Researchers identify regions of tissue-specific γ subunits that are responsible for regulating the activity of calcium-activated potassium channels.
Abstract
The large-conductance, calcium-activated potassium (BK) channels consist of the pore-forming, voltage- and Ca2+-sensing α subunits (BKα) and the tissue-specific auxiliary β and γ subunits. The BK channel γ1 subunit is a leucine-rich repeat (LRR)–containing membrane protein that potently facilitates BK channel activation in many tissues and cell types through a vast shift in the voltage dependence of channel activation by ∼140 mV in the hyperpolarizing direction. In this study, we found that the single transmembrane (TM) segment together with its flanking charged residues is sufficient to fully modulate BK channels upon its transplantation into the structurally unrelated β1 subunit. We identified Phe273 and its neighboring residues in the middle of the TM segment and a minimum of three intracellular juxtamembrane Arg residues as important for the γ1 subunit’s modulatory function and observed functional coupling between residues of these two locations. We concluded that the TM segment is a key molecular determinant for channel association and modulation and that the intracellular positively charged cluster is involved mainly in channel association, likely through its TM-anchoring effect. Our findings provide insights into the structure–function relationship of the γ1 subunit in understanding its potent modulatory effects on BK channels.
INTRODUCTION
Large-conductance, calcium- and voltage-activated potassium (BK) channels are widely expressed and play various physiological roles, for example, neuronal firing and neurotransmitter release (Gribkoff et al., 2001) and frequency tuning of auditory hair cells (Ramanathan et al., 1999). The BK channel has a large single-channel conductance and can be activated by both membrane depolarization and elevation of intracellular free calcium ([Ca2+]i). BK channels consist of homotetrameric pore-forming voltage- and calcium-sensing α subunits (BKα) and regulatory tissue-specific auxiliary β and/or γ subunits. The γ subunits (BKγ) exhibit tissue-specific mRNA expression and are thought to modulate BK channel function across these diverse tissues (Yan and Aldrich, 2012). Studies so far have shown that the γ1 subunit regulates BK channels in prostate cancer cells (Gessner et al., 2006; Yan and Aldrich, 2010), salivary gland cells (Almassy and Begenisich, 2012), airway epithelial cells (Manzanares et al., 2014, 2015), and probably arterial smooth muscle cells (Evanson et al., 2014).
BKγ proteins, as ion channel modulators, display some interesting structural and functional features. They are a group of four leucine-rich repeat (LRR)–containing membrane proteins, γ1 (LRRC26), γ2 (LRRC52), γ3 (LRRC55), and γ4 (LRRC38). The four γ subunits (∼35 kD in size) display distinct capabilities in shifting the BK channel’s voltage dependence of activation in the hyperpolarizing direction over an exceptionally large range by ∼145 mV (γ1), 100 mV (γ2), 50 mV (γ3), and 20 mV (γ4) in the absence of calcium (Yan and Aldrich, 2010, 2012). They are structurally distinct from the double membrane–spanning BK channel β subunits and other known ion channel modulatory proteins by possessing an N-terminal signal peptide, an extracellular LRR domain, a single transmembrane (TM) segment, and a short intracellular C terminus (Yan and Aldrich, 2010, 2012). The BK channel γ and β subunits can coassemble in the same channel complex and independently regulate channel gating (Gonzalez-Perez et al., 2015). β subunits have complex effects on various aspects of BK channel gating (Wallner et al., 1999; Brenner et al., 2000; Meera et al., 2000; Xia et al., 2000; Zeng et al., 2003; Savalli et al., 2007; Contreras et al., 2012; Sun et al., 2012); however, the γ1 subunit has a remarkably simple mechanism of action (Yan and Aldrich, 2010; Zhang and Yan, 2014). An analysis of the effects of γ1 on different BK channel gating properties within the framework of an allosteric HA model (Horrigan and Aldrich, 2002) suggested that its main effect was to enhance the allosteric coupling factor between voltage sensors and the channel pore by ∼20-fold (Yan and Aldrich, 2010). The γ1 subunit also exhibits an “all or none” regulation of BK channels upon variation of the molar ratio of injected BKα/γ1 RNA in Xenopus oocytes (Gonzalez-Perez et al., 2014), an effect that is fundamentally different from that of β subunits, which regulate the voltage dependence of BK channel activation in a titration-dependent manner (Wang et al., 2002).
To understand the molecular mechanisms of BK channel regulation by auxiliary γ subunits, it is critical to identify key structural elements underlying their channel-modulatory functions. By swapping structural elements among γ subunits and by mutations, we recently found that the differences in the different γ subunit–induced shifts of the BK channel V1/2 are determined mainly by their single TM segments for an approximately −100-mV shift in V1/2, in which the γ1 and γ2 TMs produced low V1/2 BK channels, whereas the γ3 and γ4 TM domains all resulted in high V1/2 channels (Li et al., 2015). We also found that their intracellular C-tails, particularly the juxtamembrane positively charged residue cluster regions, further adjust the modulatory functions of the four γ subunits by conferring on BK channels an additional approximately −40- to −50-mV shift in V1/2 from the γ1 and γ3 C-tails (Li et al., 2015). In the current study, we investigated in detail the structure and function in BK channel modulation of the γ1 subunit’s peptide region (∼40 amino acids) encompassing the single TM segment and the adjacent poly-Arg cluster. We demonstrated that this peptide region, independent of the N-terminal LRR domain and the rest of the C-terminal tail, is sufficient to fully modulate BK channels. We identified key amino acid residues in the single TM segment and the positively charged cluster essential for the BKγ1 modulatory function and observed structural and functional coupling between residues of these two locations. Our findings provide insights into the structure–function relationship of the γ1 subunit concerning its potent activating effect on BK channels.
MATERIALS AND METHODS
Expression of BKα and BKγ proteins in HEK-293 cells
Recombinant cDNA constructs of human BKα (hSlo), γ1 (LRRC26), γ2 (LRRC52), γ3 (LRRC55), and γ4 (LRRC38) subunits were used for heterologous expression experiments in HEK-293 cells. HEK-293 cells (ATCC) were transfected with plasmids using Lipofectamine 2000 (Invitrogen) and subjected to electrophysiological assays 16–72 h after transfection. Synthetic cDNA sequences of chimeric BKγ or BK β1/γ1 subunits were subcloned into the mammalian expression vector of pCDNA6 with V5 tags attached at their C termini. As previously described (Yan and Aldrich, 2010, 2012; Li et al., 2015), BKα–γ fusion cDNA constructs, which encode precursor fusion proteins of human BKα on the N-terminal side and BKγ proteins on the C-terminal side, were generated with the pCDNA6 vector and used to facilitate the cotranslational assembly of BKα–γ protein complexes after endogenous cleavage by peptidases at the linker (BKγ signal peptide) region in the mature proteins. This previously established cotranslational assembly strategy produced reproducible results comparable with those of other strategies that generated overexpression of BKγ relative to BKα in a single HEK-293 cell using either an internal ribosome entry site (IRES)–based single bicistronic expression method or the BKα stable cell line method (Yan and Aldrich, 2010, 2012). For the indicated experiments involving overexpression of BKγ relative to BKα, HEK-293 cells were cotransfected with the BKα–γ fusion cDNA construct and an additional pCDNA6 plasmid encoding the γ subunit alone at a ratio of 1:1.5 in plasmid DNA molecules.
Electrophysiology
To record the BK channel currents, we used patch-clamp recording in excised inside-out patches of HEK-293 cells with symmetric internal and external solutions of 136 mM KMeSO3, 4 mM KCl, and 20 mM HEPES, pH 7.20. The external solution was supplemented with 2 mM MgCl2, and the internal solution was supplemented with 5 mM HEDTA without Ca2+ to create a virtually Ca2+-free solution. Steady-state activation was expressed as the normalized conductance (G/Gmax) calculated from the relative amplitude of the tail currents (deactivation at −120 mV). The voltage of half-maximal activation (V1/2) and the equivalent gating charge (z) were obtained by fitting the relations of G/Gmax versus voltage with the single Boltzmann function G/Gmax = 1/(1 + e−zF(V−V1/2)/RT) or with the double Boltzmann function G/Gmax = a/(1 + e−zaF(V−Va1/2)/RT) + (1 − a)/(1 + e−zbF(V−Vb1/2)/RT). Experimental values are reported as means ± SEM.
Immunoprecipitation and immunoblotting
The BKα–γ1 complex was solubilized from HEK-293 cells with 1% n-dodecyl-β-d-maltoside in TBS buffer (50 mM Tris and 150 mM NaCl, pH 7.6). After centrifugation at 17,000 g for 10 min, the solubilized BKα–γ1 complex in the supernatant was incubated with immobilized mouse monoclonal anti-BKα antibody (L6/60; NeuroMab) for 2 h, and the immunoprecipitated channel complex was washed three times (10 min each time) and then eluted from beads with Laemmli sample buffer. Protease inhibitor cocktail (Roche) was used throughout the procedure. Immobilization of antibody was achieved by covalently cross-linking to protein-A/G agarose beads with bis(sulfosuccinimidyl)suberate (Thermo Fisher Scientific) in a procedure following the manufacturer’s instruction. The eluted proteins were separated on 40–20% gradient SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes. Immunoblotting was performed with mouse monoclonal anti-V5 antibody to detect the V5-tagged BKα and γ1 subunits.
