Abstract
The efficient and reliable evaluation of patients with acute chest pain is one of the most challenging tasks in the emergency department. Coronary computed tomography (CT) angiography may play a major role, since it permits ruling out coronary artery disease with high accuracy if performed with expertise in properly selected and prepared patients. Several randomized trials have established early cardiac CT as a viable safe and potentially more efficient alternative to functional testing in the evaluation of acute chest pain. Ongoing investigations explore whether advanced anatomic and functional assessments such as high-risk coronary plaque, resting myocardial perfusion, and left ventricular function, or the simulation of the fractional coronary flow reserve will add information to the anatomic assessment for stenosis, which would allow expanding the benefits of cardiac CT from triage to treatment decisions. Especially, the combination of high-sensitive troponins and coronary computed tomography angiography may play a valuable role in future strategies for the management of patients presenting with acute chest pain.
Keywords: Computed tomography, Acute chest pain, Acute coronary syndrome, Myocardial infarction, Diagnostic triage, Review
Background
Chest pain is one of the most common reasons to visit the emergency department (ED). The challenge for ED physicians is to accurately and efficiently identify the small proportion of patients with myocardial infarction or other life-threatening conditions, while at the same time contain the expanding logistic burden for emergency medical services. Initial triage of acute chest pain is targeted at identifying patients at very low risk, who can be safely discharged immediately. Unfortunately, neither clinical presentation, traditional cardiovascular risk factors, nor clinical risk scores allow for a safe initial triage, as the adverse event rate even in patients with the lowest scores is still around 2%.1,2 In this review, we will discuss the role of cardiac computed tomography (CT) in low to intermediate risk ED patients with suspicion of an acute coronary syndrome (ACS), focusing on early triage and subsequent management decisions.
Geographical heterogeneity in the care of acute chest pain
The organization of emergency medicine and care of suspected ACS varies between countries, hospitals, and doctors. Patients may be seen by cardiologists, ED specialists, internists, or even general surgeons in smaller hospitals. In some European countries, general practice medicine functions as a gatekeeper for low-risk chest pain, while in USA, the ED is often the first place for medical consultation on a walk-in basis. Many American hospitals, and some in Europe, have dedicated low-risk chest pain observation units as an intermediary between the ED and a full admission. Despite guideline mediated management using risk models, differences in the choice and frequency of non-invasive diagnostic testing, as well as referral rates for invasive angiography, remain. Variations in population characteristics, disease prevalence, ED logistics, observation capacity, local traditions, and financial incentives affect diagnostic management, as well as the potential role of new diagnostic tools, in the triage of acute chest pain.
Cardiac computed tomography—an opportunity to improve chest pain triage?
Over the past two decades, CT has rapidly evolved. State-of-the-art scanners acquire 64–320 cross-sections per rotation, depicting vascular details with a spatial resolution <0.5 mm. Fast scanner technology combined with heart rate reducing medication now make it possible to image the coronary arteries without motion artefacts in most patients. An electrocardiogram (ECG)-synchronized, contrast-enhanced images of the heart and coronary arteries can be acquired in one to five heart cycles. The diagnostic performance of coronary computed tomography angiography (CTA) has been investigated extensively in patients with stable coronary artery disease (CAD). Using invasive angiography as a reference, coronary CTA is more sensitive (98–100%) than any other non-invasive technique.3 Because of the high negative predictive value (99–100%), coronary CTA is recommended in patients with a low to intermediate probability of CAD, or after an inconclusive functional test.4 A normal cardiac CT examination is associated with a low adverse cardiac event rate in the following years.5 The reported per-patient specificity (≈85%) is lower due to overestimation of stenosis severity, often due to the presence of calcifications, but not inferior to other non-invasive techniques. The radiation exposure has decreased dramatically over the past years. Doses <5 mSv are now common practice using state-of-the-art technology, while very recent innovations permit doses <1 mSv in selected patients.6 Given the practical limitations of functional testing in the ED setting, and the relatively low prevalence of CAD in this setting, direct coronary visualization by CTA appears an attractive diagnostic alternative for early triage of ACS (Figure 1).
