Abstract
This paper describes the results of a 3-year study on the prevalence, enterotoxinogenicity and resistance to antimicrobials of S. aureus isolated on dairy farms with small scale production of raw cow milk cheeses. The samples of raw milk, semi-finished products and the final products as well as swabs were collected between 2011 and 2013 from nine dairy farms in Poland. A total of 244 samples were examined, of which 122 (50.0%) were contaminated with S. aureus including 18 of 26 (69.2%) mature cheese samples with log10 CFU g−1 between <1- and 7.41. In swabs collected from the staff and production environment the highest contamination rate with coagulase positive staphylococci (CPS) was detected on hands of cheese makers (4.34 log10 CFU/swab). None of the cheese samples contaminated with CPS contained staphylococcal enterotoxins (SEs). However, 55 of 122 (45.1%) S. aureus isolates possessed SEs genes, mainly (26 of 55; 47.3%) a combination of the sed, sej and ser genes. Furthermore, the sep (15 of 55; 27.3%) as well as seg and sei (9 of 55; 16.4%) genes were also identified. The remaining S. aureus isolates possessed the sea gene (one isolate), the combination of sec, seg and sei (three isolates) as well as the sed, sej, sep and ser markers together (one CPS). Resistance to penicillin (62 of 122 isolates; 50.8%) was the most common among the tested isolates. Some CPS were also resistant to chloramphenicol (7; 5.7%) and tetracycline (5; 4.1%). The obtained results indicated that the analyzed cheeses were safe for consumers. To improve the microbiological quality of traditional cheese products more attention should be paid to animal welfare and hygiene practices during the process of cheese manufacturing in some dairy farms.
Keywords: S. aureus, SE genes, antimicrobial resistance, raw milk cheese, public health
1. Introduction
Staphylococcal food poisoning (SFP) is one of the most common foodborne diseases worldwide resulting from the consumption of foods containing staphylococcal enterotoxins (SEs) produced mainly by Staphylococcus aureus [1,2]. Symptoms of SFP have a rapid onset (2–8 h) and include hypersalivation, nausea, vomiting, abdominal cramping and diarrhea [3]. In the majority of cases recovery occurs within 24–48 h without specific treatment. Occasionally, the disease can be more severe or even fatal, especially in infants, elderly or immunecompromised patients. S. aureus is ubiquitous in the environment and can be found in the air, water, humans and animals. It is also one of the major causes of bovine mastitis and therefore, raw milk and subsequently raw milk products may be contaminated with S. aureus [4]. About 10% of cheeses in Europe are made from raw milk, which presents a considerable potential threat to human health [5]. In Scotland, S. aureus was found to be the most frequent pathogen of raw milk cheeses [6]. In France, a study of foodborne disease outbreaks showed that S. aureus was one of the most common causative pathogens associated with milk-related outbreaks [7]. The lack of proper hygienic measures during food processing may also increase the probability of contamination with S. aureus, especially in manually prepared foods. Cheese makers carrying enterotoxin-producing S. aureus in their noses or on their hands are thought to be the main source of food contamination caused byphysicial contact or through respiratory secretions. André et al. isolated S. aureus from hand and nose samples of approximately 75% of cheese makers in a dairy processing plant [8].
Some S. aureus produce toxins which are potent emetic agents causing SFP. In Italy, 55% of food isolates (milk, dairy products, meat, meat products) were positive for classic SEs (SEA-SEE) [9], while in Norway 48% of isolates from bovine raw milk and raw milk products were found to be SE producers [10]. Many S. aureus strains can synthesize more than one type of toxins [1,2] and to date 22 different SEs have been described. Apart from the classic SE types several new variants of SEs or staphylococcal-like toxins have been identified [11]. All of these toxins are heat stable and therefore, they may still be present in food while S. aureus is absent [3]. Enterotoxinogenic staphylococci need to reach levels of at least 5-6 log10 CFU g−1 to produce detectable amounts of SE [12]. More than 95% of SFP outbreaks worldwide are caused by classic enterotoxins, mainly SEA. On the other hand, strains of S. aureus isolated from cow milk are mostly positive for SEC and SED [12].
According to the recent European Food Safety Authority report, a total of 386 food-borne outbreaks caused by staphylococcal toxins were identified in the European Union in 2013 [13]. Most of them (336 outbreaks) were recorded in France where consumption of unpasteurized milk cheeses is common and milk-based products are more frequently involved in food poisoning than in other countries. In Poland, in the same year, only five confirmed food-borne intoxications were notified [13]. In recent years, traditionally made food products such as cheese manufactured at farm dairies are becoming more popular in our country [14]. These products should be of high quality and safe for consumers.