RESULTS
The TM segment and its flanking charged residues are transplantable molecular determinants of the γ1 subunit for BK channel modulation
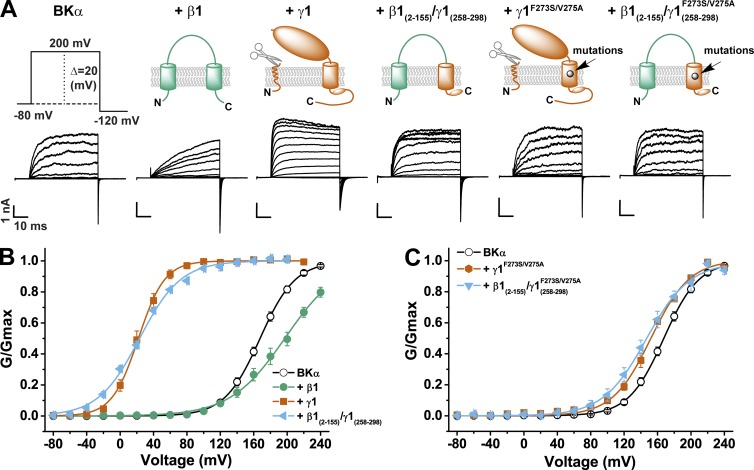
To determine whether the single TM segment and its flanking charged residues of the γ1 subunit are sufficient to modulate BK channels, we used this peptide sequence (residues 258–298) to replace the mouse BK channel β1 subunit’s second TM segment and C terminus (residues 156–191). We used the resulting β1(2–155)/γ1(258–298) chimeric construct as a tool to facilitate proper insertion and orientation in the membrane for this γ1 peptide region. Because there is no amino acid sequence similarity between the extracellular domains of the BK channel γ and β subunits, we expected no direct contribution of the β1 subunit’s first TM segment and extracellular domain on the fused γ1 peptide’s modulatory function. The BK channel γ1 and β1 subunits are distinct in their modulatory effects on BK channels. Similar to the previous observation that the β1 subunit reduced BK channel activation at low [Ca2+]i (Orio et al., 2006; Wang and Brenner, 2006), the β1 subunit caused a shift in V1/2 to the depolarizing direction, a decrease in the conductance-voltage (G-V) relationship slope, a great deceleration in channel activation, and a moderate delay in channel closure in the virtual absence of calcium (Fig. 1, A and B). In contrast, the γ1 subunit mainly caused a large shift in the G-V relationship to the hyperpolarizing direction, accompanied by acceleration in activation kinetics and deceleration in deactivation kinetics (Fig. 1, A and B). The β1(2–155)/γ1(258–298) chimeric protein overall displayed similar function of the γ1 subunit with nearly no noticeable contribution in modulatory function from the β1 subunit except that the G-V relationship became shallower than that with the γ1 wild type (Fig. 1, A and B; and Table 1). It conferred on BK channels accelerated activation kinetics and a large shift in V1/2 to 23 ± 2 mV that were very similar to the effects of the γ1 subunit. The F273S/V275A mutation in the γ1 TM segment inactivated most modulatory function of the γ1 subunit with a resultant V1/2 of 152 ± 2 mV close to that of BKα alone (V1/2 = 167 ± 2 mV; Fig. 1, A and C; and Table 1). The F273S/V275A mutation similarly eliminated most of the modulatory effect of the β1(2–155)/γ1(258–298) chimeric protein with a resultant V1/2 of 149 ± 6 mV close to that of BKα alone (Fig. 1, A and C; and Table 1), suggesting little contribution from the β1(2–155) part on the modulatory effects of the β1(2–155)/γ1(258–298) chimeric protein. These results indicate that in the absence of an LRR domain and the rest of the γ1 C-tail region, the γ1 single TM segment and its neighboring charged residues are transplantable molecular determinants that confer on the acceptor the full capacity of the γ1 subunit in BK channel modulation.
Figure 1.
Full modulation of BK channels by the BKγ1’s TM segment and neighboring positively charged cluster transplanted into the structurally unrelated β1 subunit. (A) Representative traces of recorded BK channel currents in response to the depolarization of membrane potential from −80 to 200 mV in the absence and presence of the β1, γ1, and β1/γ1 chimeras. The voltage pulse protocol used to obtain the current traces is shown on the left side, and the schematic structures in the ER membrane are shown on top. (B and C) Voltage dependence of BK channel activation (plotted from tail currents at −120 mV) in the absence and presence of wild-type β1, γ1, and β1/γ1 chimeric subunits (B) and in the absence and presence of γ1 and β1/γ1 chimeric subunits with mutation F273S/V275A in the middle of the γ1 TM segment (C). All BK channel currents were recorded in the virtual absence of [Ca2+]i and plotted as the normalized conductance (G/Gmax) against different membrane voltages. Error bars represent ±SEM.
Table 1.
Boltzmann fit parameters of the voltage-dependent BK channel activation in the presence of auxiliary BKγ wild types, chimeras, and mutants (γ1 or otherwise indicated in the names for other γ subunits) in the virtual absence of intracellular Ca2+
| Expression | Boltzmann fit parameters | ||
| V1/2 | z | n | |
| mv | |||
| BKα alone | 167 ± 2 | 1.26 ± 0.07 | 8 |
| +γ1 | 22 ± 4 | 1.75 ± 0.09 | 7 |
| +γ2 | 61 ± 3 | 1.17 ± 0.07 | 8 |
| +γ3 | 115 ± 2 | 1.36 ± 0.05 | 6 |
| +γ4 | 154 ± 3 | 1.27 ± 0.07 | 7 |
| +β1 | 197 ± 6 | 0.80 ± 0.02 | 3 |
| +β1(2–155)/ γ1(258–298) | 23 ± 2 | 1.04 ± 0.05 | 7 |
| +β1(2–155)/ γ1(258–298)F273S/V275A | 149 ± 6 | 0.96 ± 0.09 | 6 |
| +Δ262–265 | 152 ± 2 | 1.36 ± 0.01 | 3 |
| +Δ266–269 | 159 ± 6 | 1.38 ± 0.10 | 4 |
| +Δ270–273 | 157 ± 7 | 1.32 ± 0.09 | 7 |
| +Δ274–279 | 163 ± 6 | 1.23 ± 0.09 | 5 |
| +Δ280–284 | 166 ± 7 | 1.13 ± 0.08 | 6 |
| +Δ285–289 | 27 ± 3 | 1.12 ± 0.10 | 4 |
| +γ1/γ2-tail | 143 ± 3 | 1.26 ± 0.05 | 4 |
| +γ1/γ2-linker&tail | 60 ± 5 | 1.09 ± 0.07 | 3 |
| +γ1/γ4-tail | 147 ± 3 | 1.23 ± 0.08 | 5 |
| +γ1/γ4-linker&tail | 97 ± 3 | 1.03 ± 0.03 | 5 |
| +γ1/γ4-TMa | 69 ± 3 | 1.33 ± 0.05 | 3 |
| +γ1/γ4-TMb | 164 ± 5 | 1.29 ± 0.03 | 3 |
| +γ1/γ4-TMc | 59 ± 3 | 1.11 ± 0.09 | 4 |
| +γ1/γ4-TMd | 67 ± 4 | 1.22 ± 0.05 | 9 |
| +γ1/γ4-TMF273 | 103 ± 3 | 1.12 ± 0.07 | 5 |
| +γ1/γ4-TM | 155 ± 4 | 1.30 ± 0.03 | 5 |
| +γ2-F256S | 165 ± 4 | 1.26 ± 0.05 | 9 |
| +γ2-F256Sa | 159 ± 3 | 1.31 ± 0.07 | 5 |
| +γ3-S282F | 100 ± 3 | 1.54 ± 0.10 | 4 |
| +γ4-S259F | 125 ± 2 | 1.23 ± 0.09 | 4 |
| +P270F | 16 ± 2 (83%)b120 ± 9 (17%)c | 1.51 ± 0.061.04 ± 0.20c | 10 |
| +P270Fa | 22 ± 2 (91%)138 ± 14 (9%)c | 1.63 ± 0.081.22 ± 0.79c | 9 |
| +A271V | 1 ± 3 | 1.36 ± 0.16 | 3 |
| +S272V | 67 ± 2 | 1.07 ± 0.04 | 3 |
| +F273S | 18 ± 1 (79%)126 ± 8 (21%)c | 1.55 ± 0.070.99 ± 0.16c | 13 |
| +F273Sa | 20 ± 1 (91%)117 ± 17 (9%)c | 1.80 ± 0.111.36 ± 1.0c | 7 |
| +L274A | 25 ± 4 | 1.43 ± 0.13 | 3 |
| +V275A | 26 ± 2 | 1.60 ± 0.13 | 3 |
| +P270V/F273S | 155 ± 2 | 1.48 ± 0.14 | 5 |
| +P270V/F273Sa | 150 ± 3 | 1.27 ± 0.05 | 8 |
| +S272V/F273S | 65 ± 3 (80%)163 ± 3 (20%)c | 1.26 ± 0.11.75 ± 0.35c | 5 |
| +F273S/L274A | 157 ± 2 | 1.25 ± 0.19 | 3 |
| +F273S/L274Aa | 152 ± 2 | 1.31 ± 0.06 | 5 |
