Abstract
Presynaptic differentiation is a critical and poorly understood step in synapse formation. Using compartmentalized culture systems that isolate axons and nascent synapses, Pinto et al. (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201509039) show that the axonal ubiquitin–proteasome system locally regulates the accumulation of ubiquitinated substrates, triggering presynaptic differentiation.
One of the challenges associated with the study of neuronal signaling stems from the highly polarized morphology of neurons. Signaling pathways can be localized wholly within one compartment (e.g., a synapse, dendrite, axon, or soma) or can reflect coordinated interactions between different compartments. Distinguishing between these two possibilities is challenging because it is difficult to selectively manipulate proteins in individual cellular compartments. The interpretation of experiments applying signaling molecules or pharmacological reagents to neurons is complicated by the possible indirect effects manipulating one compartment could have on another. Thus, definitively establishing the spatial localization of individual physiological signaling events is difficult. This problem is particularly relevant to the study of synaptogenesis. Synaptogenesis involves the remodeling of clusters of synaptic vesicles and protein complexes in the axonal segment that ultimately becomes the presynaptic terminal (Gundelfinger et al., 2015). Numerous hints suggest that the ubiquitin–proteasome system (UPS) is involved in this process. First, nascent presynaptic terminals are enriched in ubiquitin and UPS proteins (Speese et al., 2003; Segref and Hoppe, 2009), and mice deficient in the turnover of ubiquitinated protein conjugates have defects in synapse formation (Wilson et al., 2002; Chen et al., 2009, 2011). However, studying this process in cultured neurons is complicated, because pharmacological inhibitors of the UPS lead to alterations in cell-wide functions. Thus, other approaches are needed to investigate the direct role of the axonal UPS on the presynaptic protein complex. In this issue, Pinto et al. address the functional role of the UPS in presynaptic differentiation using a microfluidic culturing system that isolates axons and spatially limits pharmacological treatments to the axonal UPS.
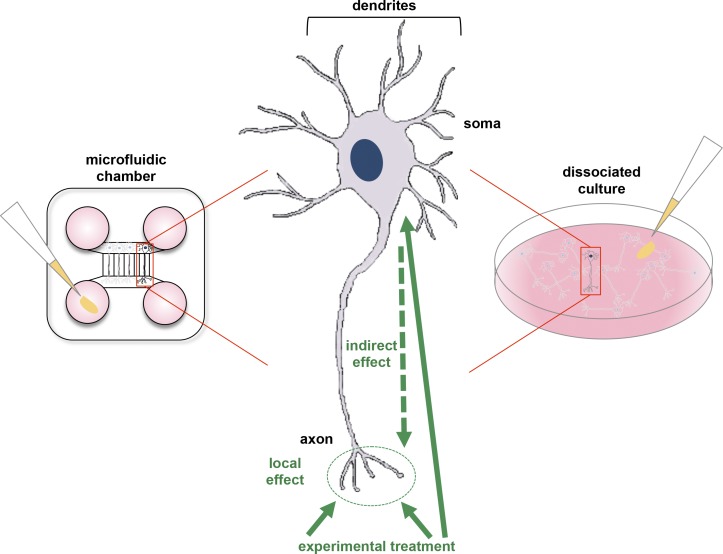
The microfluidic system used in this study has two main compartments, each with two reservoirs (Taylor et al., 2005), and the two compartments are connected by evenly spaced microgrooves (Fig. 1). Fluid flow is markedly impaired in the microgrooves, providing fluidic isolation between the two compartments. Dissociated neurons are introduced into one compartment and cultured. Only dendrites and axons fit into the microgrooves and extend into the other compartment (Fig. 1). If the microgrooves are sufficiently long, e.g., longer than 150 µm, only axons are long enough to enter the distal compartment. By manipulating the microgrooves’ length, axons are effectively separated from dendrites. Thus, culturing neurons in microfluidic chambers allows selective application of treatments to specific neuronal compartments, eliminating the ambiguity arising from stimulating axons and somata simultaneously.
Figure 1.
Differences between conventional and microfluidic chamber cultures. On the left, a microfluidic chamber is shown. The top and bottom compartments are connected with evenly spaced microgrooves. Dissociated neurons are added to the top compartment, which contains the somata. The bottom compartment contains the axons that have extended through the microgrooves. Experimental treatments, such as pharmacological reagents, are introduced into a reservoir (pink) linked to the bottom compartment (indicated with pipet tip). Only axons in the bottom compartment are exposed to these treatments. On the right, dissociated neuronal culture is illustrated. Treatments are “bath-applied,” which results in simultaneous exposure of the axons, dendrites, and somata. Such treatments trigger signaling pathways in all compartments, thereby potentially affecting axonal signaling pathways indirectly. In the middle, a diagram of a neuron is shown, indicating how bath treatments indirectly affect axons. This experimental setup fails to discern differences between globally and locally triggered signaling pathways in axons.