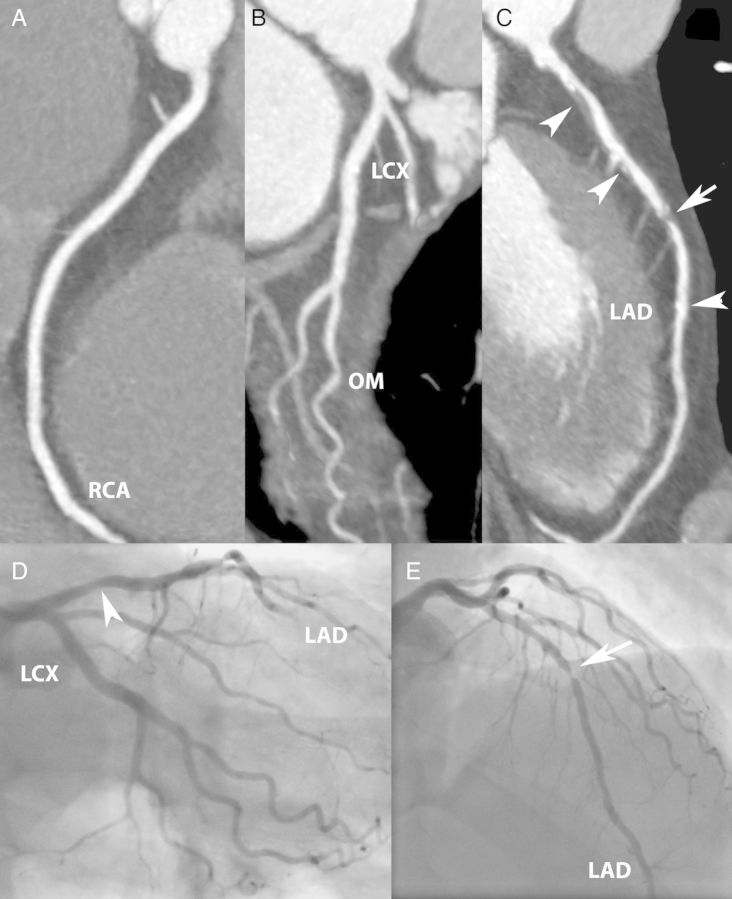
Figure 1.
Obstructive coronary disease. A 59-year-old man presenting after a prolonged episode of crushing chest pain with bilateral radiation to the arms that subsided after sublingual nitroglycerine. There were non-specific STT-segment changes on the electrocardiogram and normal initial myocardial necrosis markers. Coronary CTA (A–C) shows atherosclerotic plaque (arrowheads) and a short, severe stenosis in the mid-LAD (arrow). Second cardiac markers were positive. Catheter angiography (D and E) confirmed a stenosis in the mid-LAD.
Current data on cardiac computed tomography in the emergency department
Observational data
Between 2006 and 2012, several observational studies addressed the feasibility of cardiac CT in patients with acute chest pain (Table 1).7–11 While initial studies were performed in more selected, often high-risk patients, their results were substantiated by a large prospective observational cohort study (ROMICAT I), in which the coronary CTA results remained blinded to the caregivers and patients.9 The main conclusions from these studies were that the absence of plaque on CTA excludes ACS (sensitivity 100%), while obstructive CAD (>50% stenosis) does not (sensitivity 77%), and only half of patients with obstructive coronary disease on CTA have acute coronary disease.
Table 1.
Detection of acute coronary syndrome in observational studies
| Study | n | Population | Scanner | ACS definition | ACS rate (MI rate) | CT criterion | sens (%) | spec (%) |
|---|---|---|---|---|---|---|---|---|
| Rubinshtein et al.7 | 58 | Higher risk (incl. history of CAD) | 64-CT | Positive troponins, or >50% stenosis by invasive angiography, or positive ischaemia test | 34% | Stenosis | 100 | 92 |
| Gallagher et al.8 | 92 | Low-risk ED | 64-CT | MI, UAP | 13% | Stenosis | 86 | 92 |
| ROMICAT I9 | 368 | Low-risk ED | 64-CTa | MI (8), UAP (23) | 8.4% (2%) | Plaque | 100 | 54 |
| Stenosis | 77 | 87 | ||||||
| Hansen et al.10 | 89 | Low-risk ED | 64-DSCT | MI | 4% (4%) | Plaque | 100 | 41 |
| Stenosis | 75 | 86 | ||||||
| Dedic et al.11 | 111 | Any-risk ED (incl. low-positive troponins) | 64-DSCTa | MI (13), UAP (6) | 17% (12%) | Calcium | 89 | 41 |
| Plaque | 100 | 40 | ||||||
| Stenosis | 89 | 79 |
MI, myocardial infarction; UAP, unstable angina pectoris; sens, sensitivity; spec, specificity; DSCT, dual-source CT.
aBlinded cardiac CT exam, without affecting management.
Randomized comparative effectiveness trials
Several randomized controlled trials investigated the safety and economic performance of cardiac CT in acute chest pain (Table 2).12–14 The trial designs can be summarized as non-controlled comparative effectiveness trials, in which decisions of care were made by caregivers, and thus results reflect common practice patterns. While the CT-STAT trial compared coronary CTA with single-photon emission computed tomography (SPECT),16 the ACRIN and ROMICAT II trials compared CTA with standard of care (SOC).13,14 Patients were predominantly enrolled at academic centres in the USA (4–16 sites), without functional testing on the ED, or an accelerated care protocol. The study cohorts were at very low (ACRIN, CT-STAT) or low to intermediate risk (ROMICAT II). Between 10 and 15% of patients presenting with acute chest pain to the ED were enrolled in these trials.
Table 2.
