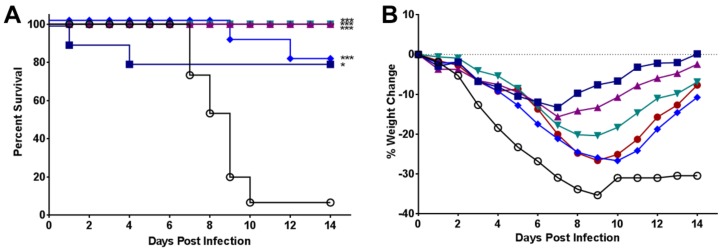
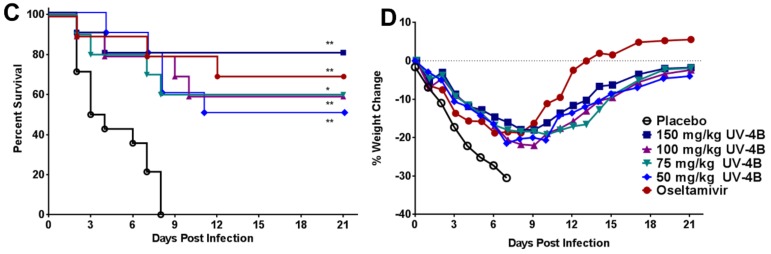
Figure 3.
Antiviral efficacy of UV-4B in lethal mouse models of influenza A (H1N1) virus infections. Female BALB/c mice were infected with ~1LD90 of influenza virus via intranasal instillation. Mice were administered UV-4B diluted in sterile water. The dose levels are expressed as the active free base form, UV-4. Mice (n = 10/group) were orally treated with 50, 75, 100 or 150 mg/kg/dose of UV-4, vehicle or oseltamivir starting one hour before infection. Efficacy data are plotted as percent survival against days post virus exposure. Average weight changes per group are plotted versus days post virus exposure. (A,B) UV-4 efficacy following infection with influenza A/CA/04/09 (H1N1pdm09). In this study, UV-4 was administered for 10 days and oseltamivir was administered at 20 mg/kg/dose BID for 5 days. Mice were monitored for through 14 days post infection. (C,D) UV-4 efficacy following infection with influenza A/New Caledonia/99 (H1N1). In this study, UV-4 was administered for 7 days and oseltamivir was administered at 10 mg/kg/dose TID for 5 days. Mice were monitored for through 21 days post infection. * p < 0.01, ** p < 0.001, *** p < 0.0001.