Introduction
Approximately 25,000 allogeneic hematopoietic stem cell transplants (HSCTs) are performed annually. Cutaneous complications are common and include a spectrum of acute and chronic graft-versus-host disease (GVHD). Mechanisms implicated in the rare development of sarcoidosis after HSCT include specific pretransplant conditioning regimens, possible donor-to-recipient transmission, genetic predisposition of sarcoidosis, and post-HSCT immune dysregulation.
Report of a case
A 58-year-old woman with a history of polymyalgia rheumatica, myelofibrosis (27 months postallogeneic HSCT from her human leukocyte antigen [HLA]-matched brother), hypothyroidism, GVHD of the gut, and noncaseating granulomas on lung biopsy, presented with the following new skin findings: white, firm lesions under her breasts (Fig 1) and an increasing number of asymptomatic red scaly bumps on her bilateral forearms and trunk (Fig 2). The lesions persisted despite use of triamcinolone 0.1% ointment. She denied family history of skin or lung disease. Her medications included omeprazole, vitamin D, calcium, duloxetine, levothyroxine, and alprazolam as needed. Importantly, she had not received any immunosuppressive therapy for almost 1 year.
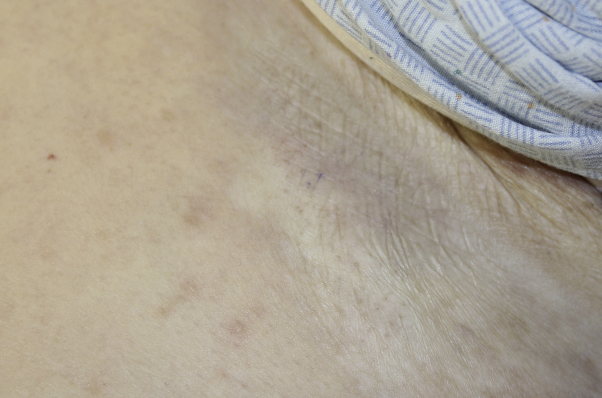
Fig 1.
White firm atrophic plaques of the inframammary skin.
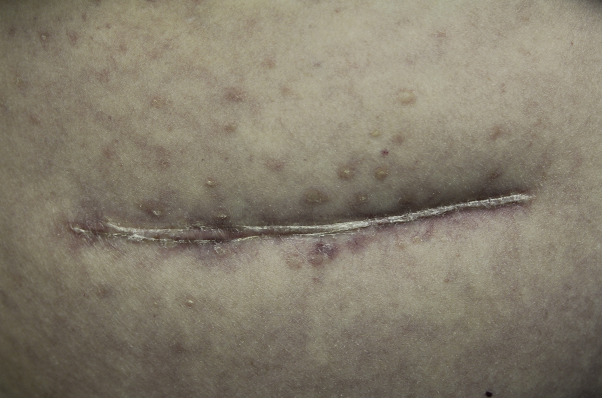
Fig 2.
Erythematous-to-violaceous papules with minimal scale centered around an old, well-healed abdominal scar.
Physical examination found bilateral inframammary white atrophic plaques and erythematous-to-violaceous guttatelike papules with minimal scale on her bilateral dorsal forearms and lower back. The papules were also becoming confluent around an old scar on her abdomen. Biopsy of an inframammary atrophic plaque found epidermal thinning and atrophy, papillary dermal hyalinization and sclerosis, telangiectasias, and sparse interstitial lymphocytic infiltrate. Biopsies of the abdominal scar and forearm found numerous epithelioid histiocytes and multinucleated giant cells forming noncaseating granulomas without polarizable material; special stains were negative for organisms.
She received a diagnosis of cutaneous chronic GVHD, manifested as lichen sclerosus, and cutaneous and pulmonary sarcoidosis. Thirty-one months after transplantation, her donor HLA-matched brother did not have any signs or symptoms of sarcoidosis.
Discussion
In 2014, the National Institutes of Health proposed updated diagnostic requirements for chronic GVHD, none of which include granulomatous phenomenon.1 Thorough literature review found 9 cases of sarcoidosis after allogeneic HSCT, all reported in nondermatologic literature (Table I).2, 3, 4, 5, 6, 7, 8, 9 Five of the 9 cases describe cutaneous findings, 4 of which had cutaneous biopsies showing noncaseating granulomas. An additional 5 cases of sarcoidosis have been reported after autologous HSCT.10
Table I.