Randomized controlled trials
| Study | CT-STAT12 (2011) |
ACRIN13 (2012) |
ROMICAT II14 (2012) |
|||
|---|---|---|---|---|---|---|
| Population | 699 TIMI risk score 0–4 MI 0.9% |
1370 TIMI risk score 0–2 MI 1% |
985 Low–intermediate risk MI 2.5% |
|||
| Randomization | 1:1 | 2:1 | 1:1 | |||
| Control group | SPECT MPI | Usual care | Usual care | |||
| CTA | Controls | CTA | Controls | CTA | Controls | |
| ACS diagnosis | 1.1% | 2.4% | 1% | 1% | 9% | 6% |
| ED discharge | 50% | 23% | 47% | 12% | ||
| ICA rate | 8.0% | 7.4% | 5% | 4% | 12% | 8% |
| Revascularization | 4.3% | 2.7% | 3% | 1% | 6% | 4% |
| Time to diagnosis (median, range) | 2.9 h a (2.1–4.0) | 6.3 h (4–19) | ||||
| Length of stay (median, range) | 18.0 h (8–27) | 24.8 h (19–31) | 23.2 ha | 30.8 h | ||
| One month MACE | 0%a | 0% | 0.4% | 1.2% | ||
| Six months MACE | 0.8% | 0.4% | ||||
| Cost (US$) | 2137b | 3458 | 4026c | 3874 | ||
TIMI, thrombolysis in myocardial infarction.
Statistically significant results in bold.
aPrimary endpoint of the study.
bOnly ED costs.
cIndex hospitalization including angiograms and interventions.
The ACRIN trial demonstrated that CTA is safe in low-risk patients, with a 0% adverse event rate at 30 days (95% CI 0–0.57%, primary endpoint). Also in the ROMICAT II and CT-STAT trials, adverse event rates were low, both in the CTA strategy and standard care groups (Table 2). In total, these three trials composed of more than 3000 patients, and follow-up analysis demonstrated that based on the CTA results, not a single patient was discharged with a missed diagnosis of ACS, supporting the conclusion that coronary CTA guided management at the ED is safe.
Another goal of these trials was to demonstrate the efficiency of CTA compared with SOC. This was primarily tested in the ROMICAT II trial, which demonstrated a reduction in length of stay, hospital admissions, and ED cost, while overall hospital costs remained similar to SOC, driven by a higher rate of invasive angiography and revascularizations. Across the trials, patients randomized to CTA more often underwent cardiac catheterization (8.4 vs. 6.3%) and percutaneous coronary intervention (4.6 vs. 2.6%). Unfortunately, the trials were not powered to prove that improved sensitivity for the detection of obstructive CAD by CTA, with subsequently increased revascularization rates, also result in a better clinical outcome. Additionally, radiation exposure will be higher for CTA when SOC consists of exercise tests and stress echocardiography. In summary, these trials have established cardiac CT as a viable alternative to functional testing for triage of low-risk patients with acute chest pain.
Prediction of mid-term outcome
Several studies demonstrated that CTA findings of plaque, stenosis, and ventricular function accurately predict adverse cardiac events over the next 6 months to 2 years (Table 3).7,15–18 While patients without CAD remain virtually event-free, non-obstructive CAD is associated with a slight increase of risk, and those with obstructive CAD are at the highest risk.9
Table 3.
Mid-term outcome after cardiac computed tomography in acute chest pain
| Author/year | n | ACS risk | Follow-up | Event definition | CT criterion (% total cohort) | Events (%) |
|---|---|---|---|---|---|---|
| Rubinshtein et al. (2007)7 | 58 | Avg TIMI 1.3 | 1 year | Death, MI, revascularization | ED discharge (55%) | 0 |
| Hollander et al. (2009)15 | 588 | TIMI 0–1 | 1 year | Death, MI, revascularization | Stenosis <50% (82%) | 0.2 |
| ROMICAT I (2011)17 | 368 | Low risk | 2 years | Death, MI, revascularization | Normal (50%) | 0 |
| <50% stenosis (32%) | 4.6 | |||||
| >50% stenosis (19%) | 30.3 | |||||
| CT-STAT (2011)12 | 361 | Average TIMI 1 | 6 months | Death, MI, revascularization | (Nearly) normal (74%) | 0.8 |
| Singer et al. (2012)16 | 507 | Average TIMI 1 | 6 months | Death, revascularization | Stenosis <50% (96%) | 0 |
| Christiaens et al. (2012)18 | 175 | Low–intermediate risk | 6 months | MACE (non-specified) | Stenosis <50% (78%) | 0 |
Challenges to implementation of cardiac computed tomography in the emergency department
While the potential diagnostic value of cardiac CT in the ED seems evident, there are practical obstacles that interfere with widespread implementation. Computed tomography equipment with sufficient cardiac imaging capabilities (minimally a single-source 64-slice system), fully trained technologists, and experienced cardiac CT readers are essential. Not all patients are eligible for coronary CTA, including those with known CAD, cardiac arrhythmia, tachycardia, or severe obesity (typically BMI > 40 kg/m2). Computed tomography angiography is associated with risks due to radiation exposure, although doses have decreased substantially over the past decade. Use of iodine-containing contrast media may be contra-indicated in case of renal dysfunction or related allergies. The guidelines emphasize that the choice of test, whether CT or another modality, should be based on local expertise and individual characteristics that affect eligibility.4 More advanced CT technology, dual-source CT systems or wider detector arrays, can improve image quality in somewhat less suitable patients. Presently, few centres have sufficient experienced personnel to offer cardiac CT around the clock. Recently published guidelines on the practice of cardiac CT in the ED specify need for certification and maintenance of certification for imaging centres, interpreting physicians, and medical staff.19
Beyond the coronary artery lumen
Myocardial enhancement and stress perfusion imaging
Resting myocardial ischaemia or myocardial infarction can be identified on routine CTA datasets as myocardial hypo-enhancement (Figure 2). The presence of a resting myocardial enhancement defect on CTA has a sensitivity and specificity of around 90% to identify patients with a myocardial infarction.20,21 While chronic myocardial scar and acute hypo-perfusion both show lower contrast enhancement on CTA, chronic infarction can often be differentiated by wall thinning or lower attenuation values (<0 HU) as a result of fat tissue within the scar.22
Figure 2.