| +F273S/V275A | 152 ± 2 | 1.14 ± 0.07 | 4 |
| +F273S/V275Aa | 145 ± 3 | 1.33 ± 0.07 | 6 |
| +Δ3R | 40 ± 1 | 1.34 ± 0.05 | 4 |
| +Δ3R/R293Q | 138 ± 2 | 1.35 ± 0.08 | 4 |
| +Δ3R/R293Qa | 12 ± 3 | 1.30 ± 0.10 | 2 |
| 23 ± 3 (46%)139 ± 4 (54%) | 1.55 ± 0.140.88 ± 0.11 | 5 | |
| 152 | 1.61 | 1 | |
| +Δ4R | 146 ± 4 | 1.15 ± 0.05 | 11 |
| +Δ4Ra | 48 ± 2 | 1.70 ± 0.11 | 4 |
| 48 ± 3 (32%)c141 ± 5 (68%) | 2.02 ± 0.39c0.93 ± 0.08 | 4 | |
| 162 ± 6 | 1.13 ± 0.18 | 2 | |
| +Δ3R/R293K | 35 ± 2 (67%)144 ± 4 (33%)c | 1.19 ± 0.061.16 ± 0.12c | 6 |
| 33 ± 11 (11%)c145 ± 2 (89%) | 1.04 ± 0.22c1.12 ± 0.02 | 4 | |
| +Δ3R/R291Q | 49 ± 3 (79%)147 ± 9 (19%)c | 1.22 ± 0.111.58 ± 0.54c | 3 |
| 53 (47%)154 (53%) | 1.071.26 | 1 | |
| 154 ± 7 | 1.31 ± 0.07 | 3 | |
| +Δ3R/R291Qa | 47 ± 2 (69%)159 ± 4 (31%)c | 1.41 ± 0.101.68 ± 0.27c | 3 |
| 32 ± 34 (10%)c138 ± 5 (90%) | 1.01 ± 0.55c0.89 ± 0.10 | 5 | |
| 141 ± 6 | 1.14 ± 0.0.14 | 4 | |
| +Δ3R/R291K | 24 ± 3 | 1.60 ± 0.10 | 3 |
| 38 ± 8 (69%)134 ± 23 (31%)c | 1.21 ± 0.200.98 ± 0.37c | 3 | |
| 33 ± 3 (27%)c158 ± 2 (73%) | 1.79 ± 0.28c1.17 ± 0.09 | 2 | |
| +Δ3R/R298Q | 37 ± 2 | 1.14 ± 0.10 | 2 |
| 53 ± 3 (79%)137 ± 8 (21%)c | 1.62 ± 0.141.93 ± 0.93c | 5 | |
| 57 ± 5 (43%)142 ± 5 (57%) | 1.35 ± 0.171.16 ± 0.18 | 5 | |
| 153 ± 2 | 0.94 ± 0.04 | 4 | |
| +Δ3R/R298Qa | 49 ± 2 (82%)129 ± 13 (18%)c | 1.87 ± 0.091.01 ± 0.34c | 9 |
| 48 ± 5 (26%)c138 ± 3 (74%) | 1.64 ± 0.28c1.15 ± 0.07 | 3 | |
| 175 | 1.00 | 1 | |
| +Δ3R/R298K | 21 ± 4 | 1.35 ± 0.08 | 5 |
| 30 ± 3 (48%)123 ± 14 (54%) | 1.70 ± 0.320.81 ± 0.30 | 5 | |
| 175 | 1.00 | 1 | |
| +F273S/Δ3R | 163 ± 7 | 1.13 ± 0.04 | 5 |
| +F273S/Δ3Ra | 157 ± 4 | 1.48 ± 0.07 | 5 |
| +P270F/Δ4R | 65 ± 2 | 1.07 ± 0.04 | 5 |
| ΔtailN291–298 | 168 ± 3 | 1.22 ± 0.11 | 5 |
| ΔtailN291–298a | 172 ± 5 | 1.34 ± 0.05 | 4 |
n values are the number of recorded excised inside-out patches from different HEK-293 cells.
The indicated γ subunit mutant was overexpressed relative to BKα.
The indicated percentage in parentheses here and elsewhere refers to the portion of the channels’ subpopulation that was obtained from a double Boltzmann function fit.
Because of the difficulty in obtaining reliable parameter values from a double Boltzmann function fit for the minor portion (e.g., ≤35%), the estimated values of the V1/2 and errors provided here are considered less reliable and used for references only.
Delineation of the TM segment
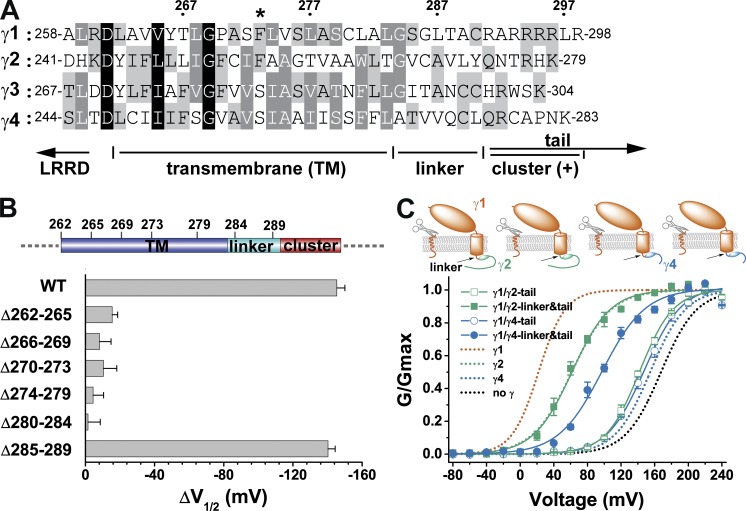
In membrane proteins, the TM segments generally exist as TM α-helices that are formed by a hydrophobic region of ∼20 amino acids in length and flanked by charged residues. Three different TM domain prediction programs, HMMTOP (Tusnády and Simon, 2001), TMHMM (Krogh et al., 2001), and TopPred (Claros and von Heijne, 1994), consistently predicted a TM segment of ∼22 amino acids in length that starts after the extracellular charged residue and stops before Gly267, Gly293, and Ala70 for the γ2, γ3, and γ4 subunits, respectively. However, the predicted γ1 TM segment is shifted to the C-terminal side to include Gly284 and five less-conserved residues (285SGLTA289) immediately in front of the poly-Arg clusters. To roughly delineate the N- and C-terminal borders of the γ1 TM segment experimentally, we performed segmental deletion to delete different parts (four or five residues together) in the region between the extracellular and intracellular charged residues (Fig. 2, A and B). We expected that most residues in the TM segment would be undeletable in function to maintain proper length and hydrophobicity of the TM segment. We found that deletion of the 285SGLTA289 segment had little effect on the BKγ1 function, whereas segmental (four to six residues together) deletions in residues 262–284 all resulted in a loss of the γ1 modulatory function (Fig. 2 B and Table 1). Therefore, we consider the residues of 284GSGLTA289 to be an intracellular linker connecting the TM segment and the intracellular poly-Arg cluster. The predicted inclusion of this linker segment in the γ1 TM segment from prediction programs was likely biased by the presence of a Pro residue in the N-terminal side and the heavily charged six Arg residues on the C-terminal side (Fig. 2 A).
Figure 2.
The presence and function of a linker region. (A) The amino acid sequence alignment of the four BKγ subunits in the region spanning the TM segment and the neighboring cytosolic positively charged cluster. The numbers on top show positions of the corresponding amino acids in the γ1 subunit. The location of the γ1 F273 residue is indicated with an asterisk. Conserved residues are shaded at three levels (from dark to light: 100, 75, and 50%). (B) Shifts in BK channel V1/2 values caused by the wild type and different mutants with deletion of amino acids in the TM and linker regions. The corresponding locations of the deleted amino acids are depicted on top. (C) Voltage dependence of BK channel activation in the presence of different BKγ subunit chimeras, whose main bodies were from γ1 and whose C-tails or C-tails together with linker regions were from γ2 or γ4. For comparison, BK channels expressed by BKα alone or together with wild-type BKγ subunits are shown with dotted lines. Error bars represent ±SEM.
For convenience, we had previously included the linker region as part of the TM region for construction of chimeric BKγ proteins (Li et al., 2015). However, we observed severe functional incompatibility between the γ1 subunit’s TM region and the γ2 and γ4 subunits’ C-tail regions that unexpectedly caused a full loss of the modulatory function in the resultant BKγ chimeric proteins (Li et al., 2015). In the current study, we found that the γ1 linker region was the main cause for the incompatibility: complete replacement of the linker together with the C-tail by those from γ2 or γ4 fully or partially rescued the expected modulatory function of the γ1 TM segment (approximate −100-mV shift in V1/2 [Li et al., 2015]; Fig. 2 C and Table 1). The resultant BK channel V1/2 in the presence of γ1/γ2-linker&tail and γ1/γ4-linker&tail were 60 ± 5 mV and 97 ± 3 mV, respectively (Fig. 2 C and Table 1).