In their new work, Pinto et al. (2016) show that the axonal UPS plays a critical role in controlling the assembly of presynaptic vesicle clusters. The authors selectively applied proteasome inhibitors to axons of neurons cultured in microfluidic chambers and found a marked increase in presynaptic assembly sites. Local suppression of the UPS leads to the accumulation of presynaptic vesicle clusters and to the formation of functional presynaptic sites, suggesting that the UPS-mediated turnover of presynaptic vesicle clusters is an early step in presynaptic site formation. To determine if the UPS is inhibited when synapse formation is initiated, the researchers triggered the formation of presynaptic clusters by axonal application of poly-d-lysine–coated beads. They monitored the activity of the UPS through live imaging using a previously published GFP reporter that is highly unstable and is degraded in a UPS-dependent manner. The reporter accumulated at sites where beads were applied, providing evidence that the axonal UPS is normally suppressed as part of synaptogenesis.
However, the physiological trigger for presynaptic differentiation is a dendrite, not a bead. Pinto et al. (2016) used a microfluidic solution to test whether dendrites cause local suppression of the UPS at sites of axon–dendrite contacts. To fluidically isolate synapses that occur as a result of axon–dendrite contacts, the authors used a microfluidic chamber in which a third compartment was added between the two compartments described above (Taylor et al., 2010). Rat embryonic hippocampal neurons were introduced into the two outer compartments. The microgrooves connecting the outer compartments to the central compartment were of two different lengths, so that axons would grow from one outer compartment and dendrites from the other outer compartment. Axons and dendrites met to form synapses in the central compartment, termed the synaptic compartment. Using a reservoir connected to the synaptic compartment, Pinto et al. (2016) selectively treated synapses with specific signals and inhibitors. This experimental design enabled the authors to identify axonally localized signaling effectors that regulate presynapse assembly. This culturing approach is notably superior to cultures of dissociated neurons, for which pharmacological inhibitors are bath-applied to the entire culture, leading to neuron-wide alterations in signaling pathways, transcription, and other processes. Using this microfluidic device, the researchers created a compartment enriched in synapse formation, allowing them to conclude that synaptogenesis is associated with physiological suppression of the axonal UPS.
What is the function of the UPS in controlling presynaptic clusters? The UPS mediates degradation of ubiquitinated proteins and it can, for instance, be inhibited by blocking the E1 Ub-activating enzyme required for protein ubiquitination or by blocking the proteasome, which removes the ubiquitin chain and degrades proteins. Interestingly, in their experimental system, Pinto et al. (2016) observed that proteasome inhibitors, but not an E1 inhibitor alone, stimulated accumulation of presynaptic clusters. This result suggested that the accumulation of ubiquitin chains is the trigger for increasing the number of presynaptic clusters. In support of this idea, the authors show that addition of deubiquitinase inhibitors, which stabilize ubiquitin chains, also led to increased presynaptic clusters. They therefore propose that the ubiquitin chains themselves are the trigger for growth and assembly of a presynaptic terminal. Moreover, the researchers performed stainings of polyubiquitinated chains in neuronal cultures and observed accumulation of these chains in newly formed presynaptic clusters. Synaptic expression of polyubiquitinated chains was confirmed to be higher during the developmental stages corresponding to the peak of synaptogenesis in the hippocampus, providing further support for the idea that polyubiquitinated chains concentrate at nascent presynaptic sites.
The microfluidic system presented in Pinto et al. (2016) is particularly valuable for central nervous system (CNS) neurons because they cannot be readily cultured in the Campenot chamber system, another device for compartmentalized culture of neurons (Campenot, 1977). Campenot chambers are used primarily with sensory and sympathetic neurons of the peripheral nervous system: these neurons’ axons are sensitive to guidance cues from neurotrophin gradients and this system uses these gradients to direct axon growth underneath a divider into a separate compartment. CNS neurons lack this type of neurotrophin dependence. The microfluidic chamber system, however, does not require neurotrophins to direct CNS axons (Taylor et al., 2005). It is not entirely clear yet what makes axons enter the microgrooves; however, this behavior is central to the success of the microfluidic culturing method. The microfluidic system used by Pinto et al. (2016) has broad applicability for studying numerous aspects of neuronal function, especially in axons and dendrites. In particular, microfluidic culturing systems are useful to distinguish between signaling components localized in axons or dendrites from signaling pathways in cell bodies. It is likely that different signaling effectors are present in synapses, as synapses rely on a small subset of locally synthesized proteins to mediate signaling (Deglincerti et al., 2015). Thus, activation of a given receptor in synapses may yield different signaling effects than its activation in cell bodies, and microfluidic compartmentalization could help differentiate between these effects. Microfluidic systems also enable the detection of long-distance signaling between axons or dendrites and cell bodies. For instance, studies using cortical neurons in microfluidic chambers revealed that neurotrophins act directly on dendrites to elicit anterograde signaling to the cell body, which induces transcription of immediate early genes and translocation of TrkB, the brain-derived neurotrophic factor receptor, to the cell body (Cohen et al., 2011).