Characteristics of patients reported to have sarcoidosis after allogeneic HSCT
| Study | Heyll2 | Sundar3 | Tauro4 | Morita5 | Bhagat6 | Gooneratne7 | Pukiat8 | Kushima9 | Current case | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) & Sex | 34 M | 37 F | 36 F | 55 F | 51 F | 45 F | 51 M | 47 M | 64 F | 58 F |
| Type of HSCT, donor | Allo, sibling | Allo, sibling | Allo, sibling | Allo, UD | Allo | Allo, UD | Allo, UD | Allo, UD | Allo, UD | Allo, sibling |
| Family history of sarcoidosis | +Donor | +Donor | +Donor & +Patient | None | None | None | None | None | None | None |
| Donor diagnosed with sarcoid before or after the HSCT? (at the time of case publication) | Yes, before the HSCT | Yes after the HSCT (Lymph node biopsies with noncaseating granulomas) | Yes, before the HSCT (sarcoidosis of the liver) | Unknown | Unknown | No | No | No | Unknown | No signs or symptoms of sarcoidosis to date (31 months posttransplant) |
| Underlying disease | NHL | B cell lymphoma | CML | FL | CML | MDS | MDS | CMML | ATL | MF |
| HLA | A1, A2, B8, B51, DR4, DR4 | A(1,26), B(8,49), Cw(7,-), DRb1*03011, DRb1*1302 | A-24(9), B-8 52(5), C-7 16; DR-17(3) 18(3) DQ-2 4 DP- |
Donor had one locus mismatch HLA phenotype & genotype (DRB1 locus) | Unknown | Unknown | Unknown | A*0101, A*2501, B*0801, B*1801, DRB1*0301, DRB1*1501, DQB1*0201, DQB1*0602 | Unknown | A*0301, B*0702, B*5501, C*0702, C*0304, DRB1*1301, DRB1*1501, DQB1*0602 |
| Post-HSCT period (mo) | 3 | 18 | 21 | 6 | 6 | 12 | 20 | 22 | 16 | 27 |
| Organs confirmed to have noncaseating granulomatous changes on biopsy | Lung & liver | Lung, (skin biopsy not done) | Bone marrow | Mediastinal lymph nodes, pleura, and a piece of lung | Lung | Liver, Skin: non-caseating granulomas |
Liver | Lymph nodes, Skin: poorly defined granulomatous inflammation in the deep dermis |
Lung, mediastinal lymph nodes, Subcutaneous mass in left upper arm: noncaseating granulomas |
Lung, skin: noncaseating granulomas |
| Clinically evident cutaneous lesions reported | No | Petechial rash owing to chronic GVHD over lower extremities; skin bx not done |
No | No | No | Purple-brown firm skin lesions on limbs, upper back, right cheek | No | Papular rash (cutaneous chronic GVHD), indurated erythematous plaque (granulomatous) |
Subcutaneous mass in left upper arm | Red papules with minimal scale on forearms and trunk; White firm atrophic patches inferior to bilateral breasts |
Allo, Allogeneic; ATL, adult T-cell leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; FL, follicular lymphoma; MDS, myelodysplastic syndrome; MF, myelofibrosis; NHL, non-Hodgkin's lymphoma; UD, unrelated donor.
Immune dysregulation, both donor derived and de novo, after allogeneic HSCT is postulated to provide the immunologic environment necessary for the development of sarcoidosis. Additionally, 2 reported cases have confirmed donor-derived sarcoidosis with chimerism analysis of sarcoidal lesions.5, 9 Genetic predisposition to sarcoidosis is well established and frequently associated with HLA types A1, B8, and DR3. Acute disease with good prognosis is associated with HLA types DRB1*0301 and DQB1*0201, whereas prolonged disease course with poor prognosis is associated with DRB1*1501 and DRB1*0602.11 Our patient and her healthy donor possess these 2 poor-prognostic HLA types. One of the donors in the 9 previously reported cases had sarcoidosis after the marrow donation, whereas 2 of the 9 had sarcoidosis diagnosed before the donation. Based on these findings, our patient's donor may be at an increased risk for sarcoidosis.