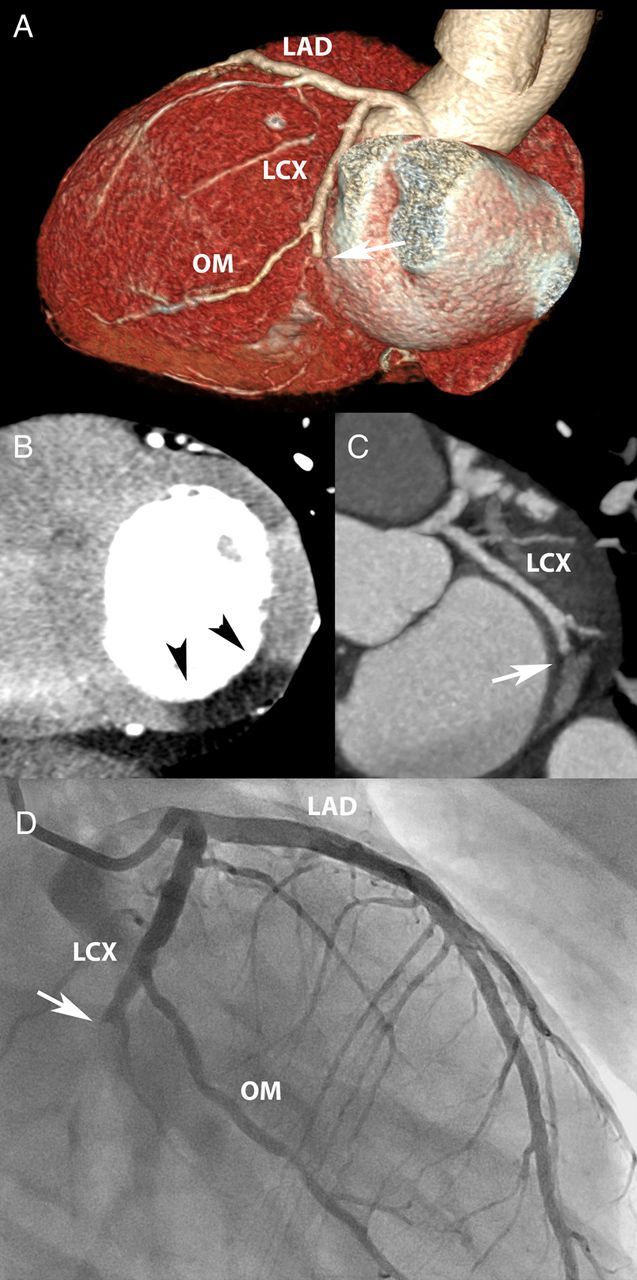
Myocardial infarction. A 48-year-old man with position dependent, burning chest pain, and gastro-intestinal complaints. The conventional 12-lead electrocardiogram showed non-specific STT-segment changes. The conventional troponin T was borderline abnormal (0.04 mg/L). Coronary CTA (A and B) revealed an occluded distal LCX (arrow), with transmural hypo-enhancement and normal thickness of the infero-lateral wall (C, arrow heads). A subsequent rise in cardiac biomarkers (max CK-MB 93 µg/L) indicated acute myocardial injury, and invasive angiography confirmed an LCX occlusion (D, arrow).
As current guidelines recommend revascularization be guided by objective evidence of ischaemia, additional information on the haemodynamic significance would benefit decision-making in patients with obstructive lesions on coronary CTA.4 Similar to other modalities, myocardial perfusion imaging can be performed using cardiac CT either in a single-phase mode for qualitative identification of ischaemic myocardium,23 or in a multi-phase mode for quantitative assessment of the myocardial blood flow (Figure 3).24 As for other methods of stress testing, stress CT myocardial perfusion imaging may be safer and logistically easier to perform at a later stage, after an ACS has been ruled out.
Figure 3.