Altogether, the results from Pinto et al. (2016) show that polyubiquitinated proteins constitute a local trigger for presynaptic formation. The authors propose that transient and local reduction of proteasome activity after contact with a postsynaptic partner at an early time point results in accumulation of polyubiquitinated proteins, and that these polyubiquitin chains function as a platform promoting the clustering of presynaptic material and presynaptic differentiation. More work will be needed to identify the polyubiquitinated proteins prompting presynaptic differentiation and to decipher the mechanisms by which these peptides instruct differentiation. Further, it will be interesting to investigate how developing axons regulate UPS activity spatiotemporally. The work by Pinto et al. (2016) provides both an important foundation and valuable tools for future research.
Acknowledgments
This work was supported by National Institutes of Health grant NS056306 to S.R. Jaffrey.
The authors declare no competing financial interests.
References
- Campenot R.B. 1977. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. USA. 74:4516–4519. 10.1073/pnas.74.10.4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.C., Qin L.N., Li X.M., Walters B.J., Wilson J.A., Mei L., and Wilson S.M.. 2009. The proteasome-associated deubiquitinating enzyme Usp14 is essential for the maintenance of synaptic ubiquitin levels and the development of neuromuscular junctions. J. Neurosci. 29:10909–10919. 10.1523/JNEUROSCI.2635-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.C., Bhattacharyya B.J., Hanna J., Minkel H., Wilson J.A., Finley D., Miller R.J., and Wilson S.M.. 2011. Ubiquitin homeostasis is critical for synaptic development and function. J. Neurosci. 31:17505–17513. 10.1523/JNEUROSCI.2922-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.S., Bas Orth C., Kim H.J., Jeon N.L., and Jaffrey S.R.. 2011. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proc. Natl. Acad. Sci. USA. 108:11246–11251. 10.1073/pnas.1012401108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglincerti A., Liu Y., Colak D., Hengst U., Xu G., and Jaffrey S.R.. 2015. Coupled local translation and degradation regulate growth cone collapse. Nat. Commun. 6:6888 10.1038/ncomms7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundelfinger E.D., Reissner C., and Garner C.C.. 2015. Role of Bassoon and Piccolo in assembly and molecular organization of the active zone. Front. Synaptic Neurosci. 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M.J., Alves P.L., Martins L., Pedro J.R., Ryu H.R., Jeon N.L., Taylor A.M., and Almeida R.D.. 2016. The proteasome controls presynaptic differentiation through modulation of an on-site pool of polyubiquitinated conjugates. J. Cell Biol. 10.1083/jcb.201509039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segref A., and Hoppe T.. 2009. Think locally: control of ubiquitin-dependent protein degradation in neurons. EMBO Rep. 10:44–50. 10.1038/embor.2008.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese S.D., Trotta N., Rodesch C.K., Aravamudan B., and Broadie K.. 2003. The ubiquitin proteasome system acutely regulates presynaptic protein turnover and synaptic efficacy. Curr. Biol. 13:899–910. 10.1016/S0960-9822(03)00338-5 [DOI] [PubMed] [Google Scholar]
- Taylor A.M., Blurton-Jones M., Rhee S.W., Cribbs D.H., Cotman C.W., and Jeon N.L.. 2005. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods. 2:599–605. 10.1038/nmeth777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.M., Dieterich D.C., Ito H.T., Kim S.A., and Schuman E.M.. 2010. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron. 66:57–68. 10.1016/j.neuron.2010.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.M., Bhattacharyya B., Rachel R.A., Coppola V., Tessarollo L., Householder D.B., Fletcher C.F., Miller R.J., Copeland N.G., and Jenkins N.A.. 2002. Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat. Genet. 32:420–425. 10.1038/ng1006 [DOI] [PubMed] [Google Scholar]