The aim of the study was to determine the occurrence of S. aureus in dairy farms with small scale production of raw cow milk cheese. Moreover, enterotoxinogenicity and resistance to antimicrobials of the isolates were investigated.
2. Results
CPS were isolated from 122 (50.0%) out of 244 tested samples (Table 1). The bacteria were detected both in raw milk (12; 46.2%) and in the final cheese products (18; 69.2%). The highest percentage of CPS positive samples was found in the formed cheese (21 of 26; 80.8%), in grains after rinsing (19 of 25; 76.0%), milk curd (19 of 27; 70.4%) and in milk after heating (8 of 12; 66.7%). Of the 25 positive swabs CPS were most frequently isolated from the hands of cheese makers (11 of 26; 42.3%) and milk tanks (7 of 26; 26.9%) (Table 1).
Table 1.
Contamination with coagulase positive staphylococci (CPS) at the different stages of cheese production.
| Collected Material | Source | No. of Samples | No. (%) of CPS Positive Samples | Ranges of CPS at Different Production Stages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farm No. | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
| Swab | Log10 CFU/swab or CFU/cm2 | |||||||||||
| Hands of cheese maker | 26 | 11 (42.3) | 0 | 0 | 0–4.34 | 0 | 0–3.23 | 0–2.72 | 0–2.70 | 0–3.81 | 0–3.12 | |
| Milk tank | 26 | 7 (26.9) | 0 | 0 | 0.24–1.70 | 0 | 0–0.18 | 0 | 0–0.30 | 0–0.81 | 0–0.30 | |
| Strainer/sacks | 26 | 3 (11.5) | 0 | 0 | 0–2.83 | 0 | 0 | 0–2.00 | 0 | 0 | 0 | |
| Cheese mould | 24 | 4 (16.7) | 0–1.40 | 0 | 0–2.54 | 0 | 0–2.10 | 0 | 0 | 0 | 0–2.00 | |
| Sample | Log10 CFU mL−1 or CFU g−1 | |||||||||||
| Raw milk | 26 | 12 (46.2) | 0 | 0 | 0–3.63 | 0–1.18 | 0–4.74 | 0–2.63 | 0 | 0–5.00 | 1.08–3.04 | |
| Milk after heat treatment | 12 | 8 (66.7) | 0–1.70 | 0–0.18 | 0.70–1.48 | 1.18 | 2.36 | 1.73 | 0 | 0 | 3.90 | |
| Curd | 27 | 19 (70.4) | <1–2.64 | 2.18–2.53 | <1–4.77 | <1–1.60 | <1–4.65 | 2.89–4.89 | <1–3.83 | <1–5.83 | 1–4.26 | |
| Grains after rinsing | 25 | 19 (76.0) | <1–3.36 | <1–2.57 | 2.61–5.92 | 1.30–2.04 | 1.74–5.15 | 4.08–5.32 | <1–4.74 | <1 | 3.28–5.00 | |
| Formed cheese | 26 | 21 (80.8) | <1–3.95 | <1–2.40 | 3.23–5.08 | 1–2.46 | 1.30–6.04 | 4.53–5.63 | <1–4.74 | <1–5.60 | 3.62–5.59 | |
| Mature cheese | 26 | 18 (69.2) | 2.60–3.82 | <1–5.53 | 1–3.08 | <1 | <1–4.91 | <1–5.74 | 2.83–6.58 | <1–7.41 | 4.81–7.11 | |
| Total | 244 | 122 (50.0) | ||||||||||
Contamination with CPS depended on the type of material (Table 1). Only on two dairy farms the number of CPS in raw milk was 0 log10 CFU mL−1, whereas on other farms contamination was ranging from 0–1.0 to 0–5.0 log10 CFU mL−1. All semi-finished products obtained after addition of rennet were positive for CPS at the maximum levels of <1–5.83, 2.61–5.92 and 1.30–6.04 log10 CFU g−1 in milk curd, grains after rinsing and formed cheese, respectively. Furthermore, the final products in all dairy farms were contaminated with staphylococci and the levels ranged from 0 to <1–7.41 log10 CFU g−1. In swabs collected from production environment the highest contamination rate with CPS was detected on hands of cheese makers (4.34 log10 CFU/swab).