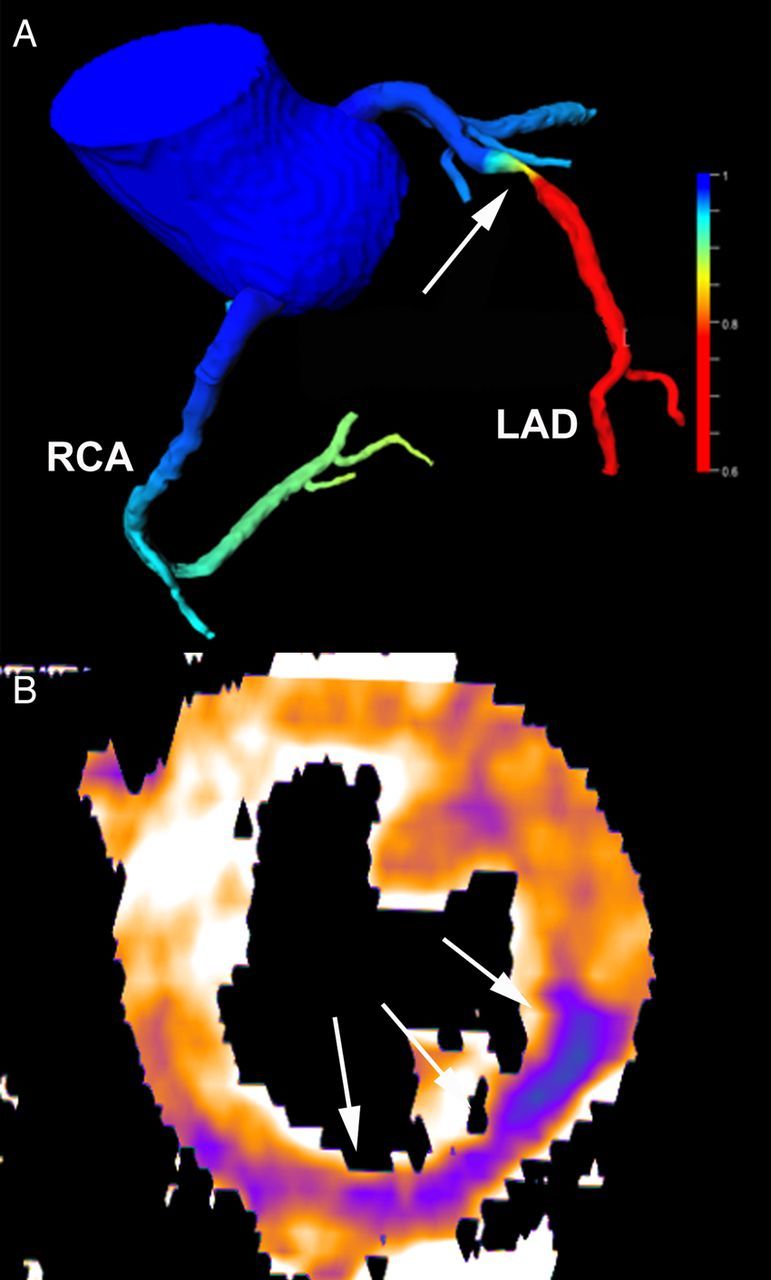
Assessment of haemodynamic relevance by computed tomography. Computed tomography angiography-derived fractional flow reserve (A) shows a haemodynamic significant (red colour) stenosis in the LAD (arrow) with a simulated FFR value of 0.72. Stress perfusion computed tomography (B) demonstrating low myocardial blood flow (during pharmacological hyperaemia) in the infero-lateral wall (arrows) caused by an RCA stenosis.
Coronary CT angiography-derived fractional flow reserve
The haemodynamic severity of CAD can also be determined by calculation of the fractional flow reserve (FFR) from CT angiograms using computational fluid dynamics.25 Computed tomography angiography-derived fractional flow reserve (CTA-FFR) could exclude haemodynamically significant CAD more confidently, and reduce the need for further functional testing or invasive angiography, but has not been investigated in patients with acute chest pain yet (Figure 3).26 While currently available CTA-FFR algorithms require processing on a powerful remote computer, point-of-care CTA-FFR with a processing time <1 h may become available in the near future.27
Calcium imaging
Calcium imaging in acute chest pain remains controversial. While the absence of calcium in low-risk acute chest pain indicates a low probability of ACS and good overall prognosis,28 a negative coronary calcium scan alone is generally not regarded as sufficient to reliably rule-out ACSs in patients with chest pain.9,11
High-risk plaque features
A unique feature of coronary CTA is the potential to non-invasively visualize and characterize coronary atherosclerotic plaque, both in stenotic and in non-obstructive lesions (Figure 4). Computed tomography angiography can classify plaque composition as calcified or non-calcified. More importantly, CT angiography can detect features associated with plaque instability, such as low attenuation (<30 HU), outward vessel remodelling, high total plaque burden, and spotty calcifications, with good correlation to intravascular imaging and histology.29 Initial data suggest that these CTA determined plaque features predict adverse events, independent of the angiographic stenosis severity.30 In a recent subanalysis of the ROMICAT II trial cohort, the presence of high-risk plaque features on coronary CTA increased the likelihood of ACS, independent of the angiographic CAD severity and clinical risk assessment.31 How these plaque features should be incorporated into individual clinical decisions requires further investigation.