Immune dysregulation after HSCT is well known to manifest as a variety of forms of GVHD. Because sarcoidosis is also attributed to immune dysregulation, it is not surprising that this common granulomatous dermatitis may be seen with increased frequency after HSCT. Our case shows the simultaneous presentation of chronic GVHD in the form of lichen sclerosus and new-onset sarcoidosis after HSCT. Dermatologists should add sarcoidosis to the list of possible posttransplant cutaneous eruptions, especially in patients with susceptible HLA types. Further research including analysis of specific HLA types, information always available in the transplant population, may lead to a better understanding of the risk factors and pathogenesis of this granulomatous conundrum.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
This work has been presented previously at the 60th Annual Georgia Society of Dermatology and Dermatologic Surgery Meeting, Amelia Island, Florida, June 5-7, 2015.
References
- 1.Jagasia M.H., Greinix H.T., Arora M. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heyll A., Meckenstock G., Aul C. Possible transmission of sarcoidosis via allogeneic bone marrow transplantation. Bone Marrow Transplant. 1994;14(1):161–164. [PubMed] [Google Scholar]
- 3.Sundar K.M., Carveth H.J., Gosselin M.V., Beatty P.G., Colby T.V., Hoidal J.R. Granulomatous pneumonitis following bone marrow transplantation. Bone Marrow Transplant. 2001;28(6):627–630. doi: 10.1038/sj.bmt.1703192. [DOI] [PubMed] [Google Scholar]
- 4.Tauro S., Mahendra P. Resolution of sarcoidosis after allogeneic bone marrow transplantation with donor lymphocyte infusions. Bone Marrow Transplant. 2001;27(7):757–759. doi: 10.1038/sj.bmt.1702874. [DOI] [PubMed] [Google Scholar]
- 5.Morita R., Hashino S., Kubota K. Donor cell-derived sarcoidosis after allogeneic BMT. Bone Marrow Transplant. 2009;43(6):507–508. doi: 10.1038/bmt.2008.340. [DOI] [PubMed] [Google Scholar]
- 6.Bhagat R., Rizzieri D.A., Vredenburgh J.J., Chao N.J., Folz R.J. Pulmonary sarcoidosis following stem cell transplantation: is it more than a chance occurrence? Chest. 2004;126(2):642–644. doi: 10.1378/chest.126.2.642. [DOI] [PubMed] [Google Scholar]
- 7.Gooneratne L., Lim Z.Y., Vivier A.d. Sarcoidosis as an unusual cause of hepatic dysfunction following reduced intensity conditioned allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39(8):511–512. doi: 10.1038/sj.bmt.1705606. [DOI] [PubMed] [Google Scholar]
- 8.Pukiat S., McCarthy P.L., Jr., Hahn T. Sarcoidosis-associated MHC Ags and the development of cutaneous and nodal granulomas following allogeneic hematopoietic cell transplant. Bone Marrow Transplant. 2011;46(7):1032–1034. doi: 10.1038/bmt.2010.235. [DOI] [PubMed] [Google Scholar]
- 9.Kushima H., Ishii H., Ikewaki J., Takano K., Ogata M., Kadota J. Sarcoidosis in donor-derived tissues after haematopoietic stem cell transplantation. Eur Respir J. 2013;41(6):1452–1453. doi: 10.1183/09031936.00136112. [DOI] [PubMed] [Google Scholar]
- 10.Daikeler T., Labopin M., Di Gioia M. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118(6):1693–1698. doi: 10.1182/blood-2011-02-336156. [DOI] [PubMed] [Google Scholar]
- 11.Voorter C.E., Amicosante M., Berretta F., Groeneveld L., Drent M., van den Berg-Loonen E.M. HLA class II amino acid epitopes as susceptibility markers of sarcoidosis. Tissue Antigens. 2007;70:18–27. doi: 10.1111/j.1399-0039.2007.00842.x. [DOI] [PubMed] [Google Scholar]