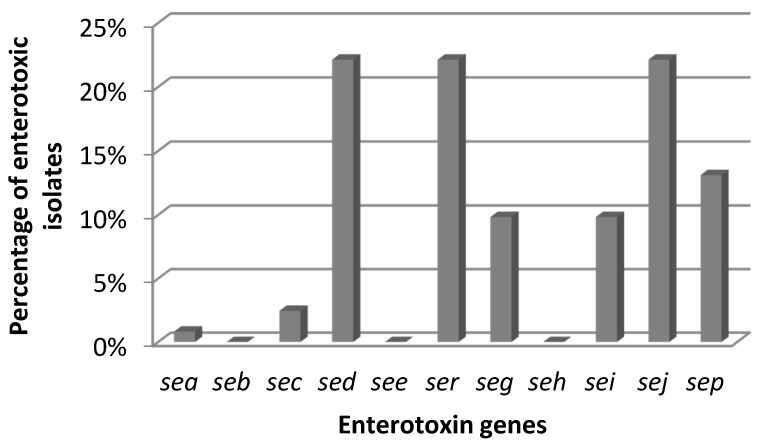
All CPS were coagulase- and catalase-positive. β-haemolysis was observed in 108 of 122 (88.5%) isolates. Based on the presence of the nuc and 16S rRNA genes all isolates were identified as S. aureus. Each sample contaminated with CPS, as well as all final products (total 105 samples) were tested for the presence of staphylococcal enterotoxins (SEs) and all of them were negative. However, SE genes were found in 55 (45.1%) isolates. Most of them were positive for three enterotoxin markers, i.e., sed + sej + ser and sec + seg + sei (26 and three isolates, respectively). Some CPS had only one gene—sep (15; 12.0%) or sea (1; 1.8%) or four sed + sej + sep + ser toxin genes (1; 1.8%) (Figure 1). Detection of SEA-SEE in culture supernatants of the isolates possessing the sea—see genes resulted in the presence of enterotoxins in supernatants of 31 of 55 (56.4%) such isolates.
Figure 1.
Enterotoxigenic S. aureus isolates.
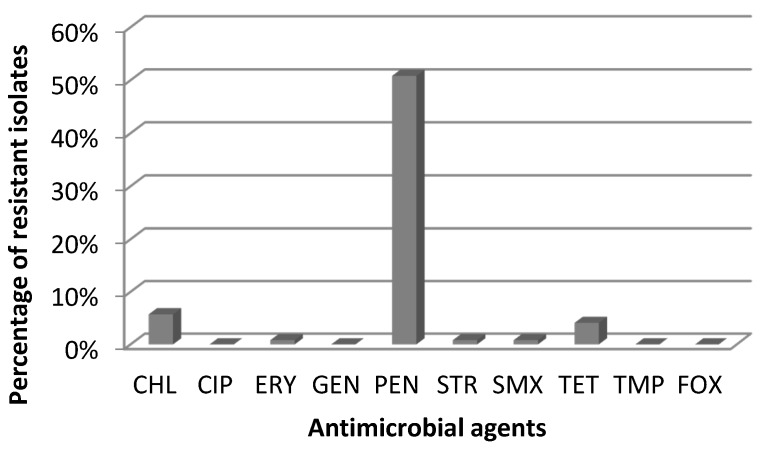
Resistance to penicillin (62 of 122 isolates; 50.8%) was the most common among the tested S. aureus, followed by chloramphenicol (7; 5.7%), tetracycline (5; 4.1%), sulphamethoxazole (1; 0.8%) erythromycin (1; 0.8%) and streptomycin (1; 0.8%) (Figure 2). Of the 62 penicillin resistant S. aureus isolates, nine were also resistant to other antimicrobials: four to chloramphenicol, three to tetracycline, one to sulphamethoxazole and one to chloramphenicol together with streptomycin.
Figure 2.
Antimicrobial resistance of S. aureus isolates.
3. Discussion
In this study, the prevalence and enterotoxigenicity of S. aureus isolated on dairy farms with small scale production of raw milk cheeses were tested. The results indicated that 46.2% of the raw milk samples were contaminated with CPS at the level up to 5 log10 CFU mL−1. It is interesting that in milk from two farms, CPS were not detected. A similar level of contamination of raw milk with CPS has been found by other authors [15,16,17,18,19]. However, there are also publications in which the rate of contamination was higher than in the present study. Peles et al. showed that bulk tank milk from 14 out of 20 farms was contaminated with S. aureus at levels up to 3.78 log10 CFU mL−1 [20]. Tondo et al. reported that S. aureus was present in 90.4% of raw milk samples with a mean number of 3.54 log10 CFU mL−1 [21]. These bacteria may be introduced to bulk milk either by direct excretion from the udder of a cow with clinical or subclinical staphylococcal mastitis or by fecal contamination [22]. In well drawn milk contamination with S. aureus ranged from 2.0 to 2.30 log10 CFU mL−1 but in the case of the presence of bacteria in the udder the number of these microorganisms may increase up to 4 log10 CFU mL−1 [23]. In the present study neither clinical nor subclinical mastitis were indicated in the cows from the dairy farms examined. Therefore, one of the possible explanations of the higher S. aureus level in some raw milk samples may be their contamination from the milking equipment or personnel involved in production.