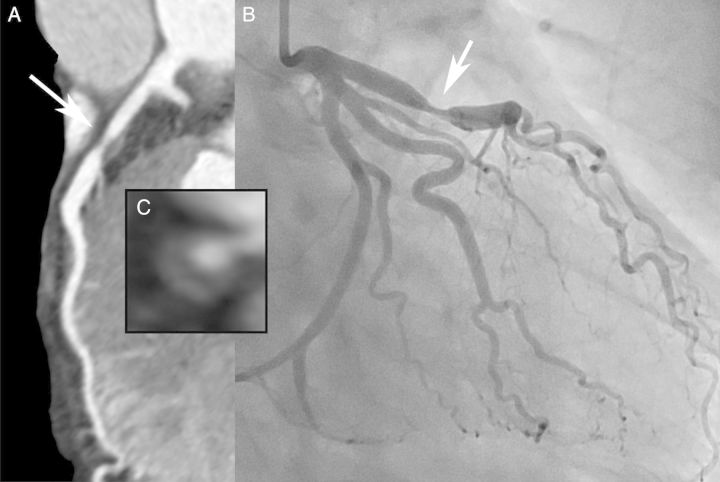
Figure 4.
Plaque imaging. In a 47-year-old man with unstable angina, cardiac computed tomography shows a plaque in the LAD with features associated with plaque instability, including severe stenosis, non-calcified plaque with a low-attenuation core (napkin ring sign) and outward vessel remodelling (A,C). Invasive angiography confirms the stenosis in the LAD (B).
Non-coronary cardiovascular emergencies
Other life-threatening conditions that need consideration in acute chest pain include pulmonary embolism, aortic dissection, pneumothorax, and pericarditis, all of which may be diagnosed by CT. An ECG-synchronized, thoracic CT scan with pulmonary and aortic contrast enhancement can rule out all of these conditions. However, this triple-rule-out protocol is not recommended for routine use in suspected ACS because of the low incidence of the non-coronary emergencies, the higher scan complexity, and the higher radiation and contrast medium dose.
Cardiac CT in the era of highly sensitive troponin assays
High-sensitive troponins that allow more sensitive and earlier detection of myocardial infarction,32 have profoundly affected the daily management of patients with acute chest pain in Europe, while none of these assays have gained approval in the USA yet. High-sensitive troponins can improve triage using an ‘accelerated diagnostic protocol’, which combines a low ACS risk score with short-interval high-sensitive-troponin measurements to identify patients without ACS.33 A challenge arises, however, from the fact that more patients will have a measurable high-sensitive troponin in the absence of acute coronary disease. The application of cardiac CT may develop as an effective means to rule-out CAD in patients with minor high-sensitive-troponin elevations (Figure 5). With the availability of high-sensitive troponins to rule-out myocardial infarction, and the development of functional CT applications, the role of cardiac CT may in the future shift from a rule-out test for low-risk patients, to a more comprehensive, and potentially therapy guiding modality.
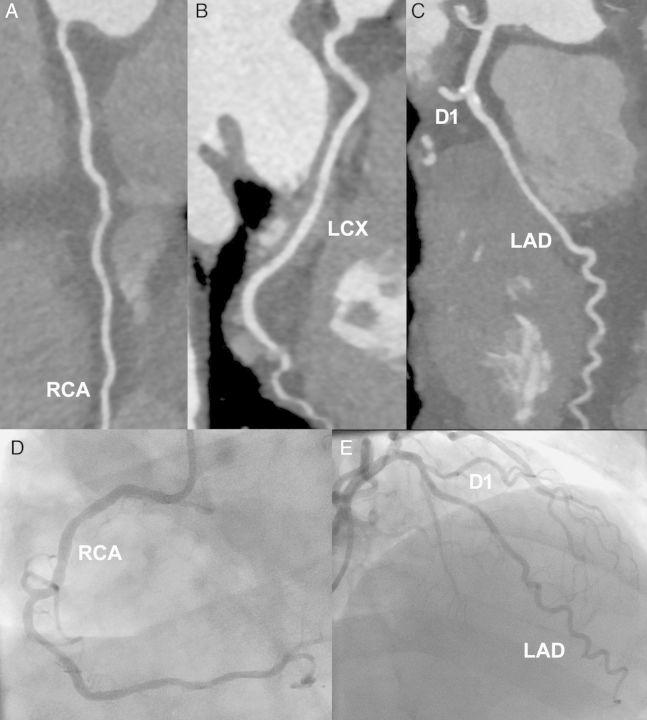
Figure 5.