Phe273 and its neighboring residues in the middle of the TM segment are critical in regulating BK channels
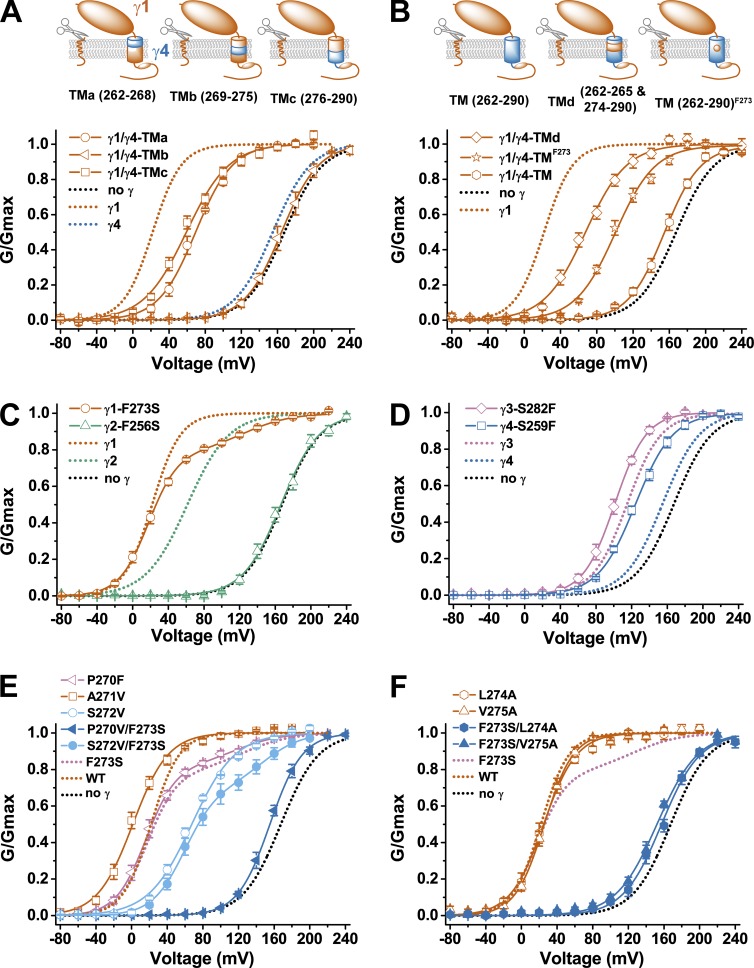
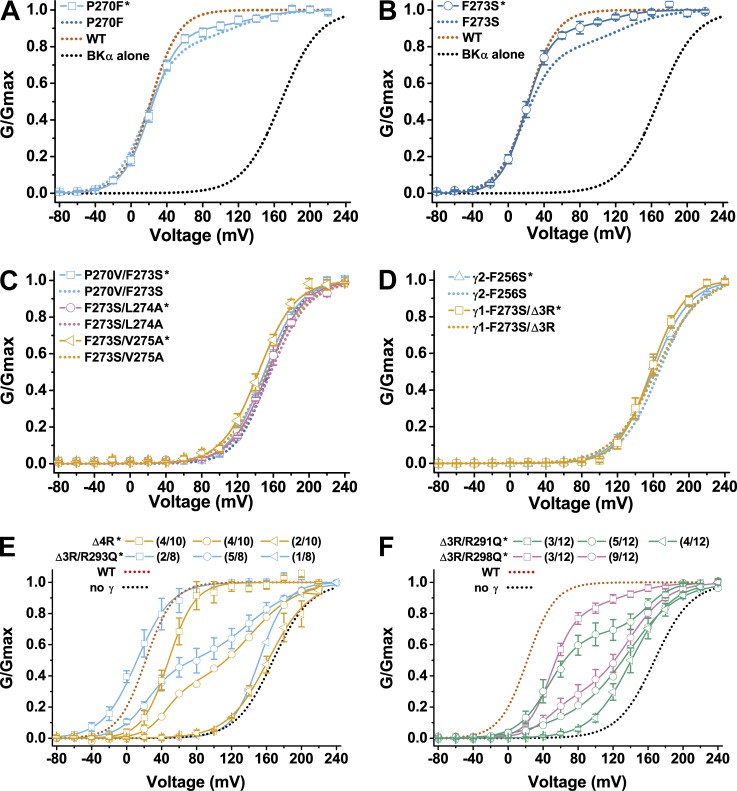
We previously reported that the replacement of the γ1 TM region, including linker region, with that of γ4 (chimera γ1/γ4-TM) converted γ1 to be similar to γ4, which is ineffective in BK channel modulation (Li et al., 2015). To identify the part in the TM region that is most important for the BKγ1’s modulatory function, we constructed three different γ1/γ4-TM(a–c) chimeras harboring different parts of the BKγ4 TM region (Fig. 3 A). We found that replacement of the first seven TM amino acid residues (TMa) alone or the last eight TM amino acid residues together with the linker (TMc) in the γ1 subunit with those from the γ4 subunit had a moderate effect on γ1 function, with a shift in BK channel V1/2 to the depolarizing direction by ∼40 mV compared with that in the presence of the wild-type γ1 subunit. However, a γ1 to γ4 swap for seven amino acid residues (TMb, residues 269–275) in the middle part of the TM segment caused a full loss of the γ1 subunit’s modulatory function, i.e., the resultant V1/2 value was not different from that of BKα alone for >10 mV. We also found that a reinstallation of the 5th to 12th residues (266–273) of the γ1 TM segment in the γ1/γ4-TM chimera restored ∼70% modulatory function of the γ1 subunit. The resultant γ1/γ4-TMd chimera shifted the BK channel V1/2 by −100 mV (V1/2 = 67 ± 4 mV; Fig. 3 B and Table 1). These results suggest that some residues in the overlapping region (residues 269–273) might be critical in determining the γ1 subunit’s ability in modulating BK channels. It is notable that the 12th TM residue (γ1-F273) in the middle of the TM segment is the only position that dramatically differs (Phe versus Ser) between the modulation-effective group of the γ1 and γ2 TM segments and the modulation-ineffective group of the γ3 and γ4 TM segments. Interestingly, a single mutation Ser↔Phe at this position in the γ1/γ4-TM chimera was able to significantly shift the BK channel V1/2 by more than −50 mV (V1/2 = 103 ± 3 mV with γ1/γ4-TMF273 compared with 155 ± 4 mV with γ1/γ4-TM; Fig. 3 B and Table 1). To test whether an amino acid difference in this position plays a key role in determining the modulatory function of the TM segment, we mutated the corresponding residues in γ1 (Phe273) and γ2 (Phe256) to Ser and in γ3 (Ser282) and γ4 (Ser259) to Phe. We found that F256S mutation in the γ2 subunit resulted in a full loss of modulatory function (Fig. 3 C and Table 1). However, F273S mutation in the γ1 subunit caused only a small portion (∼21%) of the channels to be drastically shifted in V1/2, relative to that in the presence of the wild-type γ1 subunit, to the depolarizing direction (V1/2 ≈ 125 mV), whereas the majority (∼79%) of the channels retained a low V1/2 value (V1/2 = 18 ± 1 mV) that was similar to that caused by the wild type (Fig. 3 C and Table 1). Mutation S259F in the γ4 subunit enhanced its modulatory function by ∼30 mV in shifting the BK channel V1/2 toward the negative voltage direction (V1/2 = 125 ± 2 mV with γ4-S259F), but the enhanced function was still far below the functional level of the γ2 subunit (Fig. 3 D and Table 1). Mutation S282F in the γ3 subunit only slightly enhanced the γ3 modulatory function by 15 mV in shifting the BK channel V1/2 toward the negative voltage direction (V1/2 = 100 ± 3 mV with γ3-S282F), which was far less than the expected increase to reach the functional level of the γ1 subunit (Fig. 3 D and Table 1). These results suggest that this Phe↔Ser switch at TM position 12 plays an important role but is not the sole determinant in conferring on the BKγ TM segments drastically different modulatory functions (∼100 mV difference in V1/2 shifting capability) between the two groups of TM segments (γ1 and γ2 vs. γ3 and γ4), as we previously observed with different BKγ chimera (Li et al., 2015).
Figure 3.
Identification of amino acids within the TM segment important for the BKγ modulatory function. (A and B) Voltage dependence of BK channel activation in the presence of γ1/γ4 chimeras whose main bodies were from γ1 and whose TM segment and linker regions were fully or partly from γ4. The chimera in which the single residue F273 (γ1) remained unchanged is indicated with a superscript. (C and D) Voltage dependence of BK channel activation in the presence of different BKγ subunits with a Phe↔Ser mutation at the 12th position of their TM segments. (E and F) Voltage dependence of BK channel activation in the presence of single or double mutations at or near the 12th position (Phe273) of the BKγ1 TM segment. For comparison, the γ1-F273S mutant is also shown with dotted lines in E and F. Error bars represent ±SEM.