The results of the current investigation showed that the contamination levels of the cheese gradually increased during its manufacture. It was observed that in farms in which both the quality of milk was high and the hands of cheese makers were negative for CPS, the number of staphylococci in mature cheese was lower or equal to 5 log10 CFU g−1. On the other hand, in farms with a high number of CPS both in milk and in hand’s swabs, the level of contamination increased along the production chain and peaked in the final products at as high as 6–7 log10 CFU g−1. An increase in contamination with S. aureus, of traditionally made cow milk cheese has been reported by others authors, especially at the stage from milk to curd [18]. These increasing levels may be explained by physical entrapment of S. aureus in the curd and the ability of these bacteria to grow rapidly in milk, as demonstrated by their generation time of 0.8 h at 25 °C [24,25]. Another possible source of contamination are people involved in cheese manufacturing, since S. aureus is frequently found on the skin of cheese makers [8,26]. In the present study, CPS were quite often detected on the hands of cheese makers in six out of nine tested farms and this could be the main source of contamination in the later stages of cheese manufacture.
None of the cheese contaminated with CPS contained staphylococcal enterotoxins but 45.1% isolated S. aureus harbored the SE genes. However, enterotoxins’ production by such strains is possible under appropriate environmental conditions, especially considering the temperature. The capability of the isolates for the production of enterotoxins A – E was confirmed in all sea – see-positive (31 of 122; 25.4%) isolates. Eight types of the SE genes (sea, sec, sed, seg, sei, sej, sep, ser) were detected in the isolated CPS and most of them (26 of 55; 47.3%) harbored the combination of the sed, sej and ser genes. The coexistence of the sed and sej markers has previously been reported by other authors and is due to the common location of the genes on the same plasmid [27]. The second largest subgroup of enterotoxigenic CPS detected in the present study was that with the sep gene (27.3%). Only one S. aureus strain had the sea gene. These results are similar to the study of Hummerjohann et al. who showed that S. aureus with the SE gene pattern sed, sej and ser dominated among the isolates recovered from Swiss raw milk cheeses [28]. Furthermore, several Italian studies also reported a dominance of the sed marker, which was often associated with the sea and sej genes [9,29,30]. In Norway, Jorgensen et al. indicated that 55% of CPS isolated from bovine bulk milk and 14.7% recovered from different stages of cheese production possessed SE genes but only the seg and sei markers were identified [19]. On the other hand, the present results are in contrast to the studies from Japan, Austria, France and Turkey, where the sec marker was commonly detected in S. aureus originating from milk and raw milk cheeses [10,31,32,33]. These data may possibly suggest a prevalence of certain S. aureus in different geographical regions.
The present study has shown that most S. aureus isolated from cheese were resistant to penicillin (50.8%); only a few of them showed resistance to tetracycline and sulfamethoxazole. Most of the CPS (47.5%) were resistant to one antimicrobial which is typical for strains of veterinary origin, whereas in human isolates rather multiresistant strains predominate [34]. Our results are comparable to those reported by other investigators [9,20]. The present results of antimicrobial resistance are in correlation with antibiotics that are used for treatment of bovine mastitis in Poland. Krasucka et al., based on a questionnaire completed by 109 veterinarians from the whole of Poland, showed that penicillins are mostly used in treatment of cattle infections [35]. According to the report of the European Medicines Agency, in Poland in 2012 penicillins, tetracyclines and sulfonamides accounted for over 75% of the total of veterinary antimicrobial agents sold [36].
The obtained results have also indicated resistance to chloramphenicol in 5.7% of the isolates tested, although this antibiotic is banned in the European Union for animals bred for human consumption. However, this resistance may be the result of the presence of chloramphenicol naturally occurring in the environment [37].
