Abstract
Acidic Ca2+ stores are important sources of Ca2+ during cell signaling but little is known about how Ca2+ enters these stores. In this issue, Melchionda et al. (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201510019) identify a Ca2+/H+ exchanger (CAX) that is required for Ca2+ uptake and cell migration in vertebrates.
Intracellular Ca2+ signaling is of fundamental importance in processes such as cell migration but we do not fully understand the contribution made by different intracellular Ca2+ stores to this particular function. Elevation of cytosolic Ca2+ by 10- to 100-fold the normal resting levels can occur by entry of external Ca2+ across the plasma membrane and release of Ca2+ from intracellular organelles such as the ER. Ca2+ ions are transported across membranes by ligand-gated ion channels, energy-dependent pumps, and transporters (Berridge et al., 2003; Lloyd-Evans et al., 2010). Intracellular Ca2+ levels are regulated in this manner from simple organisms, such as yeast, through to complex multicellular organisms, suggesting a degree of conservation across the taxonomic kingdoms (Patel and Cai, 2015). Recent evidence has indicated that “acidic Ca2+ stores” such as lysosomes in mammalian cells are a key intracellular Ca2+ signaling store, like the ER (Lloyd-Evans and Platt, 2011; Patel and Muallem, 2011). The Ca2+ concentration of the lysosome (500 µM) is similar to the ER (Christensen et al., 2002; Lloyd-Evans et al., 2008) but lysosomes are smaller in volume and their impact on cellular Ca2+ signaling seems localized to events that regulate endocytosis, vesicular fusion, and recycling (Ruas et al., 2010; López-Sanjurjo et al., 2013). However, there is a significant amount of evidence emerging that lysosomes are capable of triggering much larger changes in cytosolic Ca2+ during signaling via the induction of Ca2+ release from the ER. This effect appears to be mediated by the most potent intracellular Ca2+–releasing second messenger nicotinic acid adenine dinucleotide phosphate (NAADP), which triggers Ca2+ release from lysosomes via two-pore channels (Brailoiu et al., 2009; Calcraft et al., 2009). In addition to two-pore channels, acidic stores also express other Ca2+-permeable channels (summarized in Fig. 1).
Figure 1.
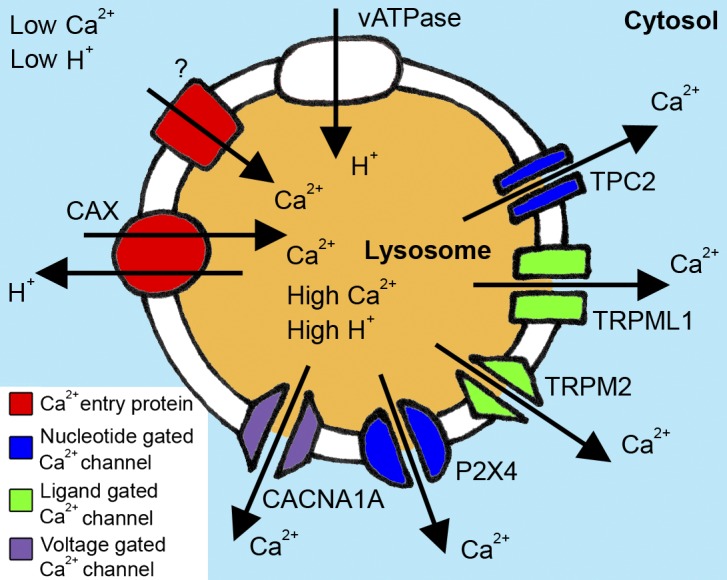
Lysosomal Ca2+ transporters and channels. Our current understanding of lysosomal Ca2+ transport and the proteins that regulate the transport of Ca2+ into and out of the lysosome is heavily stacked in favor of Ca2+ release channels. To date, voltage-gated (CaV2.1/CACNA1A), ligand-gated (TRPML1 and TRPM2), and nucleotide-gated (TPC1, TPC2, and P2X4) channels have all been identified or implicated in lysosomal Ca2+ release (Patel and Cai, 2015). Much less is known about the mechanisms of Ca2+ entry into lysosomes. In lower order organisms, CAX mediates lysosomal Ca2+ entry against the proton gradient. In this issue, Melchionda et al. (2016) provide the first evidence for a mammalian lysosomal Ca2+ uptake mechanism in nonplacental mammals. These findings provide further support for the key role of the lysosome as an intracellular Ca2+ store.
Despite recent advances in our knowledge of lysosomal Ca2+ release channels, we have so far failed to identify the transport proteins that fill the lysosome with Ca2+. Ca2+ entering the cell by endocytosis is removed by the early endosome after the initiation of endosomal acidification by the vATPase; therefore, it is likely that lysosomes have their own transporters or pumps to take up Ca2+ (Gerasimenko et al., 1998; Christensen et al., 2002). Although there have been studies suggesting the presence of ATPases and putative ion exchangers on mammalian cells (Styrt et al., 1988), the identity of the proteins that mediate lysosomal Ca2+ uptake remains elusive. In this issue, Melchionda et al. describe the first lysosomal CAX in nonplacental mammals and link lysosomal Ca2+ import via CAX to the maintenance of normal cellular migration during development.