To identify other TM residues important in determining the BKγ subunits’ modulatory functions, we generated five additional single mutants of the γ1 subunit by mutating the three residues on the N-terminal side and the two residues on the C-terminal side of Phe273 to mimic the γ3 subunit (P270F and A271V), the γ2 subunit (L274A), or both the γ3 and γ4 subunits (S272V and V275A; Fig. 2 A and Fig. 3, E and F). These additional five single mutations all produced functional BKγ1 mutants (Fig. 3, E and F). The S272V mutation caused a shift in BK channel V1/2 to the depolarizing direction by 45 mV (V1/2 = 67 ± 2 mV) relative to that modulated by the wild-type γ1 subunit (Fig. 3 E and Table 1). The A271V mutation resulted in a shift in BK channel V1/2 to the hyperpolarizing direction by 21 mV (V1/2 = 1 ± 3 mV) relative to that modulated by the wild-type γ1 subunit (Fig. 3 E and Table 1). Similar to the F273S mutation, the P270F mutation caused a significant loss of the γ1 modulatory function (V1/2 ≈ 120 mV) only in a small portion (∼17%) of the channels, whereas the majority (∼83%) of the channels retained a low V1/2 value (V1/2 = 16 ± 2 mV) that was similar to that caused by the wild type (Fig. 3 E and Table 1). The other two mutations, L274A and V275A, exerted no effect on the γ1 function (V1/2 = 25 ± 4 mV and 26 ± 2 mV, respectively; Fig. 3 F and Table 1).
Given that most single mutations appeared to be insufficient to significantly perturb the γ1 function and the potentially important role of the Phe273 residue in the γ1 subunit, we generated double mutants combining the F273S mutation and four other single mutations (P270V, S272V, L274A, and V275A). The S272V/F273S mutation showed an additive effect of F273S and S272V that produced at least two populations of channels, one major population similar to that caused by S272V and the rest similar to that noted with F273S for significant loss of the γ1 function (Fig. 3 E and Table 1). However, the other three double mutants (P270V/F273S, F273S/L274A, and F273S/V275A) all caused a nearly full loss of the γ1 subunit’s modulatory function (V1/2 = 155 ± 2 mV, 157 ± 2 mV, and 152 ± 2 mV, respectively) in spite of the limited or no effect of the single mutations (assuming P270V is similar to P270F in its effect on the γ1 function; Fig. 3, E and F; and Table 1).
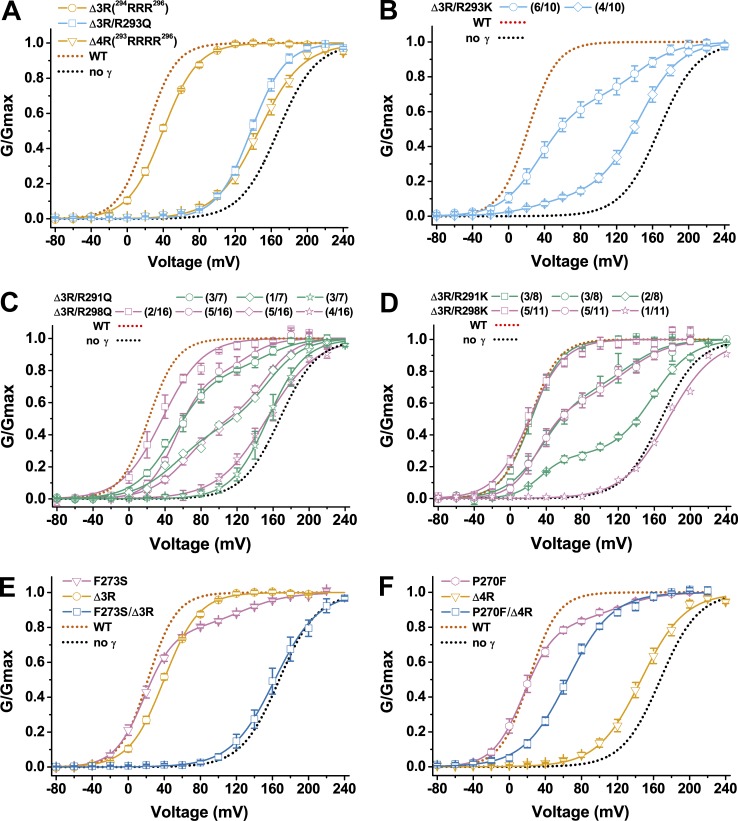
A minimum of three Arg residues in the C-tail are required for the γ1 subunit’s modulatory function
It was previously known that the poly-Arg cluster 291RARRRRLR298 in the C-tail is important for the γ1 subunit’s modulatory function, whose deletion nullified the γ1 subunit’s modulatory function (Yan and Aldrich, 2010; Li et al., 2015). To characterize the function of individual Arg residues in the γ1 poly-Arg cluster, we performed mutational analysis on these Arg residues. We found that deletion of three Arg residues (Δ3R) in the middle of the cluster had only a minor effect on the γ1 modulatory function, as indicated by only a slight decrease in the γ1-induced shift in BK channel V1/2 (V1/2 = 40 ± 1 mV; Fig. 4 A and Table 1). However, deletion of all four Arg residues (Δ4R) in the middle of the cluster (293RRRR296) caused an ∼80% loss in the γ1-induced shift in BK channel V1/2 (V1/2 = 146 ± 4 mV; Fig. 4 A and Table 1). A similar result was obtained when three Arg residues were deleted and the only Arg left in the middle of the poly-Arg cluster was neutralized by substitution with Gln (V1/2 = 138 ± 2 mV for the Δ3R/R293Q mutant; Fig. 4 A and Table 1). To determine whether Lys can replace Arg in function within the γ1 poly-Arg cluster, we replaced the residue Arg293 with Lys in the Δ3R mutant. Interestingly, only ∼40% of recorded channels retained the Δ3R modulatory function, whereas the rest (60%) lost most γ1 modulatory function (V1/2 = 145 mV; Fig. 4 B and Table 1). Additionally, the resultant Δ3R/R293K mutation caused some variation in channel properties from patch to patch, and the G-V relationships of BK channels were best fitted by at least two populations of BK channels in all recorded 10 excised patches (Fig. 4 B). In six patches, the G-V relationships were best fitted by a major population (∼67%) of low voltage–activated channels (V1/2 ≈ 35 mV) close to those associated with the unaltered γ1 subunit (BKα/γ1) and by a minor population (∼33%) of high voltage–activated channels (V1/2 ≈ 144 mV) more similar to those formed by BKα alone (Fig. 4 B and Table 1). In the other four patches, the G-V relationships were best fitted by a minor population (11%) of low voltage–activated BKα/γ1-type channels (V1/2 ≈ 33 mV) and by a major population (89%) of high voltage–activated BKα-type channels (V1/2 ≈ 145 mV; Fig. 4 B and Table 1). Overall, in the presence of the Δ3R/R293K mutant of the γ1 subunit, ∼40% of the recorded BK channels were low voltage–activated channels and 60% were high voltage–activated channels.
Figure 4.
Functional analyses of the amino acids within the poly-Arg cluster region and their relationship with the TM segment. (A–D) Voltage dependence of BK channel activation in the presence of different BKγ1 mutants with mutations in the poly-Arg cluster region. For mutants that produced heterogeneity in BK channel gating properties, membrane patches with similar channel G-V relationship were grouped together and indicated in the label with the number of grouped membrane patches over total recorded membrane patches. (E and F) Voltage dependence of BK channel activation in the presence of BKγ1 mutants with single mutation from the TM segment and poly-Arg cluster region and with combined double mutation from both regions. Error bars represent ±SEM.
To determine whether the Arg291 and Arg298 on the two ends of the clusters are required for the γ1 function, we replaced them with Gln or Lys together with the mutation Δ3R, which only had a minor effect. Similar to the Δ3R/R293K mutant, the Δ3R/R291Q, Δ3R/R298Q, Δ3R/R291K, and Δ3R/R298K mutants all caused certain patch to patch variation in BK channel gating properties, and furthermore, in most excised membrane patches, the G-V relationships needed to be best fitted by at least two populations of channels (Fig. 4, C and D; and Table 1). For example, the G-V relationships of BK channels in the presence of the γ1-Δ3R/R291Q mutant could be fitted by a single Boltzmann function with V1/2 = 154 ± 7 mV in three excised patches but needed to be fitted with a double Boltzmann function with V1/2 ≈ 53 mV (47% in fraction) and ∼154 mV (53%) in one excised patch and V1/2 = 49 ± 3 mV (79% in fraction) and ∼150 mV (19%) in another three excised patches (Fig. 4 C and Table 1). Thus, in a total of seven excised patches, the γ1-Δ3R/R291Q mutant overall resulted in ∼41% of BK channels being activated by a low voltage (V1/2 ≈ 50 mV) and ∼59% of channels being activated by a high voltage (V1/2 ≈ 150 mV). Similarly, the γ1-Δ3R/R298Q, Δ3R/R291K, and Δ3R/R298K mutants overall caused ∼51%, 70%, and 67% of BK channels to be activated by a low voltage (V1/2 ≈ 20–60 mV) and, correspondingly, ∼49%, 30%, and 33% to be activated by a high voltage (V1/2 ≈ 130–160 mV) in a total of 16, 8, and 11 excised patches, respectively (Fig. 4, C and D; and Table 1). Therefore, in the absence of three Arg residues in the middle of the poly-Arg cluster, the Arg291 and Arg298 seemed to be required to maintain the full modulatory function of the γ1 subunit, whose neutralization with Gln or substitution with Lys caused ∼50–60% and ∼30% of BK channels, respectively, to be activated at a much higher voltage (V1/2 ≥ 130 mV) that was close to that of the channel formed by BKα alone. Therefore, a minimum of three Arg residues are required to maintain the γ1 subunit’s modulatory function.