The obtained results showed that the analyzed naturally ripened rennet cheese was safe for consumers. However, a significant proportion of samples was contaminated with S. aureus and therefore, more attention should be paid to animal welfare and hygiene of cheese manufacturing, especially quality of raw milk to improve the safety of these traditional products.
4. Materials and Methods
4.1. Sample Collection
A total of 244 samples from various stages of cheese production were collected between 2011 and 2013 from nine dairy farms located in the north-eastern part of Poland. They were small dairy farms rearing 4–6 milking cows and producing naturally ripened rennet cheese from raw unpasteurized cow milk. The production of such cheeses proceeds as follows: raw milk is heated to about 32 °C, the rennin added starts the process of cloth formation. Afterwards, the milk curds are cut and the liquid whey is separated. In the next stage, cheese grains are rinsed with warm water and once again liquid whey is separated. The grains are placed in special forms where the cheese is drained off, formed and the salt is added. After this step, the cheeses are transported to the specially assigned places for ripening. The following samples were collected from cheese production stages of which the highest level of S. aureus was expected: 26 of raw milk, 90 of semi-finished products (heated milk, curd, grains, formed cheese), 26 of final products and 102 of swabs from the production environment. Once on each farm, 8–10 samples were taken from the same stages of the production chain. Raw milk and semi-finished products were collected on the day of cheese production, whereas the final products were taken after two weeks of cheese ripening. The swabs were obtained using sterile 50 cm2 sampling sponges with maximum recovery diluent (TSC Ltd., Lancashire, UK). The swabs from the production environment, i.e., milk tanks, were collected using 10 × 10 cm2 sterile propylene templates (TSC).
4.2. Enumeration of CPS
Enumeration of CPS was performed on Baird-Parker agar with rabbit plasma fibrinogen (bioMerieux, Marcy-I’Etoile, France). After 48 h of incubation at 37 ± 1 °C, one typical colony was taken for further identification [38].
4.3. Identification of S. aureus
S. aureus was identified by PCR detection of the nuc and 16S rRNA genes according to the protocols recommended by the European Union Reference Laboratory for Antimicrobial Resistance [39].
4.4. Detection of Staphylococcal Enterotoxins Genes
The presence of SE genes was determined using two multiplex PCR assays. The first one was performed with six pairs of primers allowing detection of the genes: sea, seb, sec, sed, see and ser [40]. The second reaction enabled us to identify the seg, seh, sei, sej and sep genes [41].
4.5. Detection of Staphylococcal Enterotoxins A-E
The samples contaminated with CPS and all the final products were analyzed using a two-step method consisting of extraction/concentration and detection by enzyme linked fluorescent assay (ELFA) according to the European Union Reference Laboratory for Coagulase Positive Staphylococci protocol with the Vidas SET 2 test (bioMerieux) [42]. Detection of staphylococcal enterotoxins was also performed for S. aureus isolates harboring the sea-see genes after 72 h of incubation in brain heart infusion broth (BHI, Oxoid, Hampshire, UK) at 37 °C using the Ridasceen SET Total kit (R-Biopharm, Darmstadt, Germany).
4.6. Antimicrobial Resistance
A microbroth dilution method was used to establish the minimum inhibitory concentrations (MICs) of S. aureus isolates to 10 antimicrobial agents using the DKVP microplates (Trek Diagnostic Systems, Thermo Fisher Scientific, East Grinstead, UK). Antimicrobials, dilution ranges, and cut-off values used for MIC determination are described in Table 1. The isolates were cultured on Columbia agar supplemented with 5% sheep blood (bioMerieux) at 37 °C for 24 h. The MICs were established using Mueller-Hinton broth (Trek Diagnostic Systems, Thermo Fisher Scientific, East Grinstead, UK). The microplates were incubated at 36 °C for 18–20 h and read using the Vision® system (Swin Version 3.3, Trek Diagnostic System, Thermo Fisher Scientific, East Grinstead, UK, 2011). The cut off values for the interpretation of the MIC results were in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the EURL-AR (Table 2).
Table 2.
