI] [PubMed] [Google Scholar]
- 75.Marcantonio ER, Ngo LH, O'Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 2014; 161: 554–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014; 43: 496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 2001; 13: 229–42 [DOI] [PubMed] [Google Scholar]
- 78.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg 2006; 102: 1267–73 [DOI] [PubMed] [Google Scholar]
- 79.Rashiq S, Vandermeer B, Abou-Setta AM, Beaupre LA, Jones CA, Dryden DM. Efficacy of supplemental peripheral nerve blockade for hip fracture surgery: multiple treatment comparison. Can J Anaesth 2013; 60: 230–43 [DOI] [PubMed] [Google Scholar]
- 80.Zhang H, Lu Y, Liu M, et al. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care 2013; 17: R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013; 25: 33–42 [DOI] [PubMed] [Google Scholar]
- 82.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013; 110 (Suppl. 1): i98–i105 [DOI] [PubMed] [Google Scholar]
- 83.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49: 516–22 [DOI] [PubMed] [Google Scholar]
- 84.Sieber FE, Barnett SR. Preventing postoperative complications in the elderly. Anesthesiol Clin 2011; 29: 83–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc 2012; 13: 818.e1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg 2015; 1–7. doi:10.1001/jamasurg.2015.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White SM. Ethical and legal aspects of anaesthesia for the elderly. Anaesthesia 2014; 69 (Suppl. 1): 45–53 [DOI] [PubMed] [Google Scholar]
- 88.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002; 346: 1061–6 [DOI] [PubMed] [Google Scholar]
- 89.Oxman TE, Hull JG. Social support, depression, and activities of daily living in older heart surgery patients. The J Gerontol B Psychol Sci Soc Sci 1997; 52B: P1–14 [DOI] [PubMed] [Google Scholar]
- 90.Lilley EJ, Bader AM, Cooper Z. A values-based conceptual framework for surgical appropriateness: an illustrative case report. Ann Palliat Med 2015; 4: 54–7 [DOI] [PubMed] [Google Scholar]
- 91.Pecanac KE, Kehler JM, Brasel KJ, et al. It's big surgery: preoperative expressions of risk, responsibility, and commitment to treatment after high-risk operations. Ann Surg 2014; 259: 458–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwarze ML, Bradley CT, Brasel KJ. Surgical ‘buy-in’: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med 2010; 38: 843–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwarze ML, Redmann AJ, Alexander GC, Brasel KJ. Surgeons expect patients to buy-in to postoperative life support preoperatively: results of a national survey. Crit Care Med 2013; 41: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns 2014; 94: 328–33 [DOI] [PubMed] [Google Scholar]
- 95.Cooper Z, Corso K, Bernacki R, Bader A, Gawande A, Block S. Conversations about treatment preferences before high-risk surgery: a pilot study in the preoperative testing center. J Palliat Med 2014; 17: 701–7 [DOI] [PubMed] [Google Scholar]
- 96.Ho A, Pinney SJ, Bozic K. Ethical concerns in caring for elderly patients with cognitive limitations: a capacity-adjusted shared decision-making approach. J Bone Joint Surg Am 2015; 97: e16. [DOI] [PubMed] [Google Scholar]
- 97.Fowler FJ, Jr, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood) 2011; 30: 699–706 [DOI] [PubMed] [Google Scholar]
- 98.Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surg 2012; 255: 418–23 [DOI] [PubMed] [Google Scholar]
- 99.Neuman MD, Allen S, Schwarze ML, Uy J. Using time-limited trials to improve surgical care for frail older adults. Ann Surg 2015; 261: 639–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014; 1: Cd001431. [DOI] [PubMed] [Google Scholar]
- 101.Rosenbaum L. The paternalism preference — choosing unshared decision making. N Engl J Med 2015; 373: 589–92 [DOI] [PubMed] [Google Scholar]
- 102.Schwarze ML, Brasel KJ, Mosenthal AC. Beyond 30-day mortality: aligning surgical quality with outcomes that patients value. JAMA Surg 2014; 149: 631–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan KY, Tan P, Tan L. A collaborative transdisciplinary ‘geriatric surgery service’ ensures consistent successful outcomes in elderly colorectal surgery patients. World J Surg 2011; 35: 1608–14 [DOI] [PubMed] [Google Scholar]
- 104.Zenilman ME. Geriatric consultation services for surgical patients. JAMA Surg 2014; 149: 90. [DOI] [PubMed] [Google Scholar]
- 105.Tillou A, Kelley-Quon L, Burruss S, et al. Long-term postinjury functional recovery: outcomes of geriatric consultation. JAMA Surg 2014; 149: 83–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ernst KF, Hall DE, Schmid KK, et al. Surgical palliative care consultations over time in relationship to systemwide frailty screening. JAMA Surg 2014; 149: 1121–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 2004; 52: 1929–33 [DOI] [PubMed] [Google Scholar]
- 108.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35: 526–9 [DOI] [PMC free article] [PubMed] [Google Scholar]