Positive cardiac markers. A 69-year-old man presenting after a prolonged episode of crushing chest pain accompanied by shortness of breath. The electrocardiogram showed negative T waves over the lateral leads, and initial high-sensitive troponins were low-positive (24 ng/L). Coronary CTA (A–C) demonstrated non-obstructive plaque, particularly around the LAD-diagonal bifurcation. Invasive angiography (D and E), performed after a 25% rise in troponins, confirmed the computed tomography results.
Current recommendations and future expectations of clinical use
Several guideline documents discuss the role of cardiac CT in the management of acute chest pain. The 2010 Cardiac CT Appropriateness Criteria, which included representation by the ACC, AHA, and SCCT, regarded the use of cardiac CT appropriate in low to intermediate risk acute chest pain with non-diagnostic ECG and serum biomarkers.34 The 2011 ESC Non-STE-ACS management guidelines suggest cardiac CT to be considered in patients with non-conclusive cardiac markers.35 Based on the ACRIN and ROMICAT II results, published in 2012, it is expected that upcoming guidelines will provide more specific recommendations on the triage by cardiac CT in low-risk patients. It is important to emphasize that information from cardiac CT needs to be interpreted in the context of all clinical and diagnostic information available. In the future, the diagnostic value of cardiac CT may be further strengthened by incorporation of morphologic plaque assessment and applications to assess the functional relevance of CAD on CTA. Additionally, high-sensitive-troponin assays may allow safe exclusion of ACS without the need for further diagnostic testing in a proportion of patients. Hence, early triage by cardiac CT may re-focus towards those with low elevations in high-sensitive troponins.
Conclusion
At the present time, early triage by coronary CTA is a viable, reliable, and potentially more accurate alternative to functional testing in low-risk patients with acute chest pain. Coronary CTA requires high-end equipment, sufficient expertise in data acquisition and image interpretation, and appropriate patient selection. This currently limits broad implementation of cardiac CT in emergency room setting. With the introduction of high-sensitive troponins into the early triage of acute chest pain, modifications in the use of cardiac CT as well as other diagnostic testing can be expected. Incorporation of morphological plaque characteristics, as well as the development of CT applications to assess the functional severity of CAD, could add diagnostic value to cardiac CT examinations in the ED, but requires further investigation.
Conflict of interest: K.N. has received institutional research support from Siemens Medical Solutions, Bayer Healthcare and GE Healthcare.
References
- 1.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163–1170. [DOI] [PubMed] [Google Scholar]
- 2.Kontos MC, Jesse RL. Evaluation of the emergency department chest pain patient. Am J Cardiol 2000;85:32–39. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Zhou T, Zhang R, Xu L, Peng Z, Ding J, Wang S, Li M, Sun G. Meta-analysis: diagnostic accuracy of coronary CT angiography with prospective ECG gating based on step-and-shoot, Flash and volume modes for detection of coronary artery disease. Eur Radiol 2014;24:2345–2352. [DOI] [PubMed] [Google Scholar]
- 4.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 5.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol 2011;57:1237–1247. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs TA, Stehli J, Bull S, Dougoud S, Clerc OF, Herzog BA, Buechel RR, Gaemperli O, Kaufmann PA. Coronary computed tomography angiography with model-based iterative reconstruction using a radiation exposure similar to chest X-ray examination. Eur Heart J 2014;35:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinshtein R, Halon DA, Gaspar T, Schliamser JE, Yaniv N, Ammar R, Flugelman MY, Peled N, Lewis BS. Usefulness of 64-slice multidetector computed tomography in diagnostic triage of patients with chest pain and negative or nondiagnostic exercise treadmill test result. Am J Cardiol 2007;99:925–929. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher MJ, Ross MA, Raff GL, Goldstein JA, O'Neill WW, O'Neil B. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med 2007;49:125–136. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT trial. J Am Coll Cardiol 2009;53:1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen M, Ginns J, Seneviratne S, Slaughter R, Premaranthe M, Samardhi H, Harker J, Lai T, Walters DL, Bett N. The value of dual-source 64-slice CT coronary angiography in the assessment of patients presenting to an acute chest pain service. Heart Lung Circ 2010;19:213–218. [DOI] [PubMed] [Google Scholar]
- 11.Dedic A, Ten Kate GJ, Neefjes LA, Rossi A, Dharampal A, Rood PP, Galema TW, Schultz C, Ouhlous M, Moelker A, de Feyter PJ, Nieman K. Coronary CT angiography outperforms calcium imaging in the triage of acute coronary syndrome. Int J Cardiol 2013;167:1597–1602. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O'Neil BJ, Shaw LJ, Shen MY, Valeti US, Raff GL. The CT-STAT trial. J Am Coll Cardiol 2011;58:1414–1422. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE; ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–1403. [DOI] [PubMed] [Google Scholar]