Functional coupling between the TM segment and the poly-Arg cluster
To help us understand the large effects of mutations in the γ1 poly-Arg cluster on BK channel voltage-dependent gating, we investigated the relationship between the TM segment and the poly-Arg cluster by combinational mutations in these two regions. Although both the F273S and Δ3R mutants of γ1 are close to wild type in modulatory function, we found that their combination completely nullified the γ1 modulatory function. The γ1-F273S/Δ3R mutant produced nearly no shift in the BK channel V1/2 (163 ± 7 mV) compared with the BKα channel alone (Fig. 4 E and Table 1). Furthermore, the BK channel V1/2 in the presence of the γ1-P270F/Δ4R mutant was 65 ± 2 mV (Fig. 4 F and Table 1), indicating that the P270F mutation in the TM segment restored the Δ4R mutant’s modulatory function to a level similar to that of the γ2 subunit. The γ1-P270F/Δ4R mutant may structurally mimic the γ2 subunit, in that the latter has Phe at the equivalent γ1 TM position of Pro270 and many fewer positively charged residues in the positively charged residue cluster (Fig. 2 A). These results indicate that the amino acid residues in the middle of the TM segment and the distantly located poly-Arg cluster can mutually affect each other to exert nonadditive effects on the overall γ1 modulatory function.
Mutational effects of the γ1 subunit on its association with BKα in the isolated channel complexes
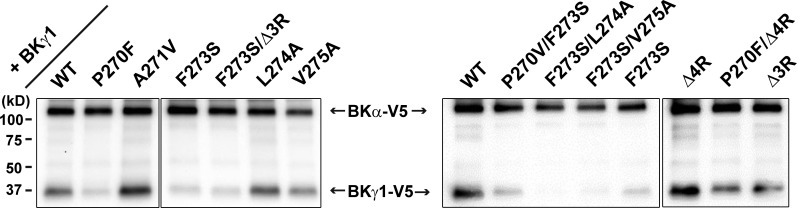
To help understand the underlying mechanisms of the observed mutational effects, we determined whether the association of the γ1 subunit with BKα was affected in some mutants. We included a V5 tag on the C termini of BKα and γ subunits and heterologously expressed them in HEK-293 cells. After immunoprecipitation of the BKα–γ1 complex with anti-BKα antibody, the relative abundance of these two subunits was immunoblotted with anti-V5 antibody. We found that compared with the wild type, both F273S and P270F mutations caused similar drastic reductions (∼80% as estimated from the changes in the immunoblotting band intensity of the γ1-V5 after normalization to that of the BKα-V5 within the same sample) in the amount of immunoprecipitated BKγ1 protein (Fig. 5), although both mutations only caused a minor portion (∼20%) of the channels to be similar to that formed by the BKα alone when they are cotranslationally expressed with BKα (Fig. 3, C and E). The effect of double mutant P270F/F273S was similar to that of the single mutant P270F or F273S in channel association in the isolated channel complexes. Compared with the F273S and P270F mutants, the L274A and V275A mutations had less effect on channel association. Their combined mutations with F273S, however, led to further loss of the associated γ1 protein as compared with the F273S mutant (Fig. 5). The Δ3R/F273S mutants were still partially associated with BKα at a level similar to that of the F273S and P270F mutants (Fig. 5). Compared with the TM mutants with greatly reduced channel association, the charged cluster mutants Δ3R, Δ4R, and P270F/Δ4R appeared to be well associated with BKα in the isolated channel complex (Fig. 5).
Figure 5.
Mutational effects of the BKγ1 subunit on its association with the BKα subunit in the isolated channel complex. Immunoprecipitation and immunoblot analyses of the BK channel complexes in the presence of the γ1 wild type or different mutants (indicated on top). BKα and γ1 were both tagged with V5 at their C termini, cotranslationally expressed in HEK-293 cells, immunoprecipitated with anti-BKα antibody, and then immunoblotted with anti-V5 antibody.
Effects of overexpression on modulatory function of γ subunit mutants
To minimize the cell to cell variation in the relative expression levels of BKα and γ subunits, we had used the BKα–γ fusion cDNA construct to coexpress BKα and γ subunits in the experimental data presented so far. The fusion cDNA construct was designed to express a precursor fusion protein of human BKα and BKγ protein, which produces a 1:1 expression of the α and γ subunit molecules after endogenous cleavage by peptidases at the linker (γ signal peptide). Compared with the commonly used cotransfection method, this convenient cotranslational expression strategy had largely eliminated the cell to cell variation in electrophysiological properties of recorded BK channels in HEK-293 cells in the presence of the wild type and most γ subunit chimera or mutants. However, for the γ subunit mutants that have impaired function in proper coassembly with BKα, the level of cotranslationally expressed BKγ protein might be not high enough to maximally occupy and modulate BKα. Overexpression of these γ subunit mutants by transfecting cells with the BKα–γ fusion cDNA construct and an additional plasmid encoding the γ subunit alone should be helpful in enhancing their occupancy and modulatory function on BK channels. Therefore, we overexpressed and reexamined the modulatory effects of the TM and charged cluster mutants that had conferred on BK channels either heterogeneity or a virtually full loss in modulation by the γ subunit when they were cotranslationally expressed with BKα. For the P270F and F273S mutants, their overexpression enhanced their modulatory functions by increasing the portion of the low V1/2 channel from 83 to 91% and from 79 to 91%, respectively (Fig. 6, A and B; and Table 1), agreeing with the aforementioned observation of a weakened association of these two mutants on BK channels in the isolated channel complex. However, as with the expression of the BKα–γ fusion cDNA constructs alone, overexpression of the three γ1 TM double mutants, F270V/F273S, F273S/L274A, and F273S/V275A conferred on BK channel gating properties that were still similar to the BKα channel alone (Fig. 6 C). Similarly, overexpression of the γ2-F256S mutant and the equivalent γ1-Δ3R/F273S mutant was also unable to alter the modulatory function of these loss-of-function mutants (Fig. 6 D). Thus, for these TM mutants with a virtually full loss in the γ1 modulatory function, their overexpression was unable to enhance their modulatory function, suggesting a virtually full impairment in either their channel gating–related modulatory functions or their association with BKα or both.
Figure 6.
Effects of protein overexpression on the modulatory function of selected γ1 mutants. (A–F) Voltage dependence of BK channel activation in the presence of selected BKγ1 mutants with mutations in the TM segment (A–D) and poly-Arg cluster region (E and F). Overexpressed mutants are indicated with asterisks on the right side of their names. For comparison, the voltage dependence of BK channel activation in the absence or presence of the non-overexpressed γ1 wild type or mutants are included as dotted lines. As in Fig. 4, membrane patches with similar channel G-V relationship (E and F) were grouped together and indicated in the label with the number of grouped membrane patches over total recorded membrane patches. Error bars represent ±SEM.
Interestingly, overexpression of the Δ4R and Δ3R/R293Q mutants partially rescued their modulatory functions. With four Arg residues removed or neutralized in the γ1 subunit’s intracellular poly-Arg region, these two mutants were largely ineffective (ΔV1/2 (γ mutant−BKα alone) ≤ −30 mV) in BK channel modulation when cotranslationally expressed with BKα (Fig. 4 A). However, with overexpression, the Δ4R and Δ3R/R293Q mutants were able to effectively modulate the BK channels to allow the channels’ G-V relationship to be fitted by a single Boltzmann function with a low V1/2 value, 48 ± 2 mV for Δ4R and ∼12 mV for Δ3R/R293Q, in 4 out of 10 and 2 out of 8 excised membrane patches, respectively (Fig. 6 E and Table 1). A mixture of low voltage– and high voltage–activated channels was also observed in 4 out of 10 (Δ4R) and 5 out of 8 (Δ3R/R293Q) excised membrane patches for these two mutants (Fig. 6 E and Table 1). These results suggest that the Δ4R and Δ3R/R293Q mutations mainly affected the association of the γ1 subunit with the BKα. The channel gating–related function of the γ1 subunit was only slightly compromised by the Δ4R but not by the Δ3R/R293Q mutation, suggesting some difference in gating effects between the deletion and substitution of the Arg293 residue upon deletion of the other three Arg residues in the middle of the poly-Arg cluster. For the Δ3R/R291Q and Δ3R/R298Q mutants, their overexpression still produced a mixture of the low voltage– and high voltage–activated channels in most excised membrane patches with no or only a slight increase in the overall portion of the low voltage–activated channels (Fig. 6 F and Table 1). Thus, in contrast to the Δ4R and Δ3R/R293Q mutants, overexpression of the Δ3R/R291Q and Δ3R/R298Q mutants failed to drastically enhance their modulatory functions. Previously, deletion of the whole poly-Arg cluster was found to cause the nearly full loss of the γ1 modulatory function upon cotranslational expression and also in condition of cotransfection of the γ1 subunit with BKα (Yan and Aldrich, 2010; Li et al., 2015). Similarly, we found overexpression of the whole poly-Arg cluster deletion mutant (ΔtailN291–298) was unable to rescue the lost modulatory function of the γ1 subunit (Table 1), confirming the requirement of some positively charged residues in this poly-Arg cluster in maintaining the γ1 subunit’s modulatory function.