Antimicrobials, dilution ranges and cut-off values used for minimum inhibitory concentrations (MIC) determination of S. aureus.
| Antimicrobial Class | Antimicrobials | Dilution Range (mg/L) | Cut off Values (mg/L) Resistant > |
|---|---|---|---|
| Amphenicols | Chloramphenicol (CHL) | 2–64 | 16 |
| Fluoroqinolones | Ciprofloxacin (CIP) | 0.12–8 | 1 |
| Macrolides | Erythromycin (ERY) | 0.25–16 | 1 |
| Aminoglycosides | Gentamicin (GEN) | 0.25–16 | 2 |
| Streptomycin (STR) | 4–64 | 16 | |
| β-Lactames | Penicillin (PEN) | 0.06–16 | 0.125 |
| Cephalosporins | Cefoxitin (FOX) | 0.5–32 | 4 |
| Tetracyclines | Tetracycline (TET) | 0.5–32 | 1 |
| Sulfonamides | Sulfamethoxazole (SMX) | 32–512 | 128 |
| Other | Trimethoprim (TMP) | 0.5–32 | 4 |
Author Contributions
J.G.R. conceived the study, designed the experiments, coordinated the investigation, analyzed the data and wrote the paper. A.C. and W.K-D. performed laboratory analysis and drafted the experimental section. J.O. critically reviewed the manuscript. All the authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Argudin M.A., Mendoza M.C., Rodicio M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins. 2010;2:1751–1773. doi: 10.3390/toxins2071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennekinne J.A., de Buyser M.L., Dragacci S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012;36:815–836. doi: 10.1111/j.1574-6976.2011.00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Le Loir Y., Baron F., Gautier M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2003;2:63–76. [PubMed] [Google Scholar]
- 4.Waage S., Mørk T., Røros A., Aasland D., Hunshamar A., Odegaard S.A. Bacteria associated with clinical mastitis in dairy heifers. J. Dairy Sci. 1999;82:712–719. doi: 10.3168/jds.S0022-0302(99)75288-4. [DOI] [PubMed] [Google Scholar]
- 5.Beuvier E., Buchin S. Raw milk cheeses. In: Fox P.F., McSweeney P.L.H., Cogan T.M., Guinee T.P., editors. Cheese: Chemistry, Physics and Microbiology. Elsevier Academic Press; Amsterdam, The Netherlands: 2004. pp. 319–345. [Google Scholar]
- 6.Williams A.G., Withers S.E. Microbiological characterisation of artisanal farm house cheeses manufactured in Scotland. Int. J. Dairy Technol. 2010;63:356–369. doi: 10.1111/j.1471-0307.2010.00596.x. [DOI] [Google Scholar]
- 7.De Buyser M.L., Dufour B., Maire M., Lafarge V. Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. Int. J. Food Microbiol. 2001;67:1–17. doi: 10.1016/S0168-1605(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 8.André M.C.D.P.B., Campos M.R.H., Borges L.J., Kipnis A., Pimenta F.C., Serafini A.B. Comparison of Staphylococcus aureus isolates from food handlers, raw bovine milk and Minas Frescal cheese by antibiogram and pulsed-field gel electrophoresis following Smal digestion. Food Control. 2008;19:200–207. doi: 10.1016/j.foodcont.2007.03.010. [DOI] [Google Scholar]
- 9.Normanno G., Corrente M., La Salandra G., Dambrosio A., Quaglia N.C., Parisi A., Greco G., Ballacicco A.L., Virgilio S., Celano G.V. Methicillin-resistant Staphylococcus aureus (MRSA) in foods of animal origin product in Italy. Int. J. Food Microbiol. 2007;117:219–222. doi: 10.1016/j.ijfoodmicro.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Loncarevic S., Jørgensen H.J., Løvseth A., Mathisen T., Rørvik L.M. Diversity of Staphylococcus aureus enterotoxin types within single samples of raw milk and raw milk products. J. Appl. Microbiol. 2005;98:344–350. doi: 10.1111/j.1365-2672.2004.02467.x. [DOI] [PubMed] [Google Scholar]
- 11.Pinchuk I.V., Beswick E.J., Reyes V.E. Staphylococcal enterotoxins . Toxins. 2010;2:2177–2197. doi: 10.3390/toxins2082177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EC—European Commission Opinion on Staphylococcal Enterotoxins in milk products, particularly cheeses (adopted on 26–27 March 2003) 2003. [(accessed on 26–27 March 2003)]. Available online: http://ec.europa.eu/food/fs/sc/scv/out61_en.pdf.