To identify novel regulators of Ca2+ transport in vertebrates, Melchionda et al. (2016) searched gene databases for homologues of the CAX proteins, which are known to use the proton gradient across the vacuole to drive Ca2+ uptake in plant and yeast cells (Dunn et al., 1994). They identified putative CAX genes in many species, from sea urchins and frogs to reptiles and birds. The CAX homologues discovered in the genomes of the platypus and Tasmanian devil are the first lysosomal Ca2+ exchangers to be identified in any mammalian species. This new work is a significant finding as it suggests that these mechanisms do clearly exist in some mammalian cells and are required for lysosomal Ca2+ store filling. To examine the regulation of lysosomal Ca2+ uptake by vertebrate CAX transporters, the authors cloned full-length CAX from the frog and found that expression of frog CAX could rescue Ca2+ transport in yeast lacking their own CAX. Furthermore, the authors show that the frog CAX channels correctly localize to lysosomes when expressed in human cell lines and that these CAX are capable of manipulating lysosomal and cytosolic Ca2+ levels (in a manner perhaps comparable to plasma membrane Ca2+ ATPases). The findings reported in Melchionda et al. (2016) also have significance for researchers who are using simpler model organisms to characterize mechanisms regulating acidic store Ca2+. A study by Churchill et al. (2002) that used acidic stores purified from sea urchin egg homogenate to monitor acidic store Ca2+ entry concluded that vanadate-sensitive Ca2+ pumps were absent and suggested instead the presence of a CAX. This now appears to be the case through the reported cloning of sea urchin CAX. The findings of Melchionda et al. (2016) are a step forward in unraveling the molecular mechanisms of Ca2+ handling in model animals.
Ca2+ signaling plays an important role in development, particularly for cellular migration, where localized elevations in intracellular Ca2+ drive rearrangement of the cytoskeleton, cellular contraction, and adhesion (Wei et al., 2009; Sumoza-Toledo et al., 2011; Praitis et al., 2013). A concentration gradient of Ca2+ exists across the migrating cell, with higher levels at the rear that contribute to cellular detachment and contraction (Praitis et al., 2013). Recent evidence has highlighted the presence of Ca2+ flickers at the leading edge of the migrating cell that have been shown to underlie changes in direction (Wei et al., 2009). Despite the clear importance of Ca2+ in mediating cellular migration events and the emergent role of lysosomes in maintaining intracellular Ca2+ signaling, very little is known about the roles of lysosomal Ca2+ stores in cellular migration. ER Ca2+ channels including the inositol 1,4,5-trisphosphate receptors and ryanodine receptors as well as the secretory pathway Ca2+ ATPase and lysosomal TRPM2 have all been implicated in regulating changes in intracellular Ca2+ to mediate cellular migration, but to date no lysosomal transporters have been implicated in this process (Wei et al., 2009; Sumoza-Toledo et al., 2011; Praitis et al., 2013). Melchionda et al. (2016) investigated the migration of neural crest cells during frog development to find out whether or not CAX transporters control cell motility. CAX proteins are expressed in the neural crest of developing frogs and morpholino-mediated knockdown of CAX expression increased cytosolic Ca2+ levels and impeded neural crest cell migration. Confocal imaging of neural crest tissue in vitro revealed the dynamic recruitment of CAX-containing vesicles to the protrusions that contain focal adhesion complexes at the leading edge of the migrating neural cells. Loss of CAX protein expression reduced the ability of neural crest cells to form stable focal adhesions and undergo the initial cell spreading required for migration. The work presented by Melchionda et al. (2016) is a significant discovery providing evidence that lysosomal Ca2+ uptake is involved in cell migration and that lower organisms are useful model systems to investigate the role of acidic store Ca2+ in this critical cellular function during embryo development.
Melchionda et al. (2016) have made a significant step forward in our understanding of the mechanisms that regulate lysosomal/vacuolar Ca2+ entry. However, we remain in the dark about the identity of the transporters that pump Ca2+ into the lysosomes of placental mammals. What led to the loss of CAX genes in these organisms is as much a mystery as the identity of the transporters that have replaced CAX. Evidence from a study using purified mammalian lysosomes to observe Ca2+ uptake indicates that the process is ATP-dependent (Styrt et al., 1988). Placental mammals may have completely different ATP-dependent mechanisms governing lysosomal Ca2+ uptake compared with lower order organisms and nonplacental mammals. Interestingly, defects in lysosomal Ca2+ uptake are associated with two human diseases, Niemann-Pick type C and Chediak-Higashi syndrome (CHS; Styrt et al., 1988; Lloyd-Evans et al., 2008). The lysosomal accumulation of sphingosine, a Ca2+ ATPase inhibitor (Lloyd-Evans and Platt, 2011), leads to reduced lysosomal Ca2+ levels in Niemann-Pick type C disease cells and defects in NAADP-mediated lysosomal Ca2+ release (Lloyd-Evans et al., 2008). In CHS, there have been reports of enhanced lysosomal Ca2+ ATPase transporter activity in neutrophils (Styrt et al., 1988). Interestingly, CHS leukocytes show alterations in chemotaxis with a reduced response to chemotactic factors (Clark and Kimball, 1971), which is supportive of the findings of Melchionda et al. (2016). Much remains to be elucidated about the enigma of mammalian lysosomal Ca2+ uptake, but the work of Melchionda et al. (2016) begins to pick this mystery apart.