Overall, the results obtained with overexpression of the selected γ subunit mutants largely validate the results obtained with the method of BKα–γ fusion cotranslational expression. A combinational use of these two expression methods provides a means to evaluate the effect of some mutations on the association of the γ subunit with the BKα, which in theory should be improved by overexpression if there was simply an increase in the dissociation constant (Kd).
DISCUSSION
The BK channel auxiliary γ subunits each consist of ∼300 (334 for γ1) amino acids, including a relatively large extracellular LRR domain. Within the BKγ proteins, we had recently identified a peptide sequence of ∼40 amino acids, including the TM segment and the adjacent intracellular poly-Arg cluster as the key determinant for the different modulatory effects of the four auxiliary γ subunits on the BK channel’s voltage dependence of channel activation (Li et al., 2015). However, it was difficult to know whether this peptide region is also the most important determinant for the BKγ subunits’ modulatory function on BK channels because deletions in other regions, particularly the LRR domain, also caused loss of modulatory functions (Yan and Aldrich, 2010). Here, we demonstrated that upon a replacement of the BK channel β1 subunit’s second TM segment and the intracellular C terminus with this peptide sequence, the resultant β1/γ1 chimera fully retained the γ1 subunit’s modulatory function, whereas the β1 subunit’s modulatory effects were largely lost (Fig. 1). This result agrees with a recent report that both TM segments are essential for the β1 subunit’s function, which cannot be functionally replaced even by those from the β4 subunit (Kuntamallappanavar et al., 2014). Importantly, because of the lack of amino acid sequence similarity between the auxiliary β and γ subunits, this result showed that the peptide sequence including the TM segment and the adjacent intracellular poly-Arg cluster is sufficient, in the absence of the extracellular LRR domain and the rest of the intracellular C-tail, to induce the full modulatory effect of the γ1 subunit. The extracellular LRR domain of the auxiliary γ1 subunit may mainly play a nonfunctional role by maintaining proper protein assembly and surface expression, which can be replaced by some other protein sequence, such as the extracellular region of the β1 subunit.
We performed mutational analyses in the TM segment and intracellular poly-Arg cluster to identify key amino acids critical for the γ1 subunit’s modulation on BK channels. We found that the single mutations P270F and F273S and the double mutations P270V/F273S, F273S/L274A, and F273S/V275A caused marked reduction or nearly full loss in association of the γ1 subunit with BKα, as shown in the immunoprecipitated BKα–γ1 complex (Fig. 5), suggesting that the TM segment, particularly the Pro270 and Phe273 residues, plays a key role in the γ1 subunit’s association with BK channels. However, instead of an expected proportional loss in the γ1 modulatory function, the P270F and F273S mutations only led to a small portion (∼20%) of the channels displaying a major loss in modulation by the γ1 subunit (Fig. 3, C and E). Assuming that the isolated BK channels were similar to those on native membrane in protein stoichiometry, these results would suggest that even a significantly reduced presence of the γ1 subunit on the BK channel complex might still be sufficient to fully modulate the channel. This interpretation is consistent with the previously reported “all or none” gating shift induced by the γ1 subunit when the molar ratio of the injected BKα/γ1 RNA to Xenopus oocytes was varied (Gonzalez-Perez et al., 2014). Alternatively, the F270F and F273S mutants might remain largely unaffected in their association with BK channels in the lipid membrane, but they were more vulnerable than wild type to the detergent-induced dissociation during immunoprecipitation. In stark contrast to those observed with the TM mutants P270F and F273S, we found that deletion of four Arg residues in the poly-Arg cluster (Δ4R) caused no reduction in association of the γ1 subunit with BKα in the isolated channel complex but a loss in channel modulation that could be partially rescued by γ1 overexpression (Figs. 4 A, 5, and 6 E). The disparate results for the Δ4R mutant between the electrophysiological assay on cell membranes and the biochemical assay on isolated channel complexes clearly suggest that the mutational effect of the γ1 subunit on its association with BKα can be quite different when the channel complex exists in detergent micelles than in a native lipid membrane environment.
We previously reported that the γ1 and γ2 TM segments, independent of the presence of different C-tails, were both potent in modulating the BK channels (Li et al., 2015). However, the γ1 and γ2 TM segments showed very little amino acid sequence similarity, and the γ1 subunit contains a Pro (P270) at the ninth amino acid position of the TM segment (Fig. 2 A) that inevitably breaks or kinks the TM α-helix. The presence of a proline in the γ1 TM segment results in at least two differences in structure–function relationships between the γ1 and γ2 subunits. First, a minimum of three Arg residues in the poly-Arg cluster seems to be required for efficient association of the γ1 subunit with BKα. Second, the 12th residue (Phe) in the γ2 TM segment (Phe256) plays a more important role in modulating BK channels than that in the γ1 TM segment (Phe273). Its replacement with a Ser caused a full loss of the γ2 subunit’s modulatory function but only some effects on channel association for the γ1 subunit. Notably, when the γ1-Pro270 was mutated to a Phe to mimic the γ2 subunit, the γ1-Phe273 residue became essential, whose replacement with Ser led to a full loss of the γ1 subunit’s modulatory function. Therefore, our observed drastic effects of the γ2-F256S, γ1-P270F/F273S, γ1-F273S/L274A, and γ1-F273S/V275A mutations on the γ subunit’s modulatory function, together with moderate effects of the γ1 single point mutations (e.g., A271V and S272V) on voltage dependence of BK channel gating, support a critical role of the TM segment in modulating the voltage dependence of BK channel activation.
The present study found that the poly-Arg cluster is involved in the γ1 subunit’s association with BKα and also modulation of BK channel gating. A minimum of three Arg residues appeared to be required for efficient association of the γ1 subunit with BK channels such that even overexpression of the Δ3R/R291Q, Δ3R/R293Q, Δ3R/R298Q, and Δ4R mutants could not or could only partially restore the γ1 subunit’s modulatory function. The Δ3R/R291Q, Δ3R/R298Q, and Δ4R mutants also displayed an ∼30-mV reduction in the maximal shift of BK channel V1/2 toward the hyperpolarizing direction as compared with the wild type. The P270F/Δ4R mutation, as compared with the wild type or the P270F mutant, also induced an ∼40-mV reduction in the shift of BK channel V1/2 toward the hyperpolarizing direction. The observed effects of the mutations in the γ1 poly-Arg cluster on BK channel gating in this study in principle agree with our previous report that the BKγ chimeras containing the positively charged cluster of the γ1 or γ3 subunit were 40–50 mV more effective, compared with those containing the positively charged cluster of the γ2 or γ4 subunit, in shifting BK channel V1/2 in the hyperpolarizing direction (Li et al., 2015). It remains unclear how the Arg residues in the γ1 poly-Arg cluster might affect the γ1 subunit’s association with BKα and its regulation of the BK channel gating. However, because the equivalent intracellular positively charged cluster in the γ2 subunit can be fully deleted without major effect on modulatory function (Li et al., 2015), we tend to rule out a dominant role of the γ1 poly-Arg cluster in directly interacting with and modulating BKα subunits.
As seen with the F273S/Δ3R and P270F/Δ4R mutants, the present study observed functional coupling between the TM segment and the intracellular poly-Arg cluster. Thus, as we previously proposed (Li et al., 2015), the positively charged residue cluster likely exerts its influence on the γ subunit’s modulatory function via its interactions with the TM segment through the general “positive-inside rule” for proper membrane anchor of the TM segment (White and von Heijne, 2004). In this scenario, the positively charged cluster may act on the γ subunit’s modulatory function though interactions with membrane lipids. The potentially labile nature of interactions between the positively charged residues and membrane lipids might explain our observation of a large heterogeneity in the channel gating property that was so far only seen with the positively charged cluster mutants in this study or C-tail chimeric mutants in a previous study (Li et al., 2015). This channel heterogeneity has been observed in the same batch of cells transfected with either the BKα–γ1 fusion alone or together with a γ1-overexpressing plasmid, and we found no obvious correlation with posttransfection time or cell batches, suggesting that it was less likely caused by cell to cell variation in the expression level of the γ1 subunit relative to BKα. Neutralization or deletion of more than half of the six Arg residues in the poly-Arg cluster may increase the sensitivity of the γ1 subunit’s structure and function to membrane lipid composition, which may vary from cell to cell, patch to patch, and even microdomain to microdomain in the same excised membrane patch. This explanation agrees with our finding that the function of the Arg residue in the γ1 poly-Arg cluster can be only partially fulfilled by the substituted Lys residue. It is well recognized that the side chains of Arg and Lys behave differently in their interactions with membrane lipids (Hong and Su, 2011; Li et al., 2013; Wu et al., 2013), e.g., the primary amine of Lys interacts much more weakly with zwitterionic phospholipids than the guanidine group of Arg (Yang et al., 2003).