- 13.EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015;13:3991. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rola J.G., Korpysa-Dzirba W., Osek J. Prevalence of Staphylococcus aureus and staphylococcal enterotoxins at different stages of production of raw milk cheeses—Preliminary results. Bull. Vet. Inst. Pulawy. 2013;57:341–345. doi: 10.2478/bvip-2013-0059. [DOI] [Google Scholar]
- 15.Bianchi D.M., Gallina S., Bellio A., Chiesa F., Civera T., Decastelli L. Enterotoxin gene profiles of Staphylococcus aureus isolated from milk and dairy products in Italy. Lett. Appl. Microbiol. 2014;58:190–196. doi: 10.1111/lam.12182. [DOI] [PubMed] [Google Scholar]
- 16.Hill B., Smythe B., Lindsay D., Shepherd J. Microbiology of raw milk in New Zealand. Int. J. Food Microbiol. 2012;157:305–308. doi: 10.1016/j.ijfoodmicro.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Hunt K., Schelin J., Radström P., Butler F., Jordan K. Classical enterotoxins of coagulase-positive Staphylococcus aureus isolates from raw milk and products for raw milk cheese production in Ireland. Dairy Sci. Technol. 2012;92:487–499. doi: 10.1007/s13594-012-0067-4. [DOI] [Google Scholar]
- 18.Jakobsen R.A., Heggebo R., Sunde E.B., Skjervheim M. Staphylococcus aureus and Listeria monocytogenes in Norwegian raw milk cheese production. Food Microbiol. 2011;28:492–496. doi: 10.1016/j.fm.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen H.J., Mork T., Hogasen H.R., Rorvik L.M. Enterotoxigenic Staphylococcus aureus in bulk milk in Norway. J. Appl. Microbiol. 2005;99:158–166. doi: 10.1111/j.1365-2672.2005.02569.x. [DOI] [PubMed] [Google Scholar]
- 20.Peles F., Wagner M., Varga L., Hein I., Rieck P., Gutser K., Kereszturi P., Kardos G., Turcsanyi I., Beri B., et al. Characterization of Staphylococcus aureus strains isolated from bovine milk in Hungary. Int. J. Food Microbiol. 2007;118:186–193. doi: 10.1016/j.ijfoodmicro.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Tondo E.C., Guimaraes M.C., Henriques J.A., Ayub M.A. Assessing and analysing contamination of a dairy products processing plant by Staphylococcus aureus using antibiotic resistance and PFGE. Can. J. Microbiol. 2000;46:1108–1114. doi: 10.1139/w00-111. [DOI] [PubMed] [Google Scholar]
- 22.Callon C., Gilbert F.B., Cremoux R.D., Montel M.C. Application of variable number of tandem repeat analysis to determine the origin of S. aureus contamination from milk to cheese in goat cheese farms. Food Control. 2008;19:143–150. doi: 10.1016/j.foodcont.2007.02.014. [DOI] [Google Scholar]
- 23.Asperger H., Zangerl P. Staphylococcus aureus . In: Roginski H., Fuquay J.W., Fox P.F., editors. Encyclopaedia of Dairy Sciences. Academic Press; San Diego, CA, USA: 2003. pp. 2563–2569. [Google Scholar]
- 24.Tatini S.R., Jezeski J.J., Morris H.A., Olson J.C., Jr., Casman E.P. Production of staphylococcal enterotoxin A in cheddar and Colby cheese. J. Dairy Sci. 1971;54:815–825. doi: 10.3168/jds.S0022-0302(71)85925-8. [DOI] [PubMed] [Google Scholar]
- 25.Le Marc Y., Valík L., Medveďová A. Modelling the effect of the starter culture on the growth of Staphylococcus aureus in milk. Int. J. Food Microbiol. 2009;129:306–311. doi: 10.1016/j.ijfoodmicro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Lawrynowicz-Paciorek M., Kochman M., Piekarska K., Grochowska A., Windyga B. The distribution of enterotoxin and enterotoxin-like genes in Staphylococcus aureus strains isolated from nasal carriers and food samples. Int. J. Food Microbiol. 2007;117:319–323. doi: 10.1016/j.ijfoodmicro.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S., Iandolo J.J., Stewart G.C. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej) FEMS Microbiol. Lett. 1998;168:227–233. doi: 10.1111/j.1574-6968.1998.tb13278.x. [DOI] [PubMed] [Google Scholar]
- 28.Hummerjohann J., Naskova J., Baumgartner A., Graber H.U. Enterotoxin-producing Staphylococcus aureus genotype B as a major contaminant in Swiss raw milk cheese. J. Dairy Sci. 2014;97:1305–1312. doi: 10.3168/jds.2013-7643. [DOI] [PubMed] [Google Scholar]