- 15.Hollander JE, Chang AM, Shofer FS, Collin MJ, Walsh KM, McCusker CM, Baxt WG, Litt HI. One-year outcomes following coronary computerized tomographic angiography for evaluation of emergency department patients with potential acute coronary syndrome. Acad Emerg Med 2009;16:693–698. [DOI] [PubMed] [Google Scholar]
- 16.Singer AJ, Domingo A, Thode HC, Jr, Daubert M, Vainrib AF, Ferraro S, Minton A, Poon A, Henry MC, Poon M. Utilization of coronary computed tomography angiography for exclusion of coronary artery disease in ED patients with low- to intermediate-risk chest pain: a 1-year experience. Am J Emerg Med 2012;30:1706–1711. [DOI] [PubMed] [Google Scholar]
- 17.Schlett CL, Banerji D, Siegel E, Bamberg F, Lehman SJ, Ferencik M, Brady TJ, Nagurney JT, Hoffmann U, Truong QA. Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMICAT trial. JACC Cardiovasc Imaging 2011;4:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christiaens L, Duchat F, Boudiaf M, Tasu JP, Fargeaudou Y, Ledref O, Soyer P, Sirol M. Impact of 64-slice coronary CT on the management of patients presenting with acute chest pain: results of a prospective two-centre study. Eur Radiol 2012;22:1050–1058. [DOI] [PubMed] [Google Scholar]
- 19.Raff GL, Chinnaiyan KM, Cury RC, Garcia MT, Hecht HS, Hollander JE, O'Neil B, Taylor AJ, Hoffmann U. SCCT guidelines on the use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department. J Cardiovasc Comput Tomogr 2014;8:254–271. [DOI] [PubMed] [Google Scholar]
- 20.Schepis T, Achenbach S, Marwan M, Muschiol G, Ropers D, Daniel WG, Pflederer T. Prevalence of first-pass myocardial perfusion defects detected by contrast-enhanced dual-source CT in patients with non-ST segment elevation acute coronary syndromes. Eur Radiol 2010;20:1607–1614. [DOI] [PubMed] [Google Scholar]
- 21.Feuchtner GM, Plank F, Pena C, Battle J, Min J, Leipsic J, Labounty T, Janowitz W, Katzen B, Ziffer J, Cury RC. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart 2012;98:1510–1517. [DOI] [PubMed] [Google Scholar]
- 22.Nieman K, Cury RC, Ferencik M, Nomura CH, Abbara S, Hoffmann U, Gold HK, Jang IK, Brady TJ. Differentiation of recent and chronic myocardial infarction by cardiac computed tomography. Am J Cardiol 2006;98:303–308. [DOI] [PubMed] [Google Scholar]
- 23.Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM, Niinuma H, Yoshioka K, Kitagawa K, Nakamori S, Laham R, Vavere AL, Cerci RJ, Mehra VC, Nomura C, Kofoed KF, Jinzaki M, Kuribayashi S, de Roos A, Laule M, Tan SY, Hoe J, Paul N, Rybicki FJ, Brinker JA, Arai AE, Cox C, Clouse ME, Di Carli MF, Lima JA. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 2014;35:1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamberg F, Hinkel R, Schwarz F, Sandner TA, Baloch E, Marcus R, Becker A, Kupatt C, Wintersperger BJ, Johnson TR, Theisen D, Klotz E, Reiser MF, Nikolaou K. Accuracy of dynamic computed tomography adenosine stress myocardial perfusion imaging in estimating myocardial blood flow at various degrees of coronary artery stenosis using a porcine animal model. Invest Radiol 2012;47:71–77. [DOI] [PubMed] [Google Scholar]
- 25.Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–2241. [DOI] [PubMed] [Google Scholar]
- 26.Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park SJ, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S; NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145–1155. [DOI] [PubMed] [Google Scholar]
- 27.Coenen A, Lubbers MM, Kurata A, Kono A, Dedic A, Chelu RG, Dijkshoorn ML, Gijsen FJ, Ouhlous M, van Geuns RJ, Nieman K. Fractional flow reserve computed from noninvasive CT angiography data: diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology 2014. Oct 13:140992. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Laudon DA, Vukov LF, Breen JF, Rumberger JA, Wollan PC, Sheedy PF., II Use of electron-beam computed tomography in the evaluation of chest pain patients in the emergency department. Ann Emerg Med 1999;33:15–21. [DOI] [PubMed] [Google Scholar]
- 29.Achenbach S, Ropers D, Hoffmann U, MacNeill B, Baum U, Pohle K, Brady TJ, Pomerantsev E, Ludwig J, Flachskampf FA, Wicky S, Jang IK, Daniel WG. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol 2004;43:842–847. [DOI] [PubMed] [Google Scholar]
- 30.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319–326. [DOI] [PubMed] [Google Scholar]
- 31.Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858–867. [DOI] [PubMed] [Google Scholar]
- 33.Than M, Aldous S, Lord SJ, Goodacre S, Frampton CM, Troughton R, George P, Florkowski CM, Ardagh M, Smyth D, Jardine DL, Peacock WF, Young J, Hamilton G, Deely JM, Cullen L, Richards AM. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med 2014;174:51–58. [DOI] [PubMed] [Google Scholar]
- 34.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Cardiovasc Comput Tomogr 2010;4:407.e1–33. [DOI] [PubMed] [Google Scholar]
- 35.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]