Acknowledgments
The author thanks Dr. Helen Waller-Evans for critical reading of the manuscript and assistance with the figure.
The author declares no competing financial interests.
References
- Berridge M.J., Bootman M.D., and Roderick H.L.. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Brailoiu E., Churamani D., Cai X., Schrlau M.G., Brailoiu G.C., Gao X., Hooper R., Boulware M.J., Dun N.J., Marchant J.S., and Patel S.. 2009. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 186:201–209. 10.1083/jcb.200904073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft P.J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.T., et al. 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 459:596–600. 10.1038/nature08030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K.A., Myers J.T., and Swanson J.A.. 2002. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 115:599–607. [DOI] [PubMed] [Google Scholar]
- Churchill G.C., Okada Y., Thomas J.M., Genazzani A.A., Patel S., and Galione A.. 2002. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 111:703–708. 10.1016/S0092-8674(02)01082-6 [DOI] [PubMed] [Google Scholar]
- Clark R.A., and Kimball H.R.. 1971. Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. J. Clin. Invest. 50:2645–2652. 10.1172/JCI106765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T., Gable K., and Beeler T.. 1994. Regulation of cellular Ca2+ by yeast vacuoles. J. Biol. Chem. 269:7273–7278. [PubMed] [Google Scholar]
- Gerasimenko J.V., Tepikin A.V., Petersen O.H., and Gerasimenko O.V.. 1998. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr. Biol. 8:1335–1338. 10.1016/S0960-9822(07)00565-9 [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E., and Platt F.M.. 2011. Lysosomal Ca2+ homeostasis: role in pathogenesis of lysosomal storage diseases. Cell Calcium. 50:200–205. 10.1016/j.ceca.2011.03.010 [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E., Morgan A.J., He X., Smith D.A., Elliot-Smith E., Sillence D.J., Churchill G.C., Schuchman E.H., Galione A., and Platt F.M.. 2008. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 14:1247–1255. 10.1038/nm.1876 [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E., Waller-Evans H., Peterneva K., and Platt F.M.. 2010. Endolysosomal calcium regulation and disease. Biochem. Soc. Trans. 38:1458–1464. 10.1042/BST0381458 [DOI] [PubMed] [Google Scholar]
- López-Sanjurjo C.I., Tovey S.C., Prole D.L., and Taylor C.W.. 2013. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 126:289–300. 10.1242/jcs.116103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda M., Pittman J.K., Mayor R., and Patel S.. 2016. Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J. Cell Biol. 10.1083/jcb.201510019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., and Cai X.. 2015. Evolution of acidic Ca2+ stores and their resident Ca2+-permeable channels. Cell Calcium. 57:222–230. 10.1016/j.ceca.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Patel S., and Muallem S.. 2011. Acidic Ca2+ stores come to the fore. Cell Calcium. 50:109–112. 10.1016/j.ceca.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Praitis V., Simske J., Kniss S., Mandt R., Imlay L., Feddersen C., Miller M.B., Mushi J., Liszewski W., Weinstein R., et al. 2013. The secretory pathway calcium ATPase PMR-1/SPCA1 has essential roles in cell migration during Caenorhabditis elegans embryonic development. PLoS Genet. 9:e1003506 10.1371/journal.pgen.1003506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M., Rietdorf K., Arredouani A., Davis L.C., Lloyd-Evans E., Koegel H., Funnell T.M., Morgan A.J., Ward J.A., Watanabe K., et al. 2010. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr. Biol. 20:703–709. 10.1016/j.cub.2010.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrt B., Pollack C.R., and Klempner M.S.. 1988. An abnormal calcium uptake pump in Chediak-Higashi neutrophil lysosomes. J. Leukoc. Biol. 44:130–135. [DOI] [PubMed] [Google Scholar]
- Sumoza-Toledo A., Lange I., Cortado H., Bhagat H., Mori Y., Fleig A., Penner R., and Partida-Sánchez S.. 2011. Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J. 25:3529–3542. 10.1096/fj.10-178483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Wang X., Chen M., Ouyang K., Song L.S., and Cheng H.. 2009. Calcium flickers steer cell migration. Nature. 457:901–905. 10.1038/nature07577 [DOI] [PMC free article] [PubMed] [Google Scholar]