- 29.Cremonesi P., Perez G., Pisoni G., Moroni P., Morandi S., Luzzana M., Brasca M., Castiglioni B. Detection of enterotoxigenic Staphylococcus aureus isolates in raw milk cheese. Lett. Appl. Microbiol. 2007;45:586–591. doi: 10.1111/j.1472-765X.2007.02231.x. [DOI] [PubMed] [Google Scholar]
- 30.Morandi S., Brasca M., Lodi R., Cremonesi P., Castiglioni B. Detection of classical enterotoxins and identification of enterotoxin genes in Staphylococcus aureus from milk and dairy products. Vet. Microbiol. 2007;124:66–72. doi: 10.1016/j.vetmic.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Katsuda K., Hata E., Kobayashi H., Kohmoto M., Kawashima K., Tsunemitsu H., Eguchi M. Molecular typing of Staphylococcus aureus isolated from bovine mastitic milk on the basis of toxin genes and coagulase gene polymorphisms. Vet. Microbiol. 2005;105:301–305. doi: 10.1016/j.vetmic.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Villard L., Lamprell H., Borges E., Maurin F., Noel Y., Beuvier E., Chamba J.F., Kodjo A. Enterotoxin D producing strains of Staphylococcus aureus are typeable by pulsed-field gel electrophoresis (PFGE) Food Microbiol. 2005;22:261–265. doi: 10.1016/j.fm.2004.02.005. [DOI] [Google Scholar]
- 33.Kav K., Col R., Ardic M. Characterization of Staphylococcus aureus isolates from white-brined Urfa cheese. J. Food Prot. 2011;74:1788–1796. doi: 10.4315/0362-028X.JFP-11-179. [DOI] [PubMed] [Google Scholar]
- 34.Werckenthin C., Cardoso M., Martel J.L., Schwarz S. Antimicrobial resistance in staphylococci from animals with particular reference to bovine Staphylococcus aureus and porcine Staphylococcus hyicus and canine Staphylococcus intermedius. Vet. Res. 2001;32:341–362. doi: 10.1051/vetres:2001129. [DOI] [PubMed] [Google Scholar]
- 35.Krasucka D., Cybulski W., Klimowicz A., Dzierżawski A. Evaluation of antimicrobial agents consumption in swine and cattle in Poland based on a questionnaire in 2010. Medycyna. Wet. 2012;68:102–105. [Google Scholar]
- 36.European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption Sales of veterinary antimicrobial agents in 26 EU/EEA countries in 2012. [(accessed on 15 October 2014)]. (EMA/333921/2014) Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/10/WC500175671.pdf.
- 37.Berendsen B., Stolker L., de Jong J., Nielsen M., Ttserendorj E., Sodnomdarjaa R., Cannavan A., Elliott C. Evidence of natural occurrence of the banned antibiotic chloramfenicol in herbs and grass. Anal. Bioanal. Chem. 2010;397:1955–1963. doi: 10.1007/s00216-010-3724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Microbiology of Food Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species). Part 2: Technique Using Rabbit Plasma Fibrynogen Agar Medium. International Standard Organisation; Geneva, Switzerland: 1999. ISO 6888–2, 1999; pp. 1–7. [Google Scholar]
- 39.Anonymous. Multiplex PCR for the detection of mecA gene and identification of Staphylococcus aureus. [(accessed on 20 April 2009)]. Available online: http//www.crl-ar.eu/data/images/tc_april-2009/6-detailed%20meca-pcr_protocol.pdf.
- 40.De Buyser M.L., Grout J., Brisabois A., Assere A., Lombard B. Detection of Genes Encoding Staphylococcal Enterotoxins. Multiplex PCR for Sea to See and Ser. Method of the CRL for Coagulase Positive Staphylococci Including Staphylococcus aureus. 1st ed. CRL CPS; AFSSA; Maisons-Alfort, France: 2009. pp. 1–5. [Google Scholar]
- 41.De Buyser M.L., Grout J., Brisabois A., Assere A., Lombard B. Detection of Genes Encoding Staphylococcal Enterotoxins. Multiplex PCR for Seg to Sej and Sep. Method of the CRL for Coagulase Positive Staphylococci Including Staphylococcus aureus. 1st ed. CRL CPS; AFSSA; Maisons-Alfort, France: 2009. pp. 1–5. [Google Scholar]
- 42.Ostyn A., Prufer A.L., Papinaud J., Hennekinne J.-A., Assere A., Lombard B. Detection of Staphylococcal Enteritoxins Types SEA to SEE in All Types of Food Matrices. European Screening Method of the EU-RL for “Coagulase Positive Staphylococci Including Staphylococcus aureus”. 5th ed. EU-RL CPS; ANSES; Maisons-Alfort, France: 2010. pp. 1–12. [Google Scholar]