In summary, we have identified the TM segment and the adjacent intracellular positively charged cluster as key determinants of the BK channel γ1 subunit in channel modulation. We found that Phe273 and its neighboring residues in the middle of the TM segment play a key role in BK channel association and modulation and that a minimum of three Arg residues in the charged cluster are required for the γ1 subunit’s modulatory function. We observed allosteric coupling between the TM segment and the intracellular positively charged cluster. We concluded that the TM segment is a key molecular determinant for channel association and modulation and the intracellular positively charged cluster is involved mainly in channel association likely through its TM anchoring effect. Our findings provide insights into the structure–function relationship of the γ1 subunit for understanding its potent modulatory effect on BK channels.
Acknowledgments
This work was supported by National Institutes of Health grants NS075118 and NS078152 (to J. Yan).
The authors declare no competing financial interests.
Kenton J. Swartz served as editor.
Footnotes
Abbreviations used in this paper:
- LRR
- leucine-rich repeat
- TM
- transmembrane
References
- Almassy J., and Begenisich T.. 2012. The LRRC26 protein selectively alters the efficacy of BK channel activators. Mol. Pharmacol. 81:21–30. 10.1124/mol.111.075234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R., Jegla T.J., Wickenden A., Liu Y., and Aldrich R.W.. 2000. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275:6453–6461. 10.1074/jbc.275.9.6453 [DOI] [PubMed] [Google Scholar]
- Claros M.G., and von Heijne G.. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685–686. [DOI] [PubMed] [Google Scholar]
- Contreras G.F., Neely A., Alvarez O., Gonzalez C., and Latorre R.. 2012. Modulation of BK channel voltage gating by different auxiliary β subunits. Proc. Natl. Acad. Sci. USA. 109:18991–18996. 10.1073/pnas.1216953109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson K.W., Bannister J.P., Leo M.D., and Jaggar J.H.. 2014. LRRC26 is a functional BK channel auxiliary γ subunit in arterial smooth muscle cells. Circ. Res. 115:423–431. 10.1161/CIRCRESAHA.115.303407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner G., Schönherr K., Soom M., Hansel A., Asim M., Baniahmad A., Derst C., Hoshi T., and Heinemann S.H.. 2006. BKCa channels activating at resting potential without calcium in LNCaP prostate cancer cells. J. Membr. Biol. 208:229–240. 10.1007/s00232-005-0830-z [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez V., Xia X.M., and Lingle C.J.. 2014. Functional regulation of BK potassium channels by γ1 auxiliary subunits. Proc. Natl. Acad. Sci. USA. 111:4868–4873. 10.1073/pnas.1322123111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V., Xia X.M., and Lingle C.J.. 2015. Two classes of regulatory subunits coassemble in the same BK channel and independently regulate gating. Nat. Commun. 6:8341 10.1038/ncomms9341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff V.K., Starrett J.E. Jr., and Dworetzky S.I.. 2001. Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist. 7:166–177. 10.1177/107385840100700211 [DOI] [PubMed] [Google Scholar]
- Hong M., and Su Y.. 2011. Structure and dynamics of cationic membrane peptides and proteins: insights from solid-state NMR. Protein Sci. 20:641–655. 10.1002/pro.600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., and Aldrich R.W.. 2002. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120:267–305. 10.1085/jgp.20028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A., Larsson B., von Heijne G., and Sonnhammer E.L.. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580. 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- Kuntamallappanavar G., Toro L., and Dopico A.M.. 2014. Both transmembrane domains of BK β1 subunits are essential to confer the normal phenotype of β1-containing BK channels. PLoS One. 9:e109306 10.1371/journal.pone.0109306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Vorobyov I., and Allen T.W.. 2013. The different interactions of lysine and arginine side chains with lipid membranes. J. Phys. Chem. B. 117:11906–11920. 10.1021/jp405418y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Fan F., Kwak H.R., and Yan J.. 2015. Molecular basis for differential modulation of BK channel voltage-dependent gating by auxiliary γ subunits. J. Gen. Physiol. 145:543–554. 10.1085/jgp.201511356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares D., Srinivasan M., Salathe S.T., Ivonnet P., Baumlin N., Dennis J.S., Conner G.E., and Salathe M.. 2014. IFN-γ-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 306:L453–L462. 10.1152/ajplung.00247.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares D., Krick S., Baumlin N., Dennis J.S., Tyrrell J., Tarran R., and Salathe M.. 2015. Airway surface dehydration by transforming growth factor β (TGF-β) in cystic fibrosis is due to decreased function of a voltage-dependent potassium channel and can be rescued by the drug pirfenidone. J. Biol. Chem. 290:25710–25716. 10.1074/jbc.M115.670885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P., Wallner M., and Toro L.. 2000. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA. 97:5562–5567. 10.1073/pnas.100118597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P., Torres Y., Rojas P., Carvacho I., Garcia M.L., Toro L., Valverde M.A., and Latorre R.. 2006. Structural determinants for functional coupling between the beta and alpha subunits in the Ca2+-activated K+ (BK) channel. J. Gen. Physiol. 127:191–204. 10.1085/jgp.200509370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan K., Michael T.H., Jiang G.J., Hiel H., and Fuchs P.A.. 1999. A molecular mechanism for electrical tuning of cochlear hair cells. Science. 283:215–217. 10.1126/science.283.5399.215 [DOI] [PubMed] [Google Scholar]
- Savalli N., Kondratiev A., de Quintana S.B., Toro L., and Olcese R.. 2007. Modes of operation of the BKCa channel β2 subunit. J. Gen. Physiol. 130:117–131. 10.1085/jgp.200709803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zaydman M.A., and Cui J.. 2012. Regulation of voltage-activated K+ channel gating by transmembrane β subunits. Front. Pharmacol. 3:63 10.3389/fphar.2012.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusnády G.E., and Simon I.. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics. 17:849–850. 10.1093/bioinformatics/17.9.849 [DOI] [PubMed] [Google Scholar]
- Wallner M., Meera P., and Toro L.. 1999. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane β-subunit homolog. Proc. Natl. Acad. Sci. USA. 96:4137–4142. 10.1073/pnas.96.7.4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., and Brenner R.. 2006. An S6 mutation in BK channels reveals beta1 subunit effects on intrinsic and voltage-dependent gating. J. Gen. Physiol. 128:731–744. 10.1085/jgp.200609596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.W., Ding J.P., Xia X.M., and Lingle C.J.. 2002. Consequences of the stoichiometry of Slo1 α and auxiliary β subunits on functional properties of large-conductance Ca2+-activated K+ channels. J. Neurosci. 22:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S.H., and von Heijne G.. 2004. The machinery of membrane protein assembly. Curr. Opin. Struct. Biol. 14:397–404. 10.1016/j.sbi.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Wu Z., Cui Q., and Yethiraj A.. 2013. Why do arginine and lysine organize lipids differently? Insights from coarse-grained and atomistic simulations. J. Phys. Chem. B. 117:12145–12156. 10.1021/jp4068729 [DOI] [PubMed] [Google Scholar]
- Xia X.M., Ding J.P., Zeng X.H., Duan K.L., and Lingle C.J.. 2000. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel beta subunit. J. Neurosci. 20:4890–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., and Aldrich R.W.. 2010. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 466:513–516. 10.1038/nature09162 [DOI] [PubMed] [Google Scholar]
- Yan J., and Aldrich R.W.. 2012. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. USA. 109:7917–7922. 10.1073/pnas.1205435109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.T., Shin S.Y., Lee C.W., Kim Y.C., Hahm K.S., and Kim J.I.. 2003. Selective cytotoxicity following Arg-to-Lys substitution in tritrpticin adopting a unique amphipathic turn structure. FEBS Lett. 540:229–233. 10.1016/S0014-5793(03)00266-7 [DOI] [PubMed] [Google Scholar]
- Zeng X.H., Xia X.M., and Lingle C.J.. 2003. Redox-sensitive extracellular gates formed by auxiliary beta subunits of calcium-activated potassium channels. Nat. Struct. Biol. 10:448–454. 10.1038/nsb932 [DOI] [PubMed] [Google Scholar]
- Zhang J., and Yan J.. 2014. Regulation of BK channels by auxiliary γ subunits. Front. Physiol. 5:401 10.3389/fphys.